New Use for Old Drugs: The Protective Effect of Risperidone on Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Population-Based Study
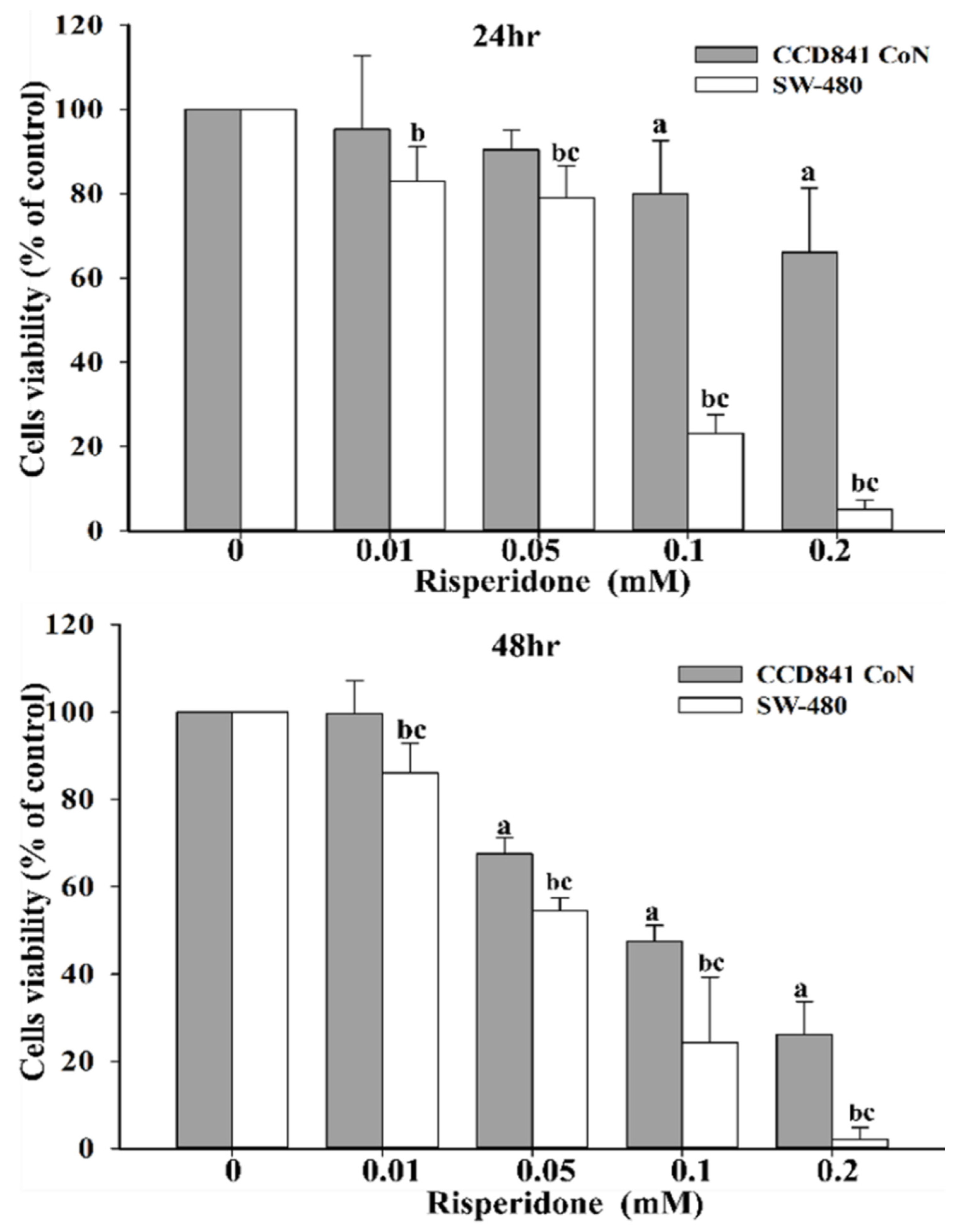
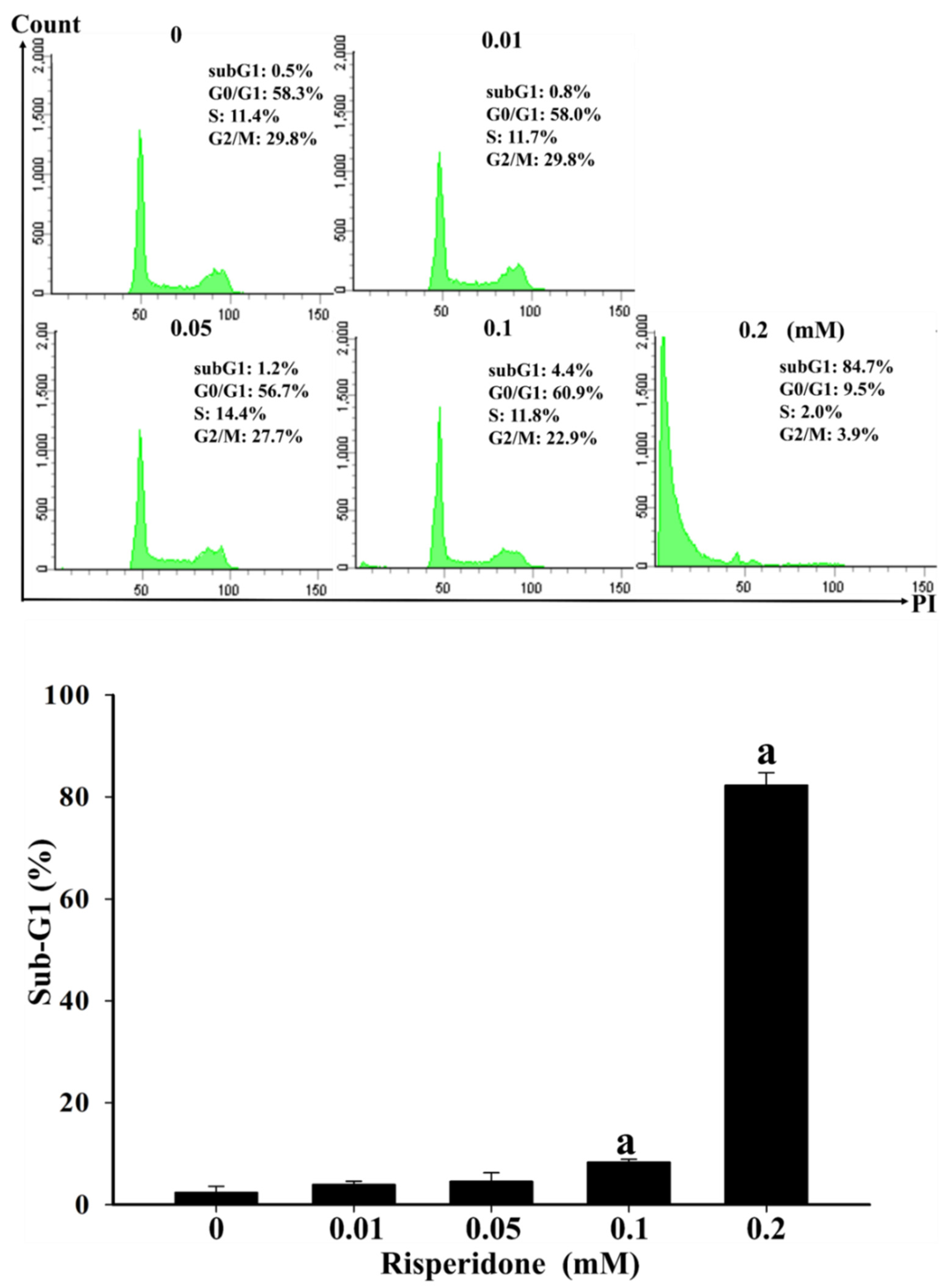
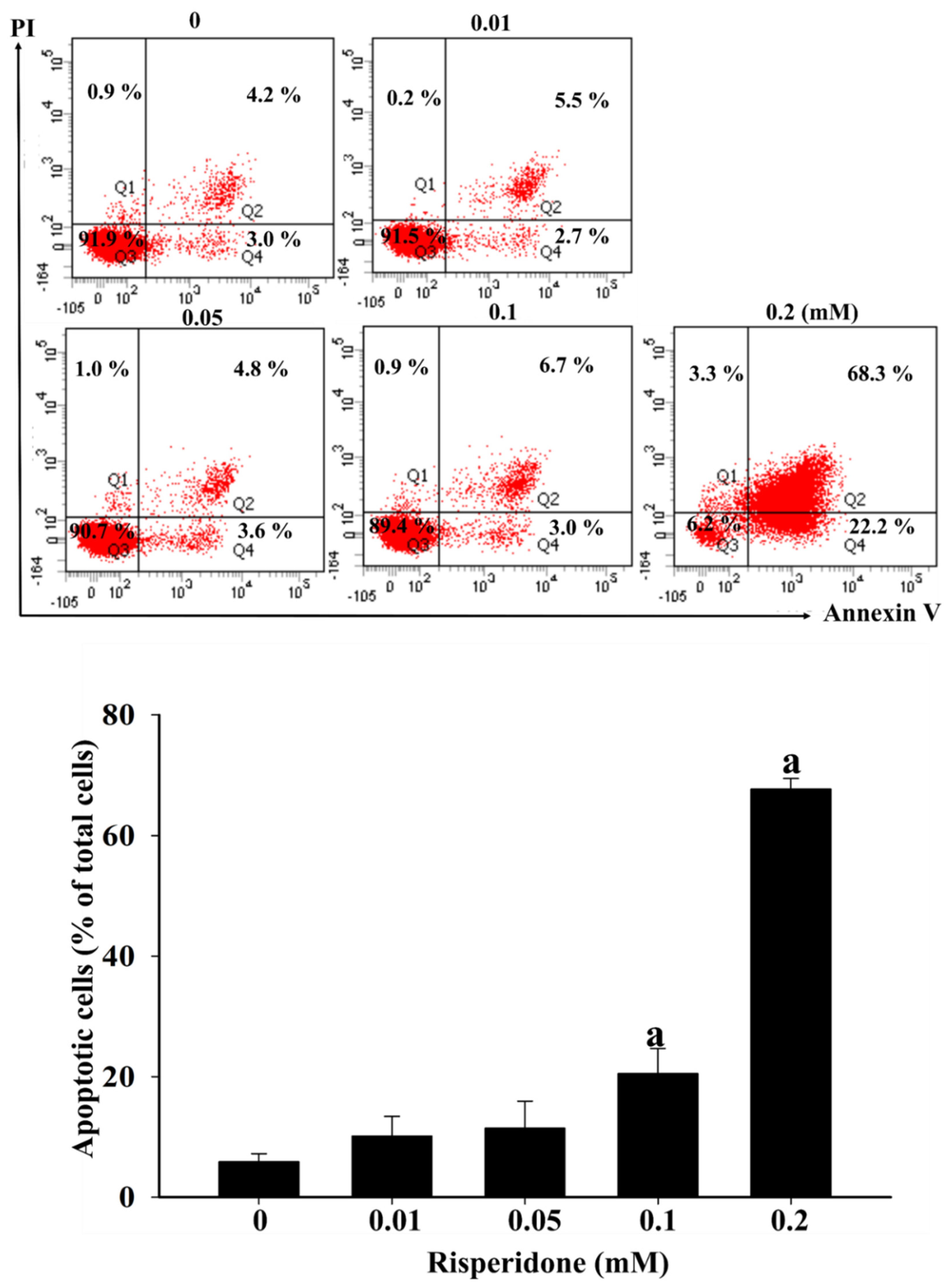
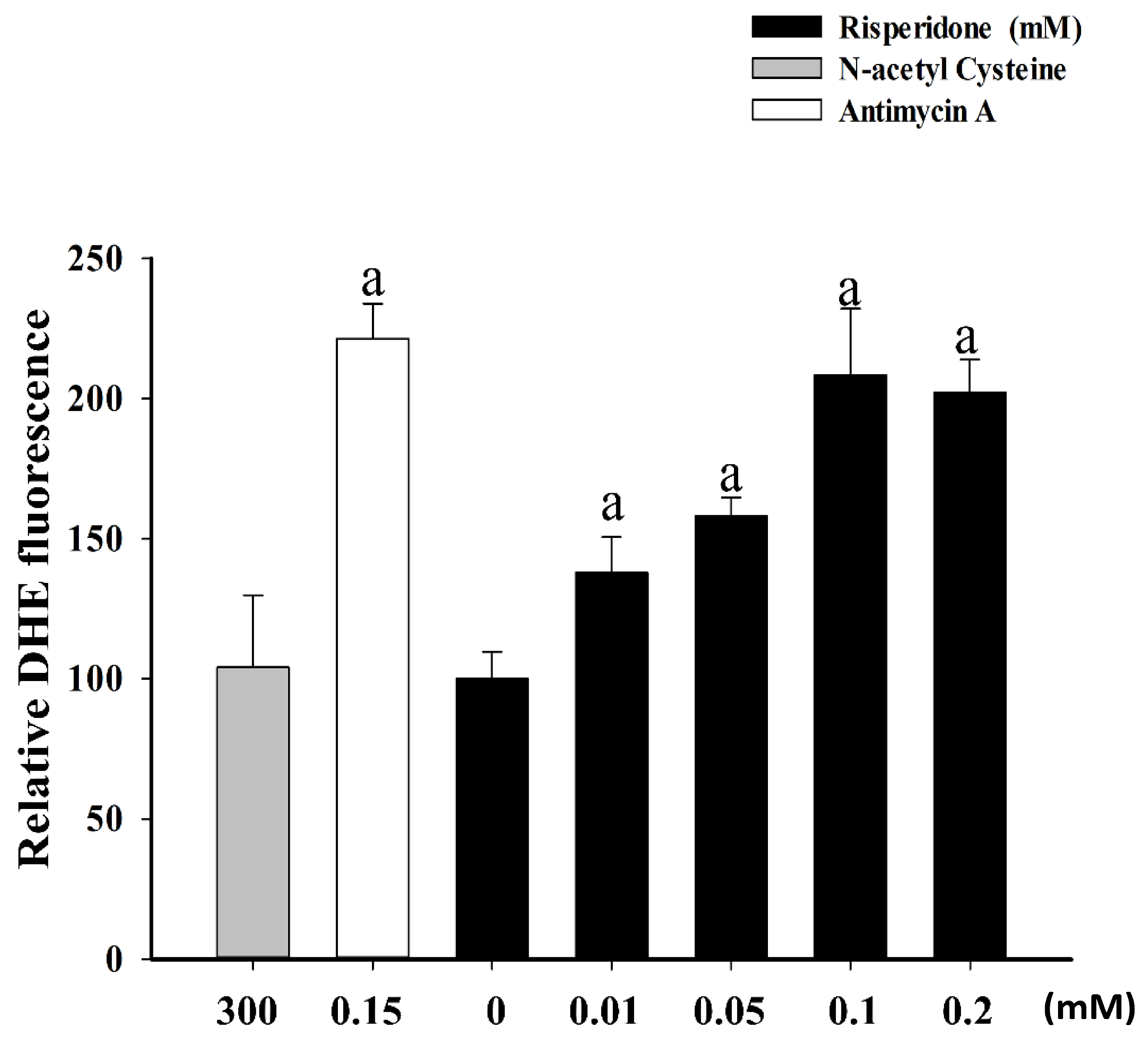
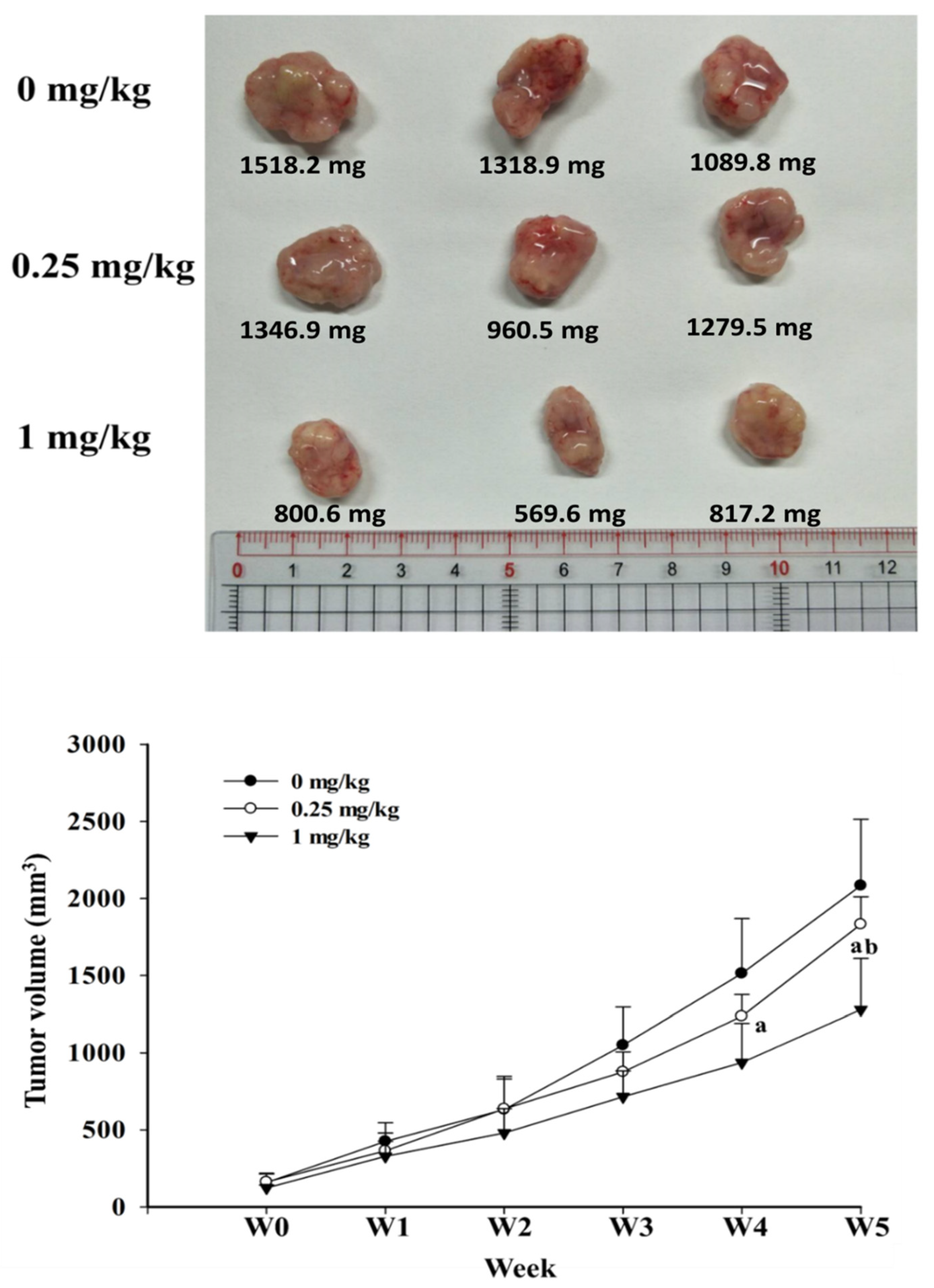
2.2. Bench Studies
3. Discussion
4. Strengths and Limitations
5. Material and Methods
5.1. Population-Based Study
5.2. Cell Study
5.2.1. MTT Assay
5.2.2. Flow Cytometry Analysis
5.2.3. Annexin V Assay
5.2.4. ROS Assay
5.2.5. Statistical Analysis
5.3. Animal Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sachs, R.E.; Ginsburg, P.B.; Goldman, D.P. Encouraging New Uses for Old Drugs. JAMA 2017, 318, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.; Chan, H.L.; Hsu, T.C.; Lu, M.L.; Lee, Y.C.; Lee, Y.; Siow, J.Y.; McIntyre, R.S.; Zhou, A.J.; Tzang, B.S.; et al. New use for old drugs: The protective effect of atypical antipsychotics on hepatocellular carcinoma. Int. J. Cancer 2019, 144, 2428–2439. [Google Scholar] [CrossRef]
- Gulbinat, W.; Dupont, A.; Jablensky, A.; Jensen, O.M.; Marsella, A.; Nakane, Y.; Sartorius, N. Cancer incidence of schizophrenic patients. Results of record linkage studies in three countries. Br. J. Psychiatry 1992, 161, 75–83. [Google Scholar] [CrossRef]
- Lichtermann, D.; Ekelund, J.; Pukkala, E.; Tanskanen, A.; Lonnqvist, J. Incidence of cancer among persons with schizophrenia and their relatives. Arch. Gen. Psychiatry 2001, 58, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B. The occurrence of cancer in first admitted schizophrenic patients. Schizophr. Res. 1994, 12, 185–194. [Google Scholar] [CrossRef]
- Dalton, S.O.; Mellemkjaer, L.; Thomassen, L.; Mortensen, P.B.; Johansen, C. Risk for cancer in a cohort of patients hospitalized for schizophrenia in Denmark, 1969–1993. Schizophr. Res. 2005, 75, 315–324. [Google Scholar] [CrossRef]
- Fleischhacker, W.W.; Cetkovich-Bakmas, M.; De Hert, M.; Hennekens, C.H.; Lambert, M.; Leucht, S.; Maj, M.; McIntyre, R.S.; Naber, D.; Newcomer, J.W.; et al. Comorbid somatic illnesses in patients with severe mental disorders: Clinical, policy, and research challenges. J. Clin. Psychiatry 2008, 69, 514–519. [Google Scholar] [CrossRef]
- Hait, W.N.; Lazo, J.S. Calmodulin: A potential target for cancer chemotherapeutic agents. J. Clin. Oncol. 1986, 4, 994–1012. [Google Scholar] [CrossRef]
- Nordenberg, J.; Fenig, E.; Landau, M.; Weizman, R.; Weizman, A. Effects of psychotropic drugs on cell proliferation and differentiation. Biochem. Pharmacol. 1999, 58, 1229–1236. [Google Scholar] [CrossRef]
- Dalton, S.O.; Johansen, C.; Poulsen, A.H.; Norgaard, M.; Sorensen, H.T.; McLaughlin, J.K.; Mortensen, P.B.; Friis, S. Cancer risk among users of neuroleptic medication: A population-based cohort study. Br. J. Cancer 2006, 95, 934–939. [Google Scholar] [CrossRef]
- Kim, D.H.; Stahl, S.M. Antipsychotic drug development. Behav. Neurobiol. Schizophr. Treat. 2010, 4, 123–139. [Google Scholar]
- Scarff, J.R.; Casey, D.A. Newer oral atypical antipsychotic agents: A review. Pharm. Ther. 2011, 36, 832–838. [Google Scholar]
- Kline, C.L.B.; Ralff, M.D.; Lulla, A.R.; Wagner, J.M.; Abbosh, P.H.; Dicker, D.T.; Allen, J.E.; El-Deiry, W.S. Role of Dopamine Receptors in the Anticancer Activity of ONC201. Neoplasia 2018, 20, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Jandaghi, P.; Najafabadi, H.S.; Bauer, A.S.; Papadakis, A.I.; Fassan, M.; Hall, A.; Monast, A.; von Knebel Doeberitz, M.; Neoptolemos, J.P.; Costello, E.; et al. Expression of DRD2 Is Increased in Human Pancreatic Ductal Adenocarcinoma and Inhibitors Slow Tumor Growth in Mice. Gastroenterology 2016, 151, 1218–1231. [Google Scholar] [CrossRef]
- Dilly, S.J.; Clark, A.J.; Marsh, A.; Mitchell, D.A.; Cain, R.; Fishwick, C.W.G.; Taylor, P.C. A chemical genomics approach to drug reprofiling in oncology: Antipsychotic drug risperidone as a potential adenocarcinoma treatment. Cancer Lett. 2017, 393, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Ciofani, G.; Conti, P. Antipsychotics reverse p-glycoprotein-mediated doxorubicin resistance in human uterine sarcoma MES-SA/Dx5 cells: A novel approach to cancer chemotherapy. J. Biol. Regul. Homeost. Agents 2015, 29, 357–365. [Google Scholar]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liaison in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta Rev. Cancer 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Seervi, M.; Rani, A.; Sharma, A.K.; Santhosh Kumar, T.R. ROS mediated ER stress induces Bax-Bak dependent and independent apoptosis in response to Thioridazine. Biomed. Pharmacother. 2018, 106, 200–209. [Google Scholar] [CrossRef]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev. 1991, 10, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Jiang, L.; Wang, X.Q.; Pan, W.; She, F.F.; Chen, Y.L. Establishment of and comparison between orthotopic xenograft and subcutaneous xenograft models of gallbladder carcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 3747–3752. [Google Scholar] [CrossRef]
- Ogata, Y.; Hara, Y.; Akagi, Y.; Ohkita, A.; Morodomi, T.; Shirouzu, K. Metastatic model of human colon cancer constructed using orthotopic implantation in nude mice. Kurume Med. J. 1998, 45, 121–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Introduction to the National Health Insurance Research Database (NHIRD). Available online: http://nhird.nhri.org.tw/date_01.html (accessed on 6 January 2020).
- Wacholder, S.; Silverman, D.T.; McLaughlin, J.K.; Mandel, J.S. Selection of controls in case-control studies. III. Design options. Am. J. Epidemiol. 1992, 135, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Chen, V.C.; Hsieh, Y.H.; Chen, L.J.; Hsu, T.C.; Tzang, B.S. Escitalopram oxalate induces apoptosis in U-87MG cells and autophagy in GBM8401 cells. J. Cell. Mol. Med. 2018, 22, 1167–1178. [Google Scholar] [CrossRef]

| Characteristic | Colorectal Cancer, n = 101,989 (%) | Non-Colorectal Cancer, n = 101,989 (%) | P-Value |
|---|---|---|---|
| Gender | 1.00 | ||
| Female | 49,357 (48.39) | 49,357 (48.39) | |
| Male | 52,632 (51.61) | 52,632 (51.61) | |
| Age at index date, year | 1.00 | ||
| 18–40 | 14,609 (14.32) | 14,609 (14.32) | |
| 40–50 | 24,788 (24.30) | 24,788 (24.30) | |
| 50–60 | 26,714 (26.19) | 26,714 (26.19) | |
| ≥60 | 35,878 (35.18) | 35,878 (35.18) | |
| Residence | 1.00 | ||
| Low | 7709 (7.56) | 7709 (7.56) | |
| Moderate | 16,871 (16.54) | 16,871 (16.54) | |
| High | 47,663 (46.73) | 47,663 (46.73) | |
| Very high | 29,746 (29.17) | 29,746 (29.17) | |
| Insurance premium (NTD a) | 1.00 | ||
| 0 | 16,937 (16.61) | 16,937 (16.61) | |
| 1–25,000 | 15,559 (15.26) | 15,559 (15.26) | |
| 25,001–40,000 | 47,181 (46.26) | 47,181 (46.26) | |
| ≥40,001 | 22,312 (21.88) | 22,312 (21.88) | |
| Aspirin, (>28 cDDD) | 21,461 (21.04) | 20,825 (20.42) | 0.0005 |
| NSAIDs, (>28 cDDD) | 69,258 (67.91) | 70,664 (69.29) | <0.0001 |
| Statin, (>28 cDDD) | 13,014 (12.76) | 12,167 (11.93) | <0.0001 |
| Antipsychotics, cDDD b | <0.0001 | ||
| 0–27 | 100,535 (98.57) | 99,647 (97.70) | |
| 28–83 | 1016 (1.00) | 1187 (1.16) | |
| 84–167 | 251 (0.25) | 389 (0.34) | |
| ≥168 | 187 (0.18) | 705 (0.69) | |
| FGAs c, cDDD b | <0.0001 | ||
| 0–27 | 100,580 (98.62) | 99760 (97.81) | |
| 28–83 | 973 (0.95) | 1173 (1.15) | |
| 84–167 | 246 (0.24) | 351 (0.31) | |
| ≥168 | 190 (0.19) | 2313 (0.75) | |
| SGAs d, cDDD b | <0.0001 | ||
| 0–27 | 101,744 (99.76) | 101,246 (99.27) | |
| 28–83 | 134 (0.13) | 272 (0.27) | |
| 84–167 | 39 (0.04) | 133 (0.13) | |
| ≥168 | 72 (0.07) | 338 (0.33) | |
| Medical diseases, yes | |||
| Hypertension | 36,970 (36.25) | 34,465 (33.79) | <0.0001 |
| Hyperlipidemia | 19,176 (18.80) | 18,467 (18.11) | <0.0001 |
| Diabetes | 19,349 (18.97) | 16,031 (15.72) | <0.0001 |
| COPD | 16,080 (15.77) | 15,845 (15.54) | 0.15 |
| Psychotic disorder | 584 (0.57) | 1386 (1.36) | <0.0001 |
| Depressive disorder | 3902 (3.83) | 4733 (4.64) | <0.0001 |
| Anxiety disorder | 17,446 (17.11) | 18,926 (18.56) | <0.0001 |
| Chronic kidney disease | 2662 (2.61) | 1686 (1.65) | <0.0001 |
| Alcohol-related disease | 479 (0.47) | 393 (0.39) | 0.0035 |
| Variable | Unadjusted Analysis | Adjusted Analysis * |
|---|---|---|
| Odds-Ratio (95% CI) | Odds-Ratio (95% CI) | |
| Antipsychotics, cDDD b | ||
| 0–27 | 1.00 (reference) | 1.00 (reference) |
| 28–83 | 0.85 (0.78–0.92) | 0.88 (0.80–0.96) |
| 84–167 | 0.64 (0.55–0.75) | 0.70 (0.60–0.83) |
| ≥168 | 0.24 (0.21–0.29) | 0.32 (0.27–0.38) |
| ATC-Code | Generic Name (cDDD a) | colorectal Cancer, n = 101989 (%) | Non-Colorectal Cancer, n = 101989 (%) | Adjusted Odds-Ratio b (95% CI) |
|---|---|---|---|---|
| FGAs | ||||
| 0–27 | 100,580 (98.62) | 99,760 (97.81) | 1.00 (reference) | |
| 28–83 | 973 (0.95) | 1173 (1.15) | 0.86 (0.78–0.93) | |
| 84–167 | 246 (0.24) | 351 (0.34) | 0.77 (0.65–0.91) | |
| ≥168 | 190 (0.19) | 705 (0.75) | 0.36 (0.30–0.43) | |
| SGAs | ||||
| 0–27 | 101,744 (99.76) | 101,246 (99.27) | 1.00 (reference) | |
| 28–83 | 134 (0.13) | 272 (0.27) | 0.57 (0.46–0.70) | |
| 84–167 | 39 (0.04) | 133 (0.13) | 0.35 (0.24–0.51) | |
| ≥168 | 72 (0.07) | 338 (0.33) | 0.32 (0.25–0.42) | |
| N05AC02 | Thioridazine | |||
| 0–27 | 101,848 (99.86) | 101,685 (99.70) | 1.00 (reference) | |
| 28–83 | 68 (0.07) | 101 (0.10) | 0.82 (0.60–1.13) | |
| 84–167 | 14 (0.01) | 49 (0.05) | 0.35 (0.19–0.63) | |
| ≥168 | 59 (0.06) | 154 (0.15) | 0.54 (0.40–0.74) | |
| N05AD01 | Haloperidol | |||
| 0–27 | 101,859 (99.87) | 101,555 (99.57) | 1.00 (reference) | |
| 28–83 | 76 (0.07) | 179 (0.18) | 0.53 (0.40–0.70) | |
| 84–167 | 31 (0.03) | 74 (0.07) | 0.55 (0.36–0.85) | |
| ≥168 | 23 (0.02) | 181 (0.18) | 0.20 (0.13–0.31) | |
| N05AL01 | Sulpiride | |||
| 0–27 | 101,053 (99.08) | 100,572 (98.61) | 1.00 (reference) | |
| 28–83 | 681 (0.67) | 852 (0.84) | 0.86 (0.78–0.96) | |
| 84–167 | 171 (0.17) | 247 (0.24) | 0.78 (0.64–0.96) | |
| ≥168 | 84 (0.08) | 318 (0.31) | 0.39 (0.30–0.49) | |
| N05AH02 | Clozapine | |||
| 0–27 | 101,983 (99.99) | 101,915 (99.93) | 1.00 (reference) | |
| ≥28 | 6 (0.01) | 74 (0.07) | 0.14 (0.06–0.33) | |
| N05AH03 | Olanzapine | |||
| 0–27 | 101,967 (99.98) | 101,864 (99.88) | 1.00 (reference) | |
| 28–83 | 5 (0.00) | 31 (0.03) | 0.22 (0.08–0.57) | |
| 84–167 | 5 (0.00) | 24 (0.02) | 0.35 (0.13–0.94) | |
| ≥168 | 12 (0.01) | 70 (0.07) | 0.32 (0.17–0.59) | |
| N05AH04 | Quetiapine | |||
| 0–27 | 101,880 (99.89) | 101,442 (99.46) | 1.00 (reference) | |
| 28–83 | 66 (0.06) | 144 (0.14) | 0.48 (0.36–0.64) | |
| 84–167 | 19 (0.02) | 93 (0.09) | 0.22 (0.13–0.36) | |
| ≥168 | 24 (0.02) | 310 (0.30) | 0.10 (0.06–0.15) | |
| N05AL05 | Amisulpride | |||
| 0–27 | 101,981 (99.99) | 101,926 (99.94) | 1.00 (reference) | |
| 28–83 | 6 (0.01) | 18 (0.02) | 0.48 (0.36–0.64) | |
| 84–167 | 0 (0.00) | 16 (0.02) | - | |
| ≥168 | 2 (0.00) | 29 (0.05) | 0.14 (0.03–0.58) | |
| N05AX08 | Risperidone | |||
| 0–27 | 101,874 (99.89) | 101,587 (99.61) | 1.00 (reference) | |
| 28–83 | 69 (0.07) | 151 (0.15) | 0.59 (0.44–0.79) | |
| 84–167 | 16 (0.02) | 70 (0.07) | 0.33 (0.19–0.57) | |
| ≥168 | 30 (0.03) | 181 (0.18) | 0.27 (0.18–0.40) | |
| N05AX12 | Aripiprazole | |||
| 0–27 | 101,979 (99.99) | 101,957 (99.97) | 1.00 (reference) | |
| 28–83 | 3 (0.00) | 13 (0.01) | 0.34 (0.09–1.22) | |
| 84–167 | 2 (0.00) | 6 (0.01) | 0.56 (0.11–2.88) | |
| ≥168 | 5 (0.00) | 13 (0.01) | 0.72 (0.25–2.06) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, V.C.-H.; Hsieh, Y.-H.; Lin, T.-C.; Lu, M.-L.; Liao, Y.-T.; Yang, Y.-H.; Hsu, T.-C.; Stewart, R.; Weng, J.-C.; Lee, M.-J.; et al. New Use for Old Drugs: The Protective Effect of Risperidone on Colorectal Cancer. Cancers 2020, 12, 1560. https://doi.org/10.3390/cancers12061560
Chen VC-H, Hsieh Y-H, Lin T-C, Lu M-L, Liao Y-T, Yang Y-H, Hsu T-C, Stewart R, Weng J-C, Lee M-J, et al. New Use for Old Drugs: The Protective Effect of Risperidone on Colorectal Cancer. Cancers. 2020; 12(6):1560. https://doi.org/10.3390/cancers12061560
Chicago/Turabian StyleChen, Vincent Chin-Hung, Yi-Hsuan Hsieh, Tzu-Chin Lin, Mong-Liang Lu, Yin-To Liao, Yao-Hsu Yang, Tsai-Ching Hsu, Robert Stewart, Jun-Cheng Weng, Min-Jing Lee, and et al. 2020. "New Use for Old Drugs: The Protective Effect of Risperidone on Colorectal Cancer" Cancers 12, no. 6: 1560. https://doi.org/10.3390/cancers12061560
APA StyleChen, V. C.-H., Hsieh, Y.-H., Lin, T.-C., Lu, M.-L., Liao, Y.-T., Yang, Y.-H., Hsu, T.-C., Stewart, R., Weng, J.-C., Lee, M.-J., Chiu, W.-C., & Tzang, B.-S. (2020). New Use for Old Drugs: The Protective Effect of Risperidone on Colorectal Cancer. Cancers, 12(6), 1560. https://doi.org/10.3390/cancers12061560





