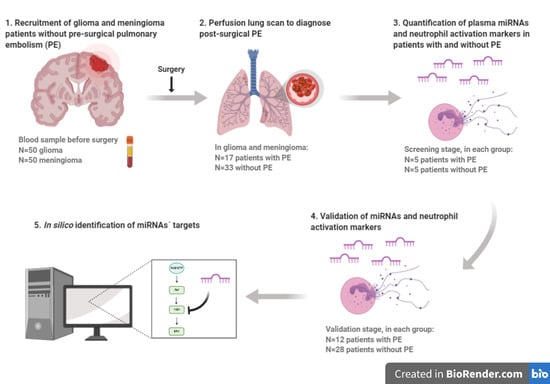
microRNAs and Markers of Neutrophil Activation as Predictors of Early Incidental Post-Surgical Pulmonary Embolism in Patients with Intracranial Tumors
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Subjects
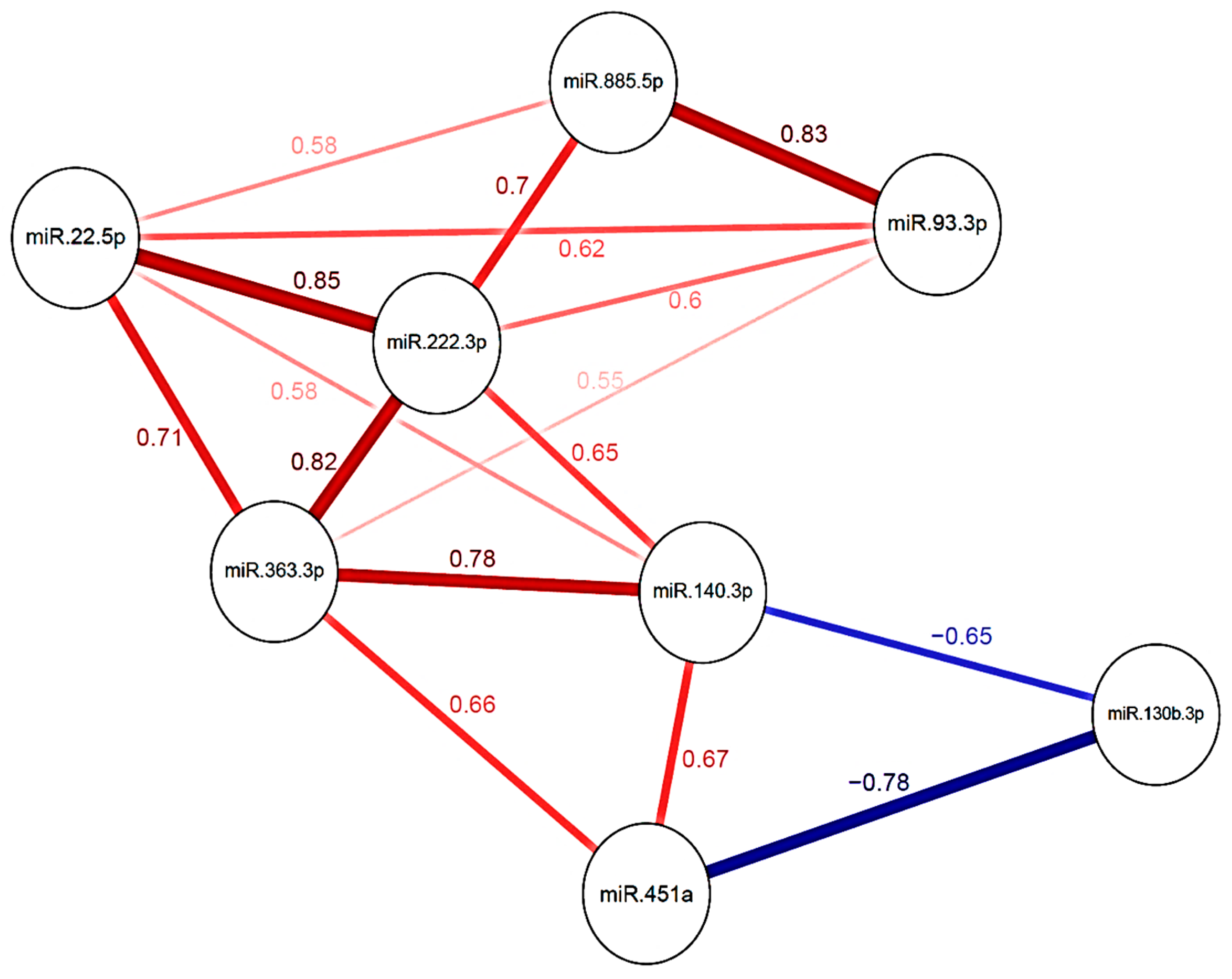
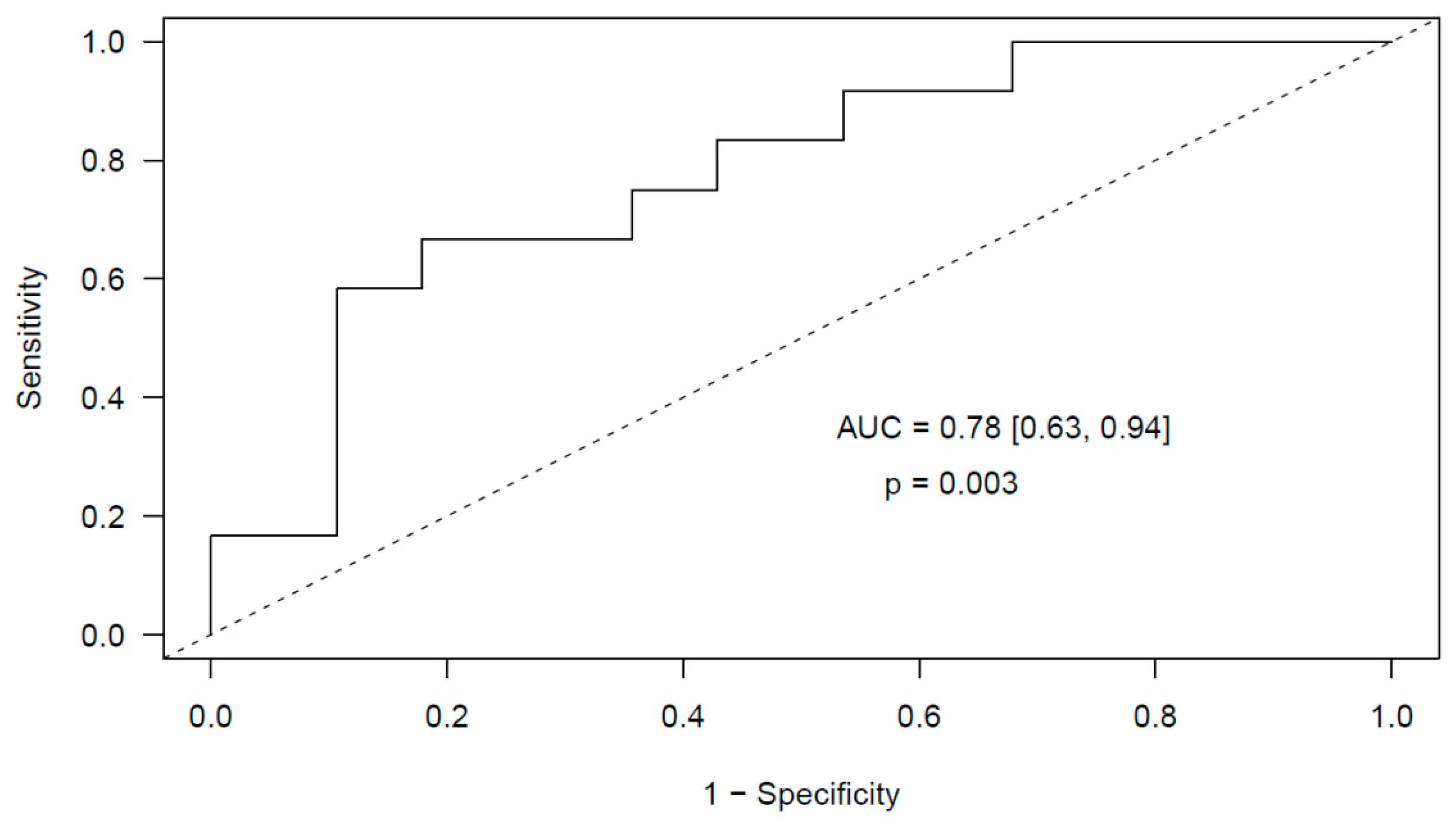
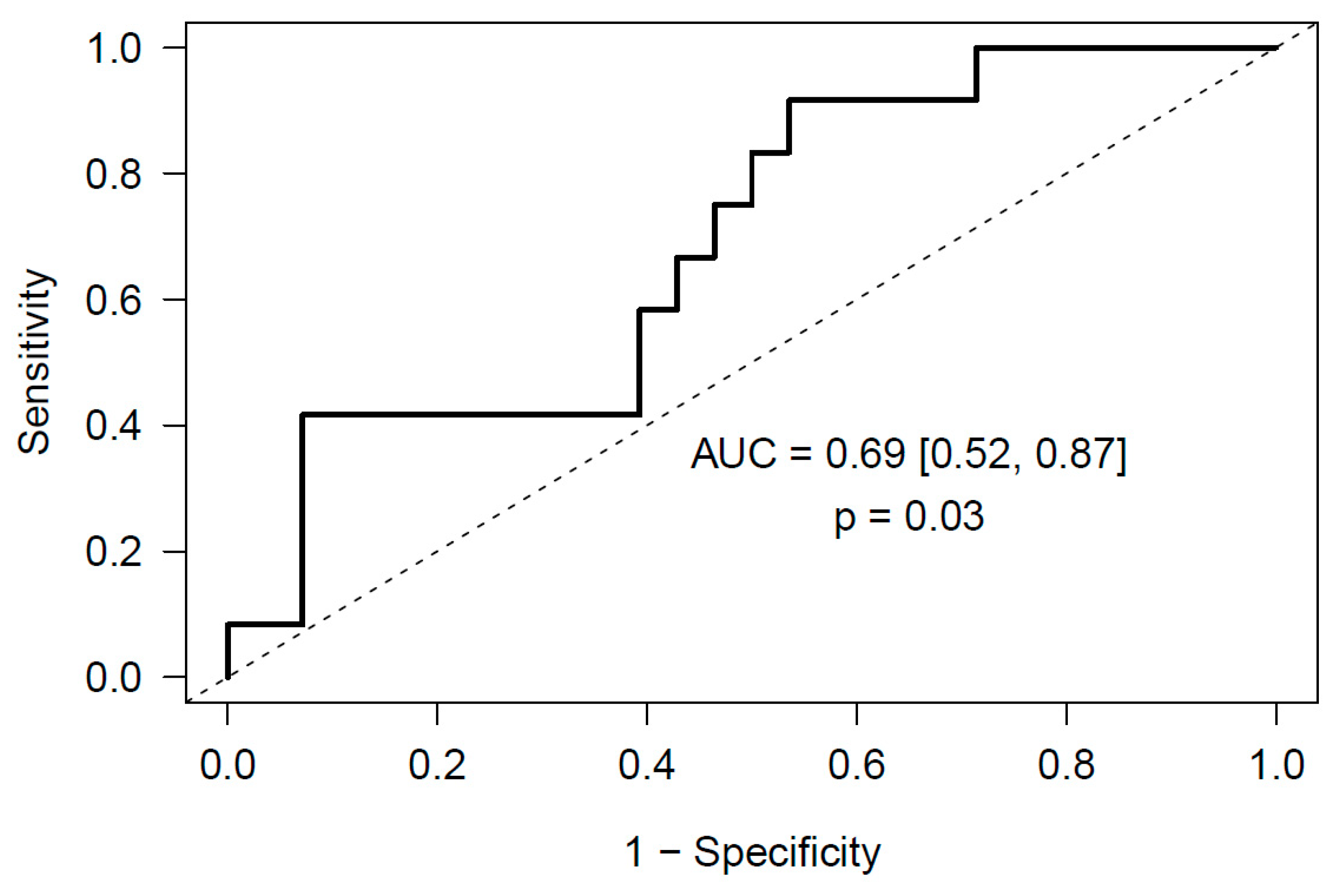
2.2. miRNA Expression Levels and Risk of Incidental Post-Surgical PE
2.2.1. Glioma
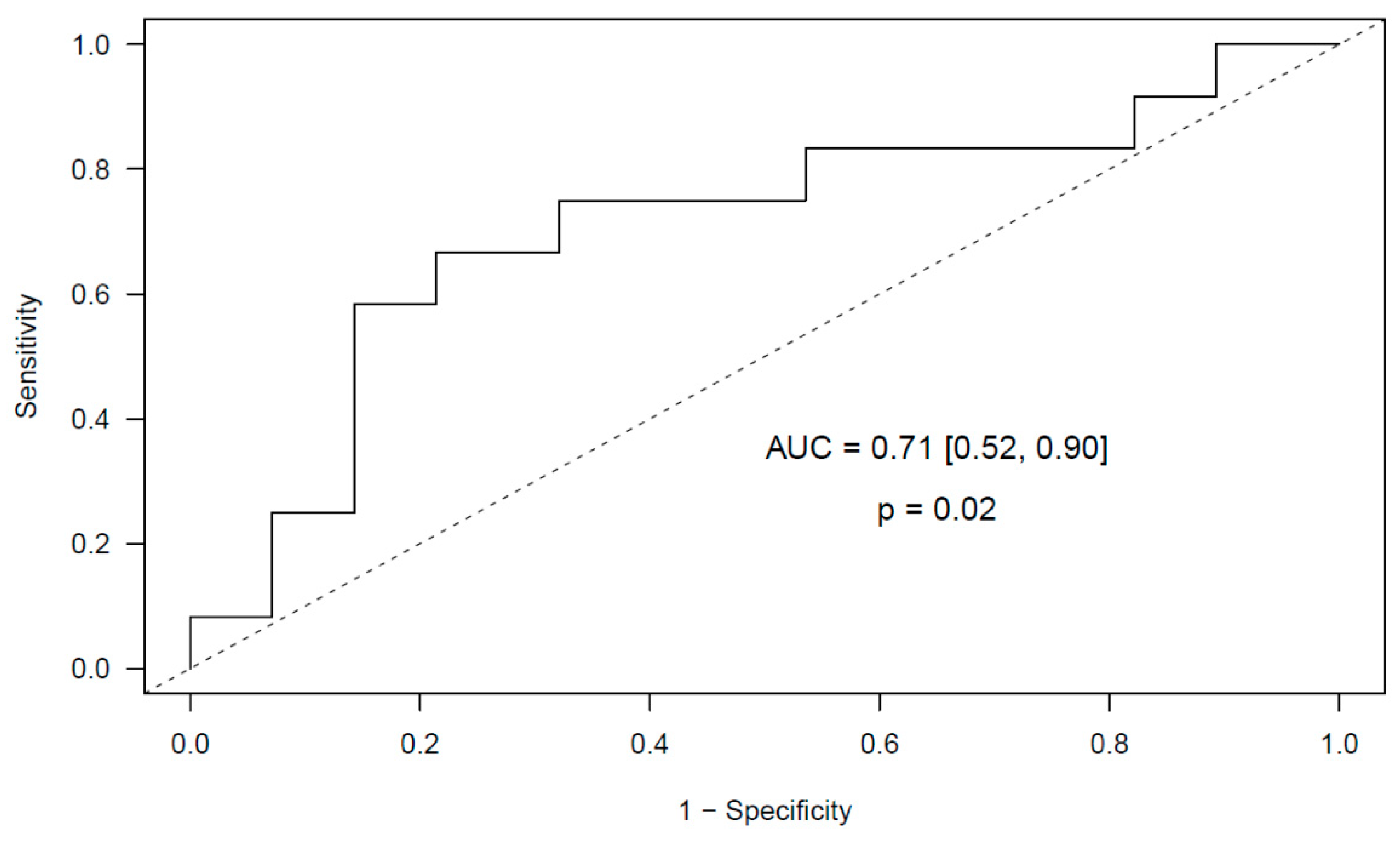
2.2.2. Meningioma
2.3. Markers of Neutrophil Activation and Risk of Incidental Post-Surgical PE
2.3.1. Glioma
2.3.2. Meningioma
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Blood Collection
4.3. miRNA Studies
4.3.1. RNA Isolation
4.3.2. Quantification of the Expression Level of miRNAs
Screening Stage
Validation Stage
4.3.3. Identification of miRNAs’ Targets
4.4. Quantification of Neutrophil Activation Markers
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ay, C.; Pabinger, I.; Cohen, A.T. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb. Haemost. 2017, 117, 219–230. [Google Scholar] [CrossRef]
- Cote, D.J.; Smith, T.R. Venous thromboembolism in brain tumor patients. J. Clin. Neurosci. 2016, 25, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, L.; Brown, D.A.; Bhargav, A.G.; Rusheen, A.E.; Naylor, R.M.; Gilder, H.E.; Monie, D.D.; Youssef, S.J.; Parney, I.F. Venous thromboembolic events in patients undergoing craniotomy for tumor resection: Incidence, predictors, and review of literature. J. Neurosurg. 2019, 132, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Streiff, M.B.; Ye, X.; Kickler, T.S.; Desideri, S.; Jani, J.; Fisher, J.; Grossman, S.A. A prospective multicenter study of venous thromboembolism in patients with newly-diagnosed high-grade glioma: Hazard rate and risk factors. J. Neurooncol. 2015, 124, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, G.; Riva, M.; Conte, V.; Di Cristofori, A.; Caroli, M.; Locatelli, M.; Castellani, M.; Bucciarelli, P.; Artoni, A.; Stocchetti, N.; et al. Risk of post-operative venous thromboembolism in patients with meningioma. J. Neurooncol. 2018, 138, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Nunno, A.; Li, Y.; Pieters, T.A.; Towner, J.E.; Schmidt, T.; Shi, M.; Walter, K.; Li, Y.M. Risk Factors and Associated Complications of Symptomatic Venous Thromboembolism in Patients with Craniotomy for Meningioma. World Neurosurg. 2019, 122, e1505–e1510. [Google Scholar] [CrossRef]
- Sousou, T.; Khorana, A.A. New insights into cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 316–320. [Google Scholar] [CrossRef]
- Nasser, N.J.; Fox, J.; Agbarya, A. Potential Mechanisms of Cancer-Related Hypercoagulability. Cancers (Basel) 2020, 12, 566. [Google Scholar] [CrossRef]
- Bluff, J.E.; Brown, N.J.; Reed, M.W.; Staton, C.A. Tissue factor, angiogenesis and tumour progression. Breast Cancer Res. 2008, 10, 204. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Liu, L.; Li, W.; Yang, Y.; Yuan, J. MicroRNA in Human Glioma. Cancers (Basel) 2013, 5, 1306–1331. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Vijayakumar, T.; Bakhshinyan, D.; Venugopal, C.; Singh, S.K. MicroRNA Regulation of Brain Tumour Initiating Cells in Central Nervous System Tumours. Stem Cells Int. 2015, 2015, 141793. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.C.; Ma, J. Role of MicroRNAs in Malignant Glioma. Chin. Med. J. (Engl.) 2015, 128, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wei, W.; Zhang, Z.; He, C.; Yang, R.; Zhang, J.; Wu, Z.; Huang, Q.; Jiang, Q. Identification of microRNAs associated with glioma diagnosis and prognosis. Oncotarget 2017, 8, 26394–26403. [Google Scholar] [CrossRef]
- Beyer, S.; Fleming, J.; Meng, W.; Singh, R.; Haque, S.J.; Chakravarti, A. The Role of miRNAs in Angiogenesis, Invasion and Metabolism and Their Therapeutic Implications in Gliomas. Cancers (Basel) 2017, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Zhou, G.; Wang, S.; Shi, Y.; Peng, Y.; Shao, N.; Guan, W.; Qu, H.; Zhang, Y.; Wang, Q.; et al. A microRNA expression signature predicts meningioma recurrence. Int. J. Cancer 2013, 132, 128–136. [Google Scholar] [CrossRef]
- Zhi, F.; Shao, N.; Li, B.; Xue, L.; Deng, D.; Xu, Y.; Lan, Q.; Peng, Y.; Yang, Y. A serum 6-miRNA panel as a novel non-invasive biomarker for meningioma. Sci. Rep. 2016, 6, 32067. [Google Scholar] [CrossRef]
- El-Gewely, M.R.; Andreassen, M.; Walquist, M.; Ursvik, A.; Knutsen, E.; Nystad, M.; Coucheron, D.H.; Myrmel, K.S.; Hennig, R.; Johansen, S.D. Differentially Expressed MicroRNAs in Meningiomas Grades I and II Suggest Shared Biomarkers with Malignant Tumors. Cancers (Basel) 2016, 8, 31. [Google Scholar] [CrossRef]
- Shea, A.; Harish, V.; Afzal, Z.; Chijioke, J.; Kedir, H.; Dusmatova, S.; Roy, A.; Ramalinga, M.; Harris, B.; Blancato, J.; et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016, 5, 1917–1946. [Google Scholar]
- Anthiya, S.; Griveau, A.; Loussouarn, C.; Baril, P.; Garnett, M.; Issartel, J.P.; Garcion, E. MicroRNA-Based Drugs for Brain Tumors. Trends Cancer 2018, 4, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Liberale, L.; Carbone, F.; Vecchié, A.; Díaz-Cañestro, C.; Camici, G.G.; Montecucco, F.; Dallegri, F. The Pathophysiological Role of Neutrophil Extracellular Traps in Inflammatory Diseases. Thromb. Haemost. 2018, 118, 006–027. [Google Scholar] [CrossRef] [PubMed]
- Folco, E.J.; Mawson, T.L.; Vromman, A.; Bernardes-Souza, B.; Franck, G.; Persson, O.; Nakamura, M.; Newton, G.; Luscinskas, F.W.; Libby, P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1alpha and Cathepsin G. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Vyas, M.V.; Gonen, L.; Tabasinejad, R.; Ostrom, Q.T.; Barnholtz-Sloan, J.; Suppiah, S.; Zadeh, G.; Aldape, K. Prognostic significance of preoperative neutrophilia on recurrence-free survival in meningioma. Neuro-Oncology 2017, 19, 1503–1510. [Google Scholar] [CrossRef]
- Mason, M.; Maurice, C.; McNamara, M.G.; Tieu, M.T.; Lwin, Z.; Millar, B.A.; Menard, C.; Laperriere, N.; Milosevic, M.; Atenafu, E.G.; et al. Neutrophil-lymphocyte ratio dynamics during concurrent chemo-radiotherapy for glioblastoma is an independent predictor for overall survival. J. Neurooncol. 2017, 132, 463–471. [Google Scholar] [CrossRef]
- Patell, R.; Rybicki, L.; McCrae, K.R.; Khorana, A.A. Predicting risk of venous thromboembolism in hospitalized cancer patients: Utility of a risk assessment tool. Am. J. Hematol. 2017, 92, 501–507. [Google Scholar] [CrossRef]
- Navone, S.E.; Guarnaccia, L.; Locatelli, M.; Rampini, P.; Caroli, M.; La Verde, N.; Gaudino, C.; Bettinardi, N.; Riboni, L.; Marfia, G.; et al. Significance and Prognostic Value of The Coagulation Profile in Patients with Glioblastoma: Implications for Personalized Therapy. World Neurosurg. 2019, 121, e621–e629. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Huang, H.; Jiang, Y.; Yang, L.; Lin, Z.; He, H.; Liu, T.; Wu, B.; Chen, J.; et al. miR-363-3p inhibits tumor growth by targeting PCNA in lung adenocarcinoma. Oncotarget 2017, 8, 20133–20144. [Google Scholar] [CrossRef]
- Floyd, D.H.; Zhang, Y.; Dey, B.K.; Kefas, B.; Breit, H.; Marks, K.; Dutta, A.; Herold-Mende, C.; Synowitz, M.; Glass, R.; et al. Novel anti-apoptotic microRNAs 582-5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS ONE 2014, 9, e96239. [Google Scholar] [CrossRef]
- Li, H.Y.; Liang, J.L.; Kuo, Y.L.; Lee, H.H.; Calkins, M.J.; Chang, H.T.; Lin, F.C.; Chen, Y.C.; Hsu, T.I.; Hsiao, M.; et al. miR-105/93-3p promotes chemoresistance and circulating miR-105/93-3p acts as a diagnostic biomarker for triple negative breast cancer. Breast Cancer Res. 2017, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, G.; Zhu, X.; Wang, Z.; Wang, H.; Bai, Y.; Sun, P.; Peng, L.; Wei, W.; Chen, G.; et al. miR-93-3p inhibition suppresses clear cell renal cell carcinoma proliferation, metastasis and invasion. Oncotarget 2017, 8, 82824–82834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Wang, D.; Wei, X. miR-22 suppresses tumorigenesis and improves radiosensitivity of breast cancer cells by targeting Sirt1. Biol. Res. 2017, 50, 27. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Takeshita, F.; Hino, Y.; Fukunaga, S.; Kudo, Y.; Tamaki, A.; Matsunaga, J.; Takahashi, R.U.; Takata, T.; Shimamoto, A.; et al. miR-22 represses cancer progression by inducing cellular senescence. J. Cell Biol. 2011, 193, 409–424. [Google Scholar] [CrossRef]
- Tian, Y.; Nan, Y.; Han, L.; Zhang, A.; Wang, G.; Jia, Z.; Hao, J.; Pu, P.; Zhong, Y.; Kang, C. MicroRNA miR-451 downregulates the PI3K/AKT pathway through CAB39 in human glioma. Int. J. Oncol. 2012, 40, 1105–1112. [Google Scholar]
- Su, Z.; Ni, L.; Yu, W.; Yu, Z.; Chen, D.; Zhang, E.; Li, Y.; Wang, Y.; Li, X.; Yang, S.; et al. MicroRNA-451a is associated with cell proliferation, migration and apoptosis in renal cell carcinoma. Mol. Med. Rep. 2015, 11, 2248–2254. [Google Scholar] [CrossRef]
- Ruhl, R.; Rana, S.; Kelley, K.; Espinosa-Diez, C.; Hudson, C.; Lanciault, C.; Thomas, C.R., Jr.; Liana Tsikitis, V.; Anand, S. microRNA-451a regulates colorectal cancer proliferation in response to radiation. BMC Cancer 2018, 18, 517. [Google Scholar] [CrossRef]
- Gillies, J.K.; Lorimer, I.A. Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle 2007, 6, 2005–2009. [Google Scholar] [CrossRef]
- Zhang, C.; Kang, C.; You, Y.; Pu, P.; Yang, W.; Zhao, P.; Wang, G.; Zhang, A.; Jia, Z.; Han, L.; et al. Co-suppression of miR-221/222 cluster suppresses human glioma cell growth by targeting p27kip1 in vitro and in vivo. Int. J. Oncol. 2009, 34, 1653–1660. [Google Scholar]
- Malzkorn, B.; Wolter, M.; Liesenberg, F.; Grzendowski, M.; Stuhler, K.; Meyer, H.E.; Reifenberger, G. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 2010, 20, 539–550. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Song, W.; Cui, H.; Chen, G.; Qiao, F.; Hu, J.; Zhou, R.; Fan, H. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J. Surg. Oncol. 2015, 13, 101. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Cao, L.; Zhang, T.; Yue, D.; Wang, L.; Ping, Y.; He, Q.; Zhang, C.; Wang, M.; et al. miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1R in hepatocellular carcinoma. Oncotarget 2017, 8, 86592–86603. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ye, Y.F.; Ruan, L.W.; Bao, L.; Wu, M.W.; Zhou, Y. Inhibition of miR-660-5p expression suppresses tumor development and metastasis in human breast cancer. Genet. Mol. Res. 2017, 16, gmr16019479. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Chen, P.; Jin, L.; Hu, J.; Li, Y.; Zhou, L.; Yang, S.; Mao, X.; Gui, Y.; Chen, Y.; et al. miR-660-5p is associated with cell migration, invasion, proliferation and apoptosis in renal cell carcinoma. Mol. Med. Rep. 2018, 17, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, J.; Zheng, L.; Ajani, J.A.; Wu, X.; Ye, Y. Serum miR-331-3p predicts tumor recurrence in esophageal adenocarcinoma. Sci. Rep. 2018, 8, 14006. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, H.; Li, X.; Tian, X.; Peng, B.; Liu, S.; Zhan, T.; Wan, Y.; Chen, W.; Li, Y.; et al. miR-331-3p functions as an oncogene by targeting ST7L in pancreatic cancer. Carcinogenesis 2018, 39, 1006–1015. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, B.; Liu, M.; Liu, Y.; Gao, R. miR-126-5p Restoration Promotes Cell Apoptosis in Cervical Cancer by Targeting Bcl2l2. Oncol. Res. 2017, 25, 463–470. [Google Scholar] [CrossRef]
- Oto, J.; Plana, E.; Sánchez-González, J.V.; García-Olaverri, J.; Fernández-Pardo, A.; España, F.; Martínez-Sarmiento, M.; Vera-Donoso, C.D.; Navarro, S.; Medina, P. Urinary microRNAs: Looking for a New Tool in Diagnosis, Prognosis, and Monitoring of Renal Cancer. Curr. Urol. Rep. 2020, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Grieco, F.A.; Sebastiani, G.; Juan-Mateu, J.; Villate, O.; Marroqui, L.; Ladriere, L.; Tugay, K.; Regazzi, R.; Bugliani, M.; Marchetti, P.; et al. MicroRNAs miR-23a-3p, miR-23b-3p, and miR-149-5p Regulate the Expression of Proapoptotic BH3-Only Proteins DP5 and PUMA in Human Pancreatic beta-Cells. Diabetes 2017, 66, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Konduri, S.D.; Rao, C.N.; Chandrasekar, N.; Tasiou, A.; Mohanam, S.; Kin, Y.; Lakka, S.S.; Dinh, D.; Olivero, W.C.; Gujrati, M.; et al. A novel function of tissue factor pathway inhibitor-2 (TFPI-2) in human glioma invasion. Oncogene 2001, 20, 6938–6945. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Kremer Hovinga, J.A.; Schatzberg, D.; Wagner, D.D.; Lämmle, B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood 2012, 120, 1157–1164. [Google Scholar] [CrossRef]
- Díaz, J.A.; Fuchs, T.A.; Jackson, T.O.; Kremer Hovinga, J.A.; Lammle, B.; Henke, P.K.; Myers, D.D., Jr.; Wagner, D.D.; Wakefield, T.W.; Michigan Research Venous Group. Plasma DNA is Elevated in Patients with Deep Vein Thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2013, 1, 341–348. [Google Scholar] [CrossRef]
- Van Montfoort, M.L.; Stephan, F.; Lauw, M.N.; Hutten, B.A.; Van Mierlo, G.J.; Solati, S.; Middeldorp, S.; Meijers, J.C.; Zeerleder, S. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 147–151. [Google Scholar] [CrossRef]
- Vallés, J.; Lago, A.; Santos, M.T.; Latorre, A.M.; Tembl, J.I.; Salom, J.B.; Nieves, C.; Moscardó, A. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: Prognostic significance. Thromb. Haemost. 2017, 117, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.B.; de los Reyes-García, A.M.; Rivera-Caravaca, J.M.; Valledor, P.; García-Barberá, N.; Roldan, V.; Vicente, V.; Martínez, C.; González-Conejero, R. miR-146a regulates neutrophil extracellular trap formation that predicts adverse cardiovascular events in patients with atrial fibrillation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 892–902. [Google Scholar] [CrossRef]
- Oto, J.; Navarro, S.; Larsen, A.C.; Solmoirago, M.J.; Plana, E.; Hervás, D.; Fernández-Pardo, A.; España, F.; Kristensen, S.R.; Thorlacius-Ussing, O.; et al. microRNAs and neutrophil activation markers predict venous thrombosis in pancreatic ductal adenocarcinoma and distal extrahepatic cholangiocarcinoma. Int. J. Mol. Sci. 2020, 21, 840. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Tafur, A.J.; Caprini, J.A.; Cote, L.; Trujillo-Santos, J.; Del Toro, J.; Garcia-Bragado, F.; Tolosa, C.; Barillari, G.; Visona, A.; Monreal, M.; et al. Predictors of active cancer thromboembolic outcomes. RIETE experience of the Khorana score in cancer-associated thrombosis. Thromb. Haemost. 2017, 117, 1192–1198. [Google Scholar] [PubMed]
- Van Es, N.; Di Nisio, M.; Cesarman, G.; Kleinjan, A.; Otten, H.M.; Mahe, I.; Wilts, I.T.; Twint, D.C.; Porreca, E.; Arrieta, O.; et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: A prospective cohort study. Haematologica 2017, 102, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Ramón-Núñez, L.A.; Martos, L.; Fernández-Pardo, A.; Oto, J.; Medina, P.; España, F.; Navarro, S. Comparison of protocols and RNA carriers for plasma miRNA isolation. Unraveling RNA carrier influence on miRNA isolation. PLoS ONE 2017, 12, e0187005. [Google Scholar] [CrossRef] [PubMed]
- Vincent, D.; Klinke, M.; Eschenburg, G.; Trochimiuk, M.; Appl, B.; Tiemann, B.; Bergholz, R.; Reinshagen, K.; Boettcher, M. NEC is likely a NETs dependent process and markers of NETosis are predictive of NEC in mice and humans. Sci. Rep. 2018, 8, 12612. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristic | Glioma Patients (n = 50) | Meningioma Patients (n = 50) |
|---|---|---|
| PE events, n (%) | 17 (34) | 17 (34) |
| Age, y | 61 (51–70) | 64 (50–71) |
| Female sex, n (%) | 22 (44) | 33 (66) |
| BMI, kg/m2 | 24.7 (22.2–27.4) | 25.9 (21.1–29.7) |
| Comorbidities, n (%) Cardiovascular Respiratory Metabolic * Miscellanea † | 20 (40) 1 (2) 8 (16) 5 (10) | 18 (36) 2 (4) 5 (10) 5 (10) |
| Pre-operative KPS ≥ 80, n (%) | 47 (94) | 49 (98) |
| Post-operative KPS ≥ 80, n (%) | 44 (88) | 47 (94) |
| WHO classification, n (%) Grade I Grade II Grade III Grade IV | 0 (--) 6 (12) 8 (16) 36 (72) | 45 (90) 5 (10) 0 (--) 0 (--) |
| Tumor location, n (%) Skull base Cerebral convexity-falx Superficial Deep-seated | 0 (--) 0 (--) 12 (24) 38 (76) | 15 (30) 35 (70) 0 (--) 0 (--) |
| Tumor dimension, cm3 | 24 (12.3–50.2) | 16 (5,8–35.2) |
| Duration of surgery, min | 240 (210–240) | 215 (176.3–277.5) |
| Khorana score, n (%) 0 1 | 27 (54) 23 (46) | 40 (80) 10 (20) |
| Hemoglobin, g/dL | 13.7 (12.7–14.9) | 12.9 (11.9–13.3) |
| WBC, ×103/mmc | 10.04 (6.64–12.10) | 6.10 (4.92–7.69) |
| Neutrophils, ×103/mmc | 6.03 (3.99–7.26) | 3.66 (2.95–4.62) |
| Platelets, ×103/mmc | 226 (188–248) | 216 (189–278) |
| PT ratio | 0.99 (0.93–1.09) | 1.02 (0.97–1.08) |
| APTT ratio | 0.81 (0.73–0.89) | 0.94 (0.86–1.01) |
| Fibrinogen, mg/dL | 229 (196–279) | 257 (222–304) |
| D-dimer, ng/mL | 218 (125–565) | 167 (100–210) |
| CRP, mg/L | 0.07 (0.03–0.18) | 0.11 (0.06–0.26) |
| eGFR, mL/min/1.73 m3 | 93.6 (79.8–113.9) | 90.8 (78.3–107.6) |
| miRNA | Sequence | Standardized OR | Fold-Change |
|---|---|---|---|
| miR-363-3p | aauugcacgguauccaucugua | 0.85 | −1.79 |
| miR-93-3p | acugcugagcuagcacuucccg | 0.91 | −1.75 |
| miR-22-5p | aguucuucaguggcaagcuuua | 0.99 | −2.50 |
| miR-130b-3p | cagugcaaugaugaaagggcau | 1.08 | 2.78 |
| miR-885-5p | uccauuacacuacccugccucu | 0.99 | −2.94 |
| miR-451a | aaaccguuaccauuacugaguu | 0.94 | −1.61 |
| miR-222-3p | agcuacaucuggcuacugggu | 0.88 | −1.54 |
| miR-140-3p | uaccacaggguagaaccacgg | 0.77 | −2.04 |
| Complement and Coagulation Cascades Pathway | ||
|---|---|---|
| miRNA | Predicted Target | Validated Target |
| miR-363-3p | C4BPA, CR2, CD55, KNG1 | - |
| miR-93-3p | PROS1, TFPI, MASP1, F9, C6, C8B, MBL2 | - |
| miR-22-5p | TFPI, F11, C8B | - |
| miR-130b-3p | SERPINA1, SERPING1, C3, MBL2, SERPINE1, C8A | F3 |
| miR-885-5p | CD59, CFI, KNG1 | - |
| miR-451a | - | - |
| miR-222-3p | - | - |
| miR-140-3p | CD59, SERPINA1, MASP1 | - |
| miRNA | Sequence | Fold-Change |
|---|---|---|
| miR-29a-3p | uagcaccaucugaaaucgguua | 1.57 |
| miR-660-5p | uacccauugcauaucggaguug | −1.59 |
| miR-331-3p | gccccugggccuauccuagaa | 2.20 |
| miR-126-5p | cauuauuacuuuugguacgcg | 1.99 |
| miR-23a-3p | aucacauugccagggauuucc | 1.91 |
| miR-23b-3p | aucacauugccagggauuaccac | 1.95 |
| Complement and Coagulation Cascades Pathway | ||
|---|---|---|
| miRNA | Predicted Target | Validated Target |
| miR-29a-3p | BDKRB1, CR1, KNG1, BDKRB2, C8G | FGA, FGB, FGG |
| miR-660-5p | C9, KNG1 | - |
| miR-331-3p | C3AR1, CFB, F10, F11, KLKB1, SERPINF2, F7 | - |
| miR-126-5p | CR2, F8, F9 | - |
| miR-23a-3p | CR1, F11, F2R, F8, MBL2, PLAU, PLAUR, PROS1, C1S, SERPINC1 | - |
| miR-23b-3p | CR1, F11, F2R, F8, MBL2, PLAUR, PROS1, C1S, SERPINC1 | PLAU |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oto, J.; Plana, E.; Solmoirago, M.J.; Fernández-Pardo, Á.; Hervás, D.; Cana, F.; España, F.; Artoni, A.; Bucciarelli, P.; Carrabba, G.; et al. microRNAs and Markers of Neutrophil Activation as Predictors of Early Incidental Post-Surgical Pulmonary Embolism in Patients with Intracranial Tumors. Cancers 2020, 12, 1536. https://doi.org/10.3390/cancers12061536
Oto J, Plana E, Solmoirago MJ, Fernández-Pardo Á, Hervás D, Cana F, España F, Artoni A, Bucciarelli P, Carrabba G, et al. microRNAs and Markers of Neutrophil Activation as Predictors of Early Incidental Post-Surgical Pulmonary Embolism in Patients with Intracranial Tumors. Cancers. 2020; 12(6):1536. https://doi.org/10.3390/cancers12061536
Chicago/Turabian StyleOto, Julia, Emma Plana, María José Solmoirago, Álvaro Fernández-Pardo, David Hervás, Fernando Cana, Francisco España, Andrea Artoni, Paolo Bucciarelli, Giorgio Carrabba, and et al. 2020. "microRNAs and Markers of Neutrophil Activation as Predictors of Early Incidental Post-Surgical Pulmonary Embolism in Patients with Intracranial Tumors" Cancers 12, no. 6: 1536. https://doi.org/10.3390/cancers12061536
APA StyleOto, J., Plana, E., Solmoirago, M. J., Fernández-Pardo, Á., Hervás, D., Cana, F., España, F., Artoni, A., Bucciarelli, P., Carrabba, G., Navarro, S., Merati, G., & Medina, P. (2020). microRNAs and Markers of Neutrophil Activation as Predictors of Early Incidental Post-Surgical Pulmonary Embolism in Patients with Intracranial Tumors. Cancers, 12(6), 1536. https://doi.org/10.3390/cancers12061536