Current Landscape of Breast Cancer Imaging and Potential Quantitative Imaging Markers of Response in ER-Positive Breast Cancers Treated with Neoadjuvant Therapy
Abstract
1. Introduction
2. Imaging of Neoadjuvant Treatment Response in Breast Cancers
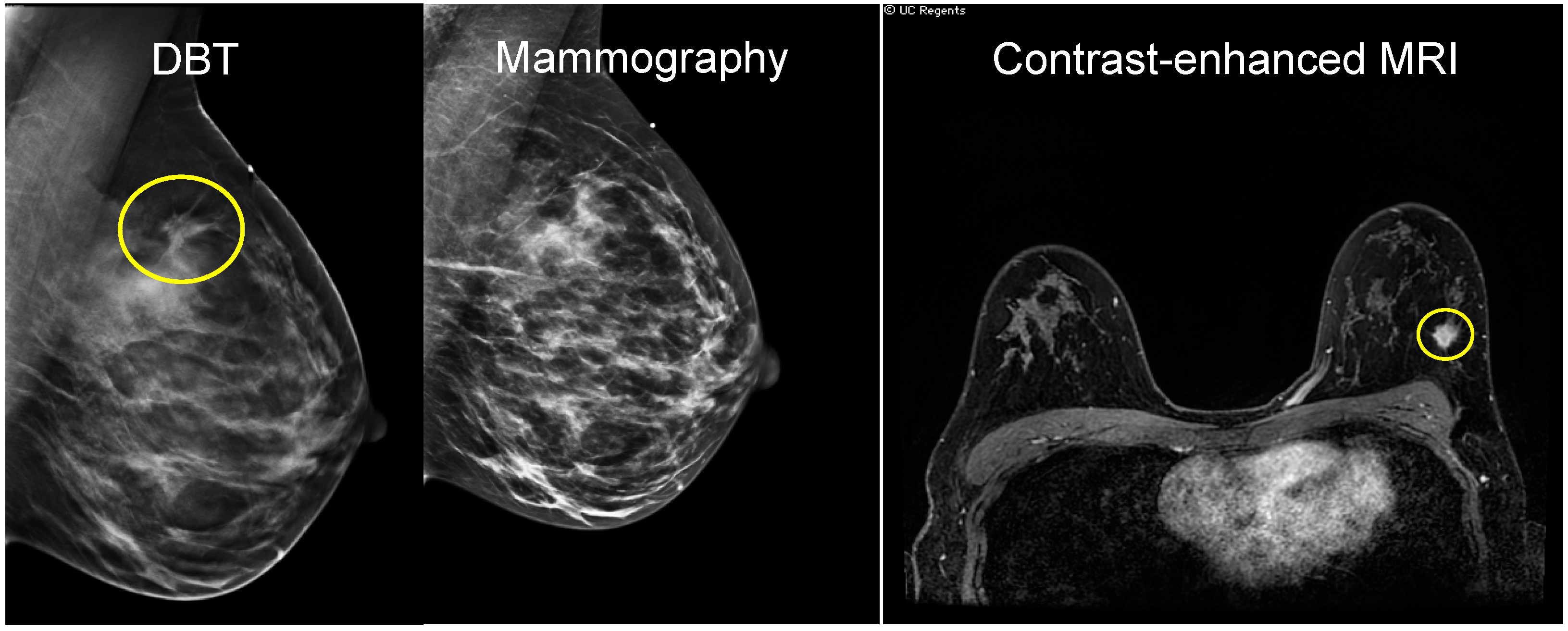
2.1. Mammography
2.2. Ultrasound
2.3. Magnetic Resonance Imaging
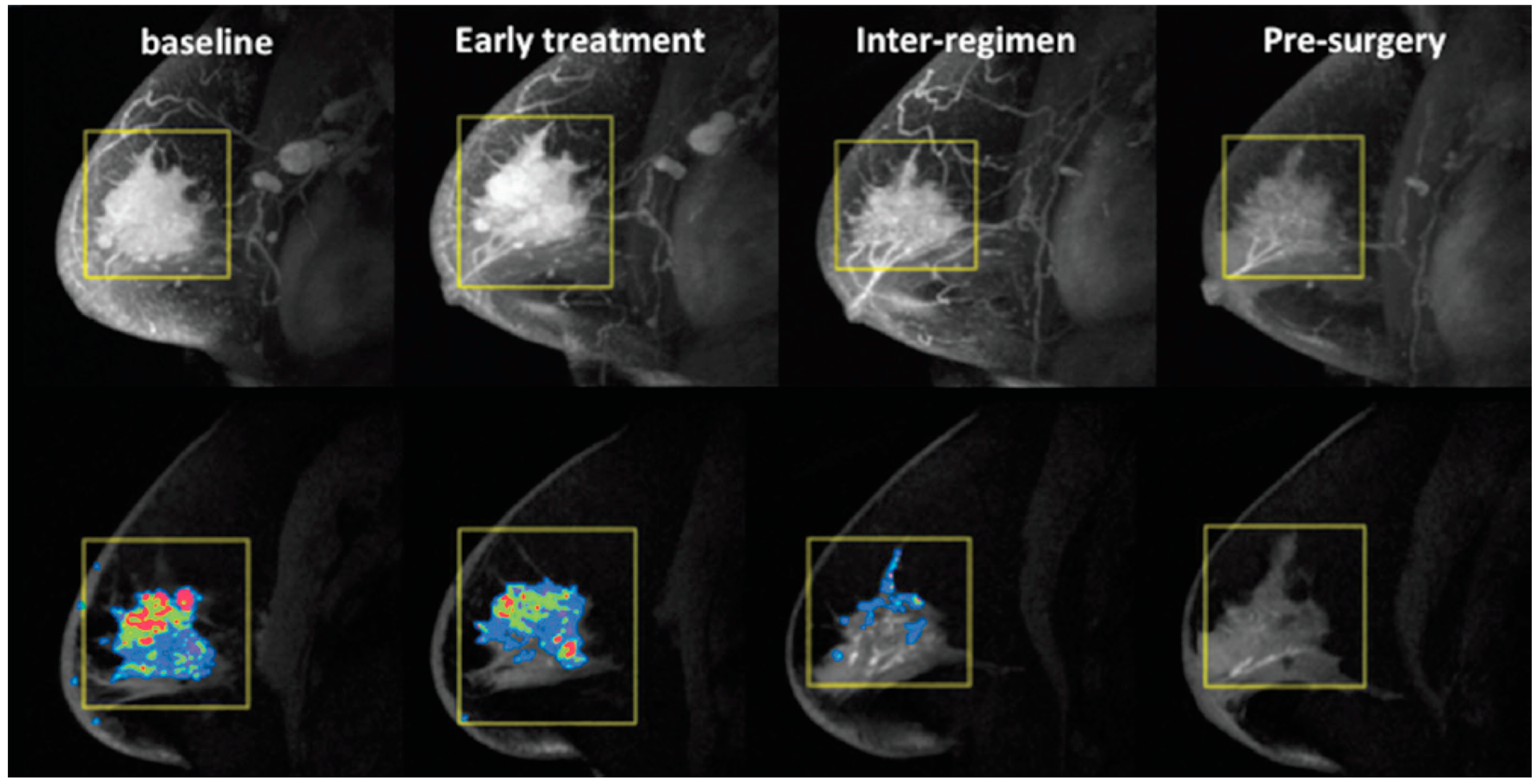
2.3.1. Tumor Volume
2.3.2. Pharmacokinetic Parameters
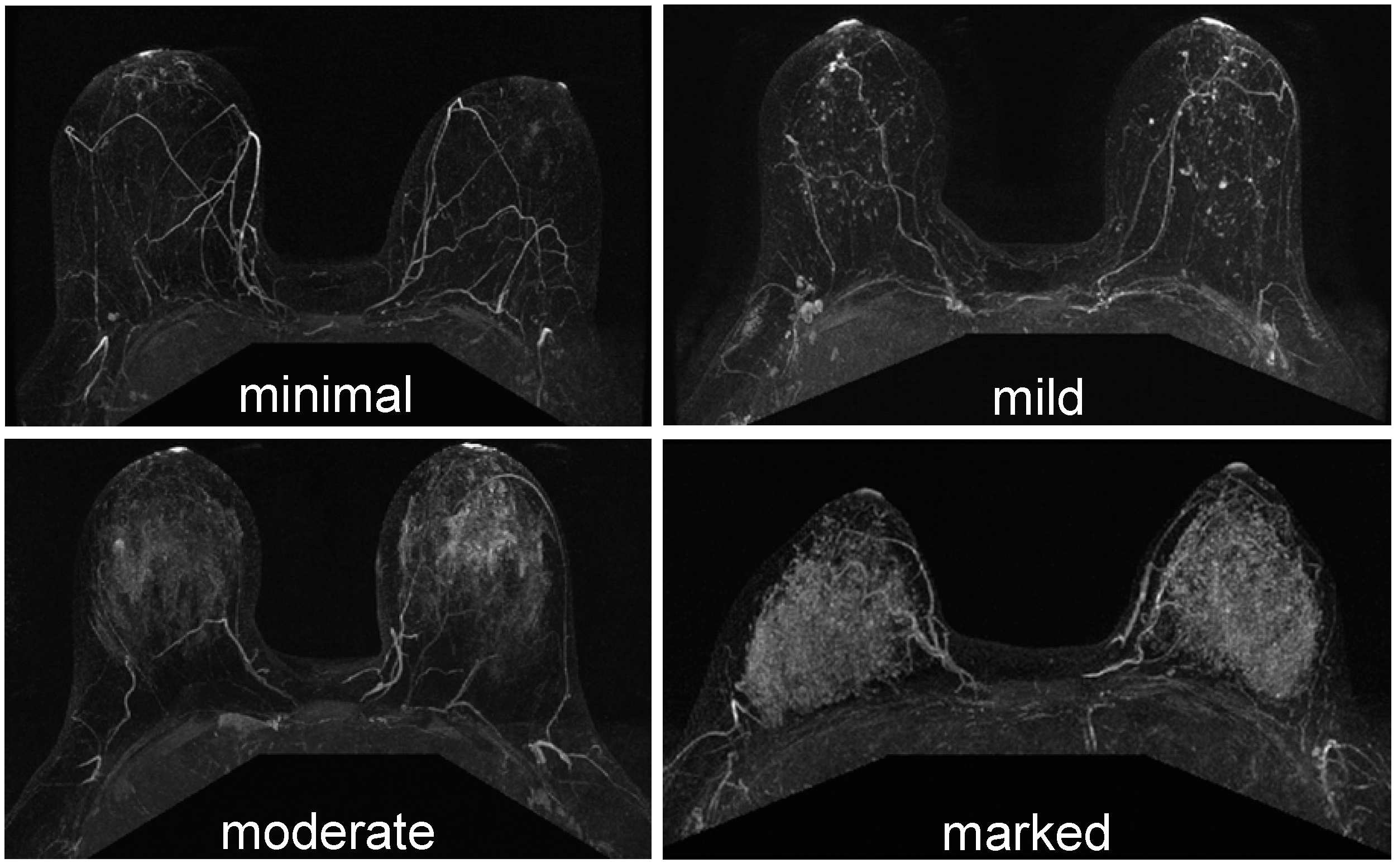
2.3.3. Background Parenchymal Enhancement
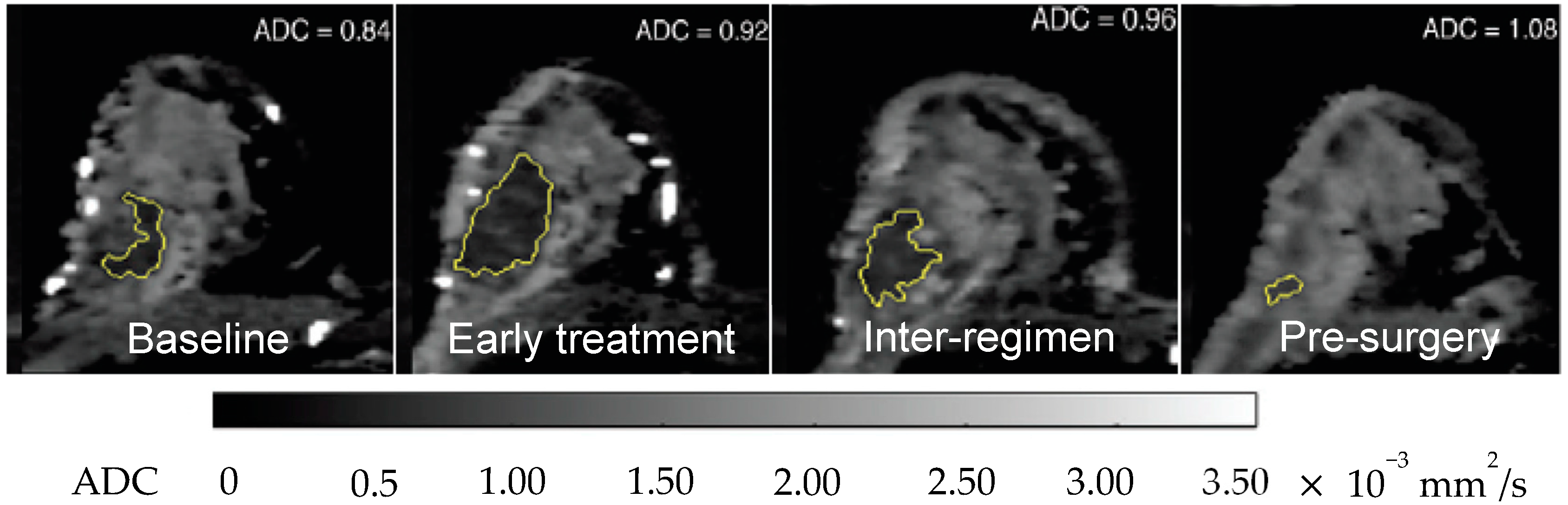
2.3.4. Apparent Diffusion Coefficient
2.3.5. Magnetic Resonance Spectroscopy
2.4. Positron Emission Tomography
2.4.1. [18F]fluorodeoxyglucose
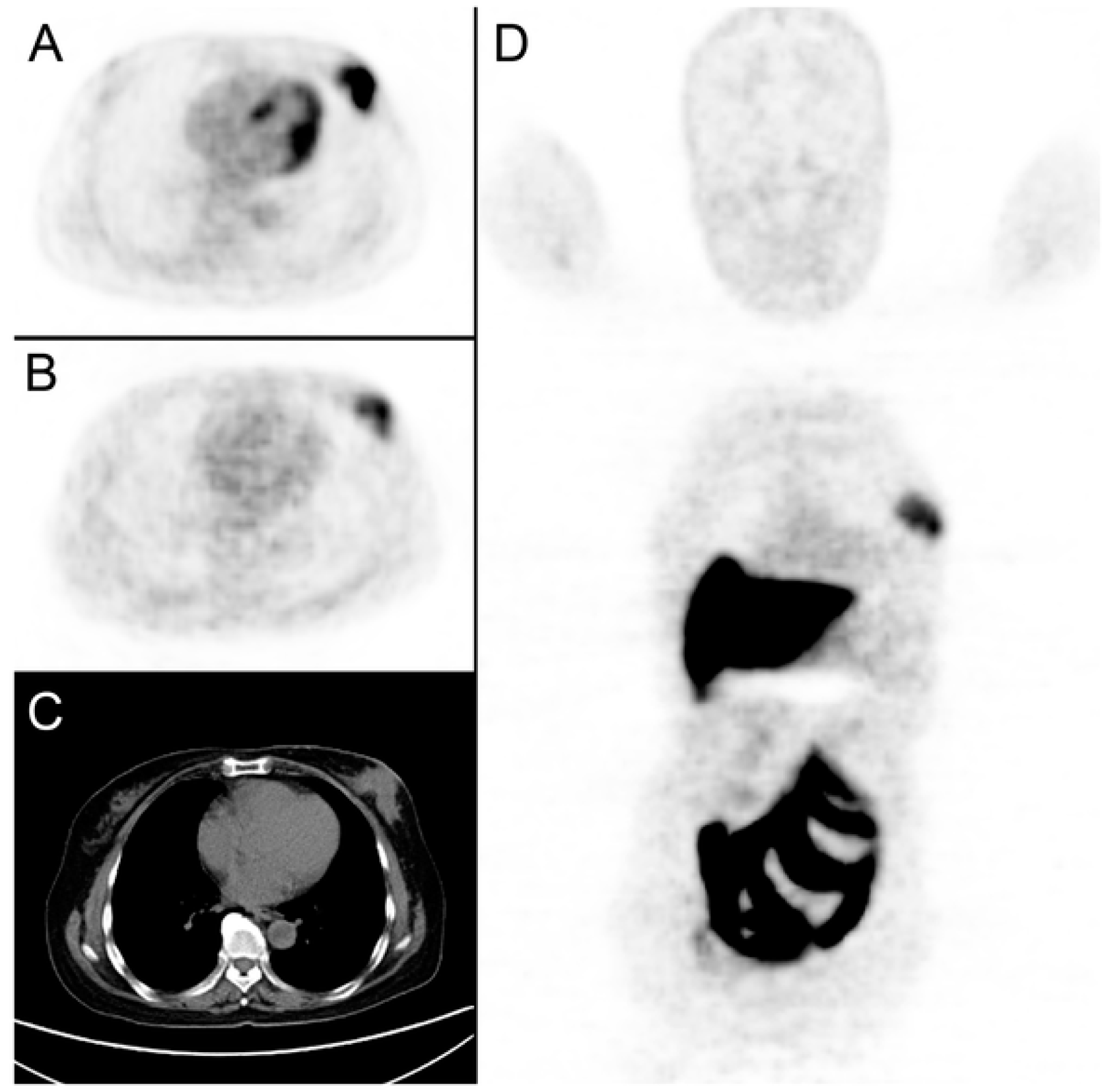
2.4.2. [18F]fluoroestradiol
2.4.3. Other Radiotracers
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Neo, S.Y.; McShane, L.M.; Korn, E.L.; Long, P.M.; Jazaeri, A.; Martiat, P.; Fox, S.B.; Harris, A.L.; Liu, E.T. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. USA 2003, 100, 10393–10398. [Google Scholar] [CrossRef] [PubMed]
- Van ‘t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Cheng, S.H.; Dressman, H.; Pittman, J.; Tsou, M.H.; Horng, C.F.; Bild, A.; Iversen, E.S.; Liao, M.; Chen, C.M.; et al. Gene expression predictors of breast cancer outcomes. Lancet 2003, 361, 1590–1596. [Google Scholar] [CrossRef]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Anderson, W.F.; Chen, B.E.; Jatoi, I.; Rosenberg, P.S. Effects of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res. Treat. 2006, 100, 121–126. [Google Scholar] [CrossRef]
- Goncalves, R.; Ma, C.; Luo, J.; Suman, V.; Ellis, M.J. Use of neoadjuvant data to design adjuvant endocrine therapy trials for breast cancer. Nat. Rev. Clin. Oncol. 2012, 9, 223–229. [Google Scholar] [CrossRef]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998, 16, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 2001, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Criscitiello, C.; Curigliano, G. Neoadjuvant Model for Testing Emerging Targeted Therapies in Breast Cancer. J. Natl. Cancer Inst. Monogr. 2015, 2015, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Kong, X.; Moran, M.S.; Zhang, N.; Haffty, B.; Yang, Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur. J. Cancer 2011, 47, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Silva, D.D.; Alessi, J.V.; Mano, M.S. Neoadjuvant endocrine therapy in breast cancer: Current role and future perspectives. Ecancermedicalscience 2016, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.H.; Ellis, M.J.; Ma, C.X. Neoadjuvant endocrine therapy in primary breast cancer: Indications and use as a research tool. Br. J. Cancer 2010, 103, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Zotano, A.L.; Arteaga, C.L. Neoadjuvant Trials in ER(+) Breast Cancer: A Tool for Acceleration of Drug Development and Discovery. Cancer Discov. 2017, 7, 561–574. [Google Scholar] [CrossRef]
- Ueno, T.; Saji, S.; Masuda, N.; Kuroi, K.; Sato, N.; Takei, H.; Yamamoto, Y.; Ohno, S.; Yamashita, H.; Hisamatsu, K.; et al. Impact of clinical response to neoadjuvant endocrine therapy on patient outcomes: A follow-up study of JFMC34-0601 multicentre prospective neoadjuvant endocrine trial. ESMO Open 2018, 3, e000314. [Google Scholar] [CrossRef]
- Guidance for Industry. Pathologic Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval; Draft Guidance. Available online: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM305501.pdf (accessed on 13 April 2020).
- Di Cosimo, S.; Arpino, G.; Generali, D. Neoadjuvant treatment of HER2 and hormone-receptor positive breast cancer—Moving beyond pathological complete response. Breast 2014, 23, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pinard, C.; Debled, M.; Ben Rejeb, H.; Velasco, V.; Tunon de Lara, C.; Hoppe, S.; Richard, E.; Brouste, V.; Bonnefoi, H.; MacGrogan, G. Residual cancer burden index and tumor-infiltrating lymphocyte subtypes in triple-negative breast cancer after neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Lopez-Tarruella, S.; Garcia-Saenz, J.A.; Khan, Q.J.; Gomez, H.L.; Prat, A.; Moreno, F.; Jerez-Gilarranz, Y.; Barnadas, A.; Picornell, A.C.; et al. Pathological Response and Survival in Triple-Negative Breast Cancer Following Neoadjuvant Carboplatin plus Docetaxel. Clin. Cancer Res. 2018, 24, 5820–5829. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef]
- Cottu, P.; D’Hondt, V.; Dureau, S.; Lerebours, F.; Desmoulins, I.; Heudel, P.E.; Duhoux, F.P.; Levy, C.; Mouret-Reynier, M.A.; Dalenc, F.; et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann. Oncol. 2018, 29, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Ellis, M.J.; Coop, A.; Singh, B.; Tao, Y.; Llombart-Cussac, A.; Janicke, F.; Mauriac, L.; Quebe-Fehling, E.; Chaudri-Ross, H.A.; Evans, D.B.; et al. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 2003, 63, 6523–6531. [Google Scholar] [PubMed]
- Dowsett, M.; Smith, I.E.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Griffith, C.; Boeddinghaus, I.; Salter, J.; Detre, S.; Hills, M.; et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin. Cancer Res. 2005, 11, 951s–958s. [Google Scholar] [PubMed]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Goncalves, R.; Sanati, S.; Creighton, C.J.; DeSchryver, K.; Crouch, E.; Brink, A.; Watson, M.; et al. Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J. Clin. Oncol. 2017, 35, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Griffith, C.; Boeddinghaus, I.; Salter, J.; Detre, S.; Hills, M.; Ashley, S.; et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: Influence of hormonal status and HER-2 in breast cancer—A study from the IMPACT trialists. J. Clin. Oncol. 2005, 23, 2477–2492. [Google Scholar] [CrossRef] [PubMed]
- Rimm, D.L.; Leung, S.C.Y.; McShane, L.M.; Bai, Y.; Bane, A.L.; Bartlett, J.M.S.; Bayani, J.; Chang, M.C.; Dean, M.; Denkert, C.; et al. An international multicenter study to evaluate reproducibility of automated scoring for assessment of Ki67 in breast cancer. Mod. Pathol. 2019, 32, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Tao, Y.; Luo, J.; A’Hern, R.; Evans, D.B.; Bhatnagar, A.S.; Chaudri Ross, H.A.; von Kameke, A.; Miller, W.R.; Smith, I.; et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J. Natl. Cancer Inst. 2008, 100, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.M.; Mankoff, D.A.; Joe, B.N. Imaging Neoadjuvant Therapy Response in Breast Cancer. Radiology 2017, 285, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Gupta, A.; Reynolds, K.L.; Gadd, M.A.; Ellisen, L.W.; Isakoff, S.J.; Moy, B.; Bardia, A. Neoadjuvant Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Savaridas, S.L.; Sim, Y.T.; Vinnicombe, S.J.; Purdie, C.A.; Thompson, A.M.; Evans, A. Are baseline ultrasound and mammographic features associated with rates of pathological completes response in patients receiving neoadjuvant chemotherapy for breast cancer? Cancers Imaging 2019, 19, 1470–7330. [Google Scholar] [CrossRef]
- Dialani, V.; Chadashvili, T.; Slanetz, P.J. Role of Imaging in Neoadjuvant Therapy for Breast Cancer. Ann. Surg. Oncol. 2015, 22, 1416–1424. [Google Scholar] [CrossRef]
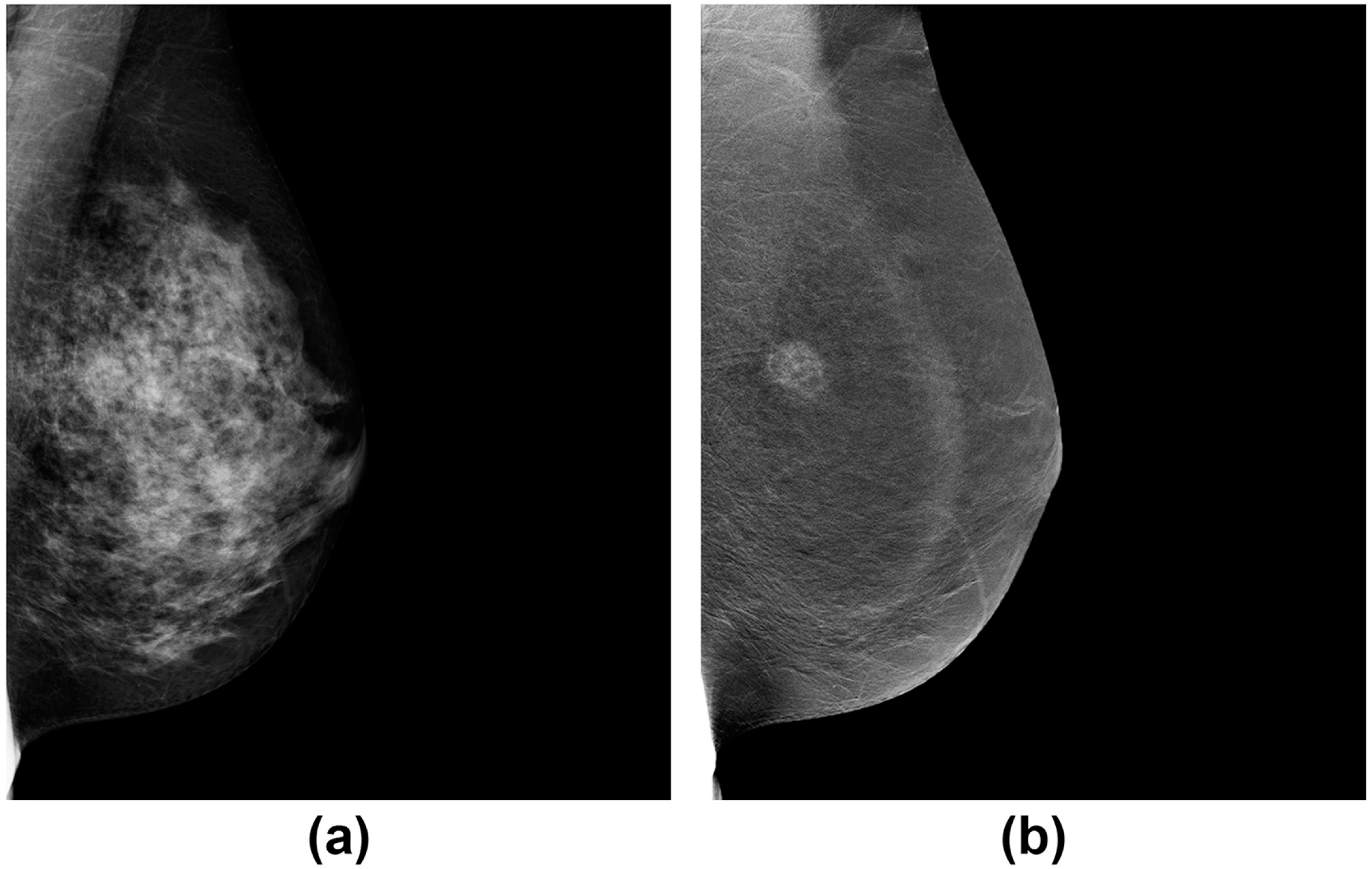
- Weiss, A.; Lee, K.C.; Romero, Y.; Ward, E.; Kim, Y.; Ojeda-Fournier, H.; Einck, J.; Blair, S.L. Calcifications on Mammogram Do Not Correlate with Tumor Size After Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2014, 21, 3310–3316. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, Y.; Mamtani, A.; Morrow, M.; Stempel, M.M.; Patil, S.; Jochelson, M.S. Do Calcifications Seen on Mammography After Neoadjuvant Chemotherapy for Breast Cancer Always Need to Be Excised? Ann. Surg. Oncol. 2017, 24, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Adrada, B.E.; Huo, L.; Lane, D.L.; Arribas, E.M.; Resetkova, E.; Yang, W. Histopathologic Correlation of Residual Mammographic Microcalcifications After Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer. Ann. Surg. Oncol. 2015, 22, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Moskovic, E.C.; Mansi, J.L.; King, D.M.; Murch, C.R.; Smith, I.E. Mammography in the assessment of response to medical treatment of large primary breast cancer. Clin. Radiol. 1993, 47, 339–344. [Google Scholar] [CrossRef]
- Haque, W.; Verma, V.; Hatch, S.; Suzanne Klimberg, V.; Brian Butler, E.; Teh, B.S. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2018, 170, 559–567. [Google Scholar] [CrossRef]
- Tot, T. Early (<10 mm) HER2-Positive Invasive Breast Carcinomas are Associated with Extensive Diffuse High-Grade DCIS: Implications for Preoperative Mapping, Extent of Surgical Intervention, and Disease-Free Survival. Ann. Surg. Oncol. 2015, 22, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Hooley, R.J.; Durand, M.A.; Philpotts, L.E. Advances in Digital Breast Tomosynthesis. Am. J. Roentgenol. 2016, 208, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Edmund, A.; Franken, J.; Garg, M.; Fajardo, L.L.; Niklason, L.T. Breast Tomosynthesis: Present Considerations and Future Applications. Radiographics 2007, 27, S231–S240. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.S.; Kim, H.H.; Shin, H.J.; Cha, J.H.; Ruppel, P.L.; Oh, H.Y.; Chae, E.Y. Assessment of extent of breast cancer: Comparison between digital breast tomosynthesis and full-field digital mammography. Clin. Radiol. 2013, 68, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chae, E.Y.; Cha, J.H.; Shin, H.J.; Choi, W.J.; Choi, Y.-W.; Kim, H.H. Comparison of mammography, digital breast tomosynthesis, automated breast ultrasound, magnetic resonance imaging in evaluation of residual tumor after neoadjuvant chemotherapy. Eur. J. Radiol. 2018, 108, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Richman, I.; Hoag, J.R.; Busch, S.; Gross, C.P. Early adoption of digital breast tomosynthesis in the United States. J. Clin. Oncol. 2018, 36, e13543. [Google Scholar] [CrossRef]
- Puong, S.; Bouchevreau, X.; Patoureaux, F.; Iordache, R.; Muller, S. Dual-energy contrast enhanced digital mammography using a new approach for breast tissue canceling. In Proceedings of the Medical Imaging, San Diego, CA, USA, 21 March 2007. [Google Scholar]
- Alexander, S.; Dulku, G.; Hashoul, S.; Taylor, D.B. Practical uses of contrast-enhanced spectral mammography in daily work: A pictorial review. J. Med. Imaging Radiat Oncol. 2019, 63, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; Balleyguier, C.; Adler, G.; Garbay, J.R.; Delaloge, S. Contrast-enhanced digital mammography. Eur. J. Radiol. 2009, 69, 34–42. [Google Scholar] [CrossRef] [PubMed]
- James, J.J.; Tennant, S.L. Contrast-enhanced spectral mammography (CESM). Clin. Radiol. 2018, 73, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Iotti, V.; Ravaioli, S.; Vacondio, R.; Coriani, C.; Caffarri, S.; Sghedoni, R.; Nitrosi, A.; Ragazzi, M.; Gasparini, E.; Masini, C.; et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: A comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Hilal, T.; Covington, M.; Zhang, N.; Kosiorek, H.E.; Lobbes, M.; Northfelt, D.W.; Pockaj, B.A. Contrast-Enhanced Spectral Mammography is Comparable to MRI in the Assessment of Residual Breast Cancer Following Neoadjuvant Systemic Therapy. Ann. Surg. Oncol. 2018, 25, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.M.; Kessels, A.G.H.; Beets, G.L.; Rupa, J.D.; Koster, D.; van Engelshoven, J.M.A.; von Meyenfeldt, M.F. Preoperative estimation of the pathological breast tumour size by physical examination, mammography and ultrasound: A prospective study on 105 invasive tumours. Eur. J. Radiol. 2003, 48, 285–292. [Google Scholar] [CrossRef]
- Keune, J.D.; Jeffe, D.B.; Schootman, M.; Hoffman, A.; Gillanders, W.E.; Aft, R.L. Accuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancer. Am. J. Surg. 2010, 199, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, R.P.; Bassett, R.L.; Symmans, W.F.; Ramineni, M.; Moulder, S.L.; Kuerer, H.M.; Thompson, A.M.; Yang, W.T. Performance of Mid-Treatment Breast Ultrasound and Axillary Ultrasound in Predicting Response to Neoadjuvant Chemotherapy by Breast Cancer Subtype. Oncologist 2017, 22, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, S.; Kamala, S.; Murthy, S.S.; Kotha, S.; Rao, Y.S.; Chaudhary, K.V. Predicting the molecular subtype of breast cancer based on mammography and ultrasound findings. Indian J. Radiol. Imaging 2018, 28, 354–361. [Google Scholar] [CrossRef]
- Vane, M.L.G.; van Nijnatten, T.J.A.; Nelemans, P.J.; Lobbes, M.B.I.; van Roozendaal, L.M.; Kooreman, L.F.S.; Keymeulen, K.B.M.I.; Smidt, M.L.; Schipper, R.J. Does the subtype of breast cancer affect the diagnostic performance of axillary ultrasound for nodal staging in breast cancer patients? Eur. J. Surg. Oncol. 2019, 45, 573–577. [Google Scholar] [CrossRef]
- Lee, S.C.; Grant, E.; Sheth, P.; Garcia, A.A.; Desai, B.; Ji, L.; Groshen, S.; Hwang, D.; Yamashita, M.; Hovanessian-Larsen, L. Accuracy of Contrast-Enhanced Ultrasound Compared With Magnetic Resonance Imaging in Assessing the Tumor Response After Neoadjuvant Chemotherapy for Breast Cancer. J. Ultrasound Med. 2017, 36, 901–911. [Google Scholar] [CrossRef]
- Jia, K.; Li, L.; Wu, X.J.; Hao, M.J.; Xue, H.Y. Contrast-enhanced ultrasound for evaluating the pathologic response of breast cancer to neoadjuvant chemotherapy: A meta-analysis. Medicine 2019, 98, e14258. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Q.; Bao, J.; Liu, D.; Huang, X.; Wang, J. Direct comparison of PET/CT and MRI to predict the pathological response to neoadjuvant chemotherapy in breast cancer: A meta-analysis. Sci. Rep. 2017, 7, 8479. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Youk, J.H.; Gweon, H.M.; Son, E.J. Shear-wave elastography in breast ultrasonography: The state of the art. Ultrasonography 2017, 36, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Armstrong, S.; Whelehan, P.; Thomson, K.; Rauchhaus, P.; Purdie, C.; Jordan, L.; Jones, L.; Thompson, A.; Vinnicombe, S. Can shear-wave elastography predict response to neoadjuvant chemotherapy in women with invasive breast cancer? Br. J. Cancer 2013, 109, 2798–2802. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chang, J.M.; Han, W.; Moon, H.G.; Koo, H.R.; Gweon, H.M.; Kim, W.H.; Noh, D.Y.; Moon, W.K. Shear-Wave Elastography for the Detection of Residual Breast Cancer After Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S376–S384. [Google Scholar] [CrossRef] [PubMed]
- Marinovich, M.L.; Houssami, N.; Macaskill, P.; Sardanelli, F.; Irwig, L.; Mamounas, E.P.; von Minckwitz, G.; Brennan, M.E.; Ciatto, S. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J. Natl. Cancer Inst. 2013, 105, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Martincich, L.; Montemurro, F.; De Rosa, G.; Marra, V.; Ponzone, R.; Cirillo, S.; Gatti, M.; Biglia, N.; Sarotto, I.; Sismondi, P.; et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res. Treat. 2004, 83, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Gibbs, J.E.; Lu, Y.; Esserman, L.J.; Tripathy, D.; Wolverton, D.S.; Rugo, H.S.; Hwang, E.S.; Ewing, C.A.; Hylton, N.M. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am. J. Roentgenol. 2005, 184, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Cho, N.; Park, I.-A.; Kwon, B.R.; Shin, S.U.; Kim, S.Y.; Lee, S.H.; Chang, J.M.; Moon, W.K. Dynamic Contrast-enhanced Breast MRI for Evaluating Residual Tumor Size after Neoadjuvant Chemotherapy. Radiology 2018, 289, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-P.; Wu, H.-K.; Chen, C.-J.; Lee, C.-W.; Chen, S.-T.; Chen, D.-R.; Chou, C.-T.; Mok, C.W.; Lai, H.-W. Higher underestimation of tumour size post-neoadjuvant chemotherapy with breast magnetic resonance imaging (MRI)—A concordance comparison cohort analysis. PLoS ONE 2019, 14, e0222917. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, L.R.; Li, X.; Levy, M.; Smith, D.; Welch, E.B.; Gore, J.C.; Yankeelov, T.E. Current and future trends in magnetic resonance imaging assessments of the response of breast tumors to neoadjuvant chemotherapy. J. Oncol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.H.; Kang, B.J. Pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: Perfusion metrics of dynamic contrast enhanced MRI. Sci. Rep. 2018, 8, 9490. [Google Scholar] [CrossRef] [PubMed]
- Tudorica, A.; Oh, K.Y.; Chui, S.Y.C.; Roy, N.; Troxell, M.L.; Naik, A.; Kemmer, K.A.; Chen, Y.; Holtorf, M.L.; Afzal, A.; et al. Early Prediction and Evaluation of Breast Cancer Response to Neoadjuvant Chemotherapy Using Quantitative DCE-MRI. Transl. Oncol. 2016, 9, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.E.; Teertstra, H.J.; Rodenhuis, S.; van de Vijver, M.J.; Hannemann, J.; Muller, S.H.; Peeters, M.-J.V.; Gilhuijs, K.G.A. Dynamic Contrast-Enhanced MRI for Prediction of Breast Cancer Response to Neoadjuvant Chemotherapy: Initial Results. Am. J. Roentgenol. 2008, 191, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Marinovich, M.L.; Sardanelli, F.; Ciatto, S.; Mamounas, E.; Brennan, M.; Macaskill, P.; Irwig, L.; von Minckwitz, G.; Houssami, N. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: Systematic review of the accuracy of MRI. Breast 2012, 21, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Heumann, E.J.; Hylton, N.M. Semi-automated analysis for MRI of breast tumors. Stud. Health Technol. Inform. 1999, 62, 259–260. [Google Scholar] [PubMed]
- Hylton, N.M.; Blume, J.D.; Bernreuter, W.K.; Pisano, E.D.; Rosen, M.A.; Morris, E.A.; Weatherall, P.T.; Lehman, C.D.; Newstead, G.M.; Polin, S.; et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—Results from ACRIN 6657/I-SPY TRIAL. Radiology 2012, 263, 663–672. [Google Scholar] [CrossRef]
- Hylton, N.M.; Gatsonis, C.A.; Rosen, M.A.; Lehman, C.D.; Newitt, D.C.; Partridge, S.C.; Bernreuter, W.K.; Pisano, E.D.; Morris, E.A.; Weatherall, P.T.; et al. Neoadjuvant Chemotherapy for Breast Cancer: Functional Tumor Volume by MR Imaging Predicts Recurrence-free Survival-Results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL. Radiology 2016, 279, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.C.; Li, W.; Jones, E.F.; Newitt, D.C.; Kornak, J.; Wilmes, L.J.; Esserman, L.J.; Hylton, N.M. Effect of Imaging Parameter Thresholds on MRI Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer Subtypes. PLoS ONE 2016, 11, e0142047. [Google Scholar] [CrossRef]
- Li, W.; Arasu, V.; Newitt, D.C.; Jones, E.F.; Wilmes, L.; Gibbs, J.; Kornak, J.; Joe, B.N.; Esserman, L.J.; Hylton, N.M. Effect of MR Imaging Contrast Thresholds on Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer Subtypes: A Subgroup Analysis of the ACRIN 6657/I-SPY 1 TRIAL. Tomography 2016, 2, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Pickles, M.D.; Gibbs, P.; Lowry, M.; Turnbull, L.W. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn. Reson. Imaging 2006, 24, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Ah-See, M.L.; Makris, A.; Taylor, N.J.; Harrison, M.; Richman, P.I.; Burcombe, R.J.; Stirling, J.J.; d’Arcy, J.A.; Collins, D.J.; Pittam, M.R.; et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin. Cancer Res. 2008, 14, 6580–6589. [Google Scholar] [CrossRef] [PubMed]
- De Bazelaire, C.; Calmon, R.; Thomassin, I.; Brunon, C.; Hamy, A.S.; Fournier, L.; Balvay, D.; Espie, M.; Siauve, N.; Clement, O.; et al. Accuracy of perfusion MRI with high spatial but low temporal resolution to assess invasive breast cancer response to neoadjuvant chemotherapy: A retrospective study. BMC Cancer 2011, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Drisis, S.; Metens, T.; Ignatiadis, M.; Stathopoulos, K.; Chao, S.L.; Lemort, M. Quantitative DCE-MRI for prediction of pathological complete response following neoadjuvant treatment for locally advanced breast cancer: The impact of breast cancer subtypes on the diagnostic accuracy. Eur. Radiol. 2016, 26, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Makris, A.; Beresford, M.J.; Taylor, N.J.; Ah-See, M.L.; Stirling, J.J.; d’Arcy, J.A.; Collins, D.J.; Kozarski, R.; Padhani, A.R. Use of dynamic contrast-enhanced MR imaging to predict survival in patients with primary breast cancer undergoing neoadjuvant chemotherapy. Radiology 2011, 260, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, H.; Arlinghaus, L.R.; Abramson, R.G.; Chakravarthy, A.B.; Abramson, V.G.; Farley, J.; Sanders, M.; Yankeelov, T.E. Analyzing Spatial Heterogeneity in DCE- and DW-MRI Parametric Maps to Optimize Prediction of Pathologic Response to Neoadjuvant Chemotherapy in Breast Cancer. Transl. Oncol. 2014, 7, 14–22. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, E.A.; Collins, D.; D’Arcy, J.; Schmidt, M.; de Souza, N.M. Multi-parametric MRI in the early prediction of response to neo-adjuvant chemotherapy in breast cancer: Value of non-modelled parameters. Eur. J. Radiol. 2016, 85, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, Q.; Miao, Y.; Li, J.; Bao, S.; Wang, H.; Wu, C.; Wang, X.; Zhu, J.; Zhong, Y.; et al. Quantitative Analysis of Clinical Dynamic Contrast-enhanced MR Imaging for Evaluating Treatment Response in Human Breast Cancer. Radiology 2010, 257, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Jun, W.; Cong, W.; Xianxin, X.; Daqing, J. Meta-Analysis of Quantitative Dynamic Contrast-Enhanced MRI for the Assessment of Neoadjuvant Chemotherapy in Breast Cancer. Am. Surg. 2019, 85, 645–653. [Google Scholar] [PubMed]
- Jansen, S.A.; Lin, V.C.; Giger, M.L.; Li, H.; Karczmar, G.S.; Newstead, G.M. Normal parenchymal enhancement patterns in women undergoing MR screening of the breast. Eur. Radiol. 2011, 21, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Bieling, H.B.; Gieseke, J.; Kreft, B.P.; Sommer, T.; Lutterbey, G.; Schild, H.H. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: Normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997, 203, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Muller-Schimpfle, M.; Ohmenhauser, K.; Stoll, P.; Dietz, K.; Claussen, C.D. Menstrual cycle and age: Influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997, 203, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.S.; Corben, A.D.; Brooks, J.D.; Edelweiss, M.; Keating, D.M.; Lin, C.; Morris, E.A.; Patel, P.; Robson, M.; Woods, M.; et al. Histopathologic characteristics of background parenchymal enhancement (BPE) on breast MRI. Breast Cancer Res. Treat. 2018, 172, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, L.; Miller, N.; Nicklee, T.; Woo, J.; Hulse-Smith, L.; Tsao, M.S.; Khokha, R.; Martin, L.; Boyd, N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.A.; Comstock, C.E.; Lee, C.H. ACR BI-RADS® Magnetic Resonance Imaging. In ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- DeMartini, W.B.; Liu, F.; Peacock, S.; Eby, P.R.; Gutierrez, R.L.; Lehman, C.D. Background Parenchymal Enhancement on Breast MRI: Impact on Diagnostic Performance. Am. J. Roentgenol. 2012, 198, W373–W380. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.J.; Henze Bancroft, L.C.; Strigel, R.M.; Chitalia, R.D.; Kontos, D.; Moy, L.; Partridge, S.C.; Rahbar, H. Background parenchymal enhancement on breast MRI: A comprehensive review. J. Magn. Reson. Imaging 2020, 51, 43–61. [Google Scholar] [CrossRef] [PubMed]
- King, V.; Brooks, J.D.; Bernstein, J.L.; Reiner, A.S.; Pike, M.C.; Morris, E.A. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011, 260, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Preibsch, H.; Wanner, L.; Bahrs, S.D.; Wietek, B.M.; Siegmann-Luz, K.C.; Oberlecher, E.; Hahn, M.; Staebler, A.; Nikolaou, K.; Wiesinger, B. Background parenchymal enhancement in breast MRI before and after neoadjuvant chemotherapy: Correlation with tumour response. Eur. Radiol. 2016, 26, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Yu, H.J.; Hsu, C.; Mehta, R.S.; Carpenter, P.M.; Su, M.Y. Background Parenchymal Enhancement of the Contralateral Normal Breast: Association with Tumor Response in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Transl. Oncol. 2015, 8, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Chae, E.Y.; Cha, J.H.; Shin, H.J.; Choi, W.J.; Kim, H.H. Relationship between background parenchymal enhancement on breast MRI and pathological tumor response in breast cancer patients receiving neoadjuvant chemotherapy. Br. J. Radiol. 2018, 91, 20170550. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Gu, Y.; Peng, W.; Li, J.; Shen, X.; Liu, G.; Peng, W. Decreased background parenchymal enhancement of the contralateral breast after two cycles of neoadjuvant chemotherapy is associated with tumor response in HER2-positive breast cancer. Acta Radiol. 2018, 59, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Ragusi, M.; Loo, C.E.; van der Velden, B.H.; Wesseling, J.; Beets-Tan, R.G.; Elias, M.S.G.; Gilhuijs, K.G. Change in Contralateral Parenchymal Enhancement during Neoadjuvant Endocrine Treatment is Associated with Tumor Response in Unilateral ER+/HER2- Breast Cancer Patients. In Proceedings of the 105th Radiological Society of North America Annual Meeting, Chicago, IL, USA, 1–6 December 2019. [Google Scholar]
- Hilal, T.; Covington, M.; Kosiorek, H.E.; Zwart, C.; Ocal, I.T.; Pockaj, B.A.; Northfelt, D.W.; Patel, B.K. Breast MRI phenotype and background parenchymal enhancement may predict tumor response to neoadjuvant endocrine therapy. Breast J. 2018, 24, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; McDonald, E.S. Diffusion weighted magnetic resonance imaging of the breast: Protocol optimization, interpretation, and clinical applications. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 601–624. [Google Scholar] [CrossRef] [PubMed]
- Chenevert, T.L.; Meyer, C.R.; Moffat, B.A.; Rehemtulla, A.; Mukherji, S.K.; Gebarski, S.S.; Quint, D.J.; Robertson, P.L.; Lawrence, T.S.; Junck, L.; et al. Diffusion MRI: A New Strategy for Assessment of Cancer Therapeutic Efficacy. Mol. Imaging 2002, 1, 15353500200221482. [Google Scholar] [CrossRef]
- Ross, B.D.; Moffat, B.A.; Lawrence, T.S.; Mukherji, S.K.; Gebarski, S.S.; Quint, D.J.; Johnson, T.D.; Junck, L.; Robertson, P.L.; Muraszko, K.M.; et al. Evaluation of Cancer Therapy Using Diffusion Magnetic Resonance Imaging. Mol. Cancer Ther. 2003, 2, 581–587. [Google Scholar]
- Padhani, A.R.; Koh, D.M. Diffusion MR imaging for monitoring of treatment response. Magn. Reson. Imaging Clin. N. Am. 2011, 19, 181–209. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: A meta-analysis. Oncotarget 2017, 8, 59492–59499. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Danishad, K.K.; Seenu, V.; Jagannathan, N.R. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009, 22, 104–113. [Google Scholar] [CrossRef]
- Park, S.H.; Moon, W.K.; Cho, N.; Song, I.C.; Chang, J.M.; Park, I.A.; Han, W.; Noh, D.Y. Diffusion-weighted MR imaging: Pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology 2010, 257, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Fangberget, A.; Nilsen, L.B.; Hole, K.H.; Holmen, M.M.; Engebraaten, O.; Naume, B.; Smith, H.J.; Olsen, D.R.; Seierstad, T. Neoadjuvant chemotherapy in breast cancer-response evaluation and prediction of response to treatment using dynamic contrast-enhanced and diffusion-weighted MR imaging. Eur. Radiol. 2011, 21, 1188–1199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.R.; Cheng, L.Q.; Liu, M.; Zhang, Y.J.; Wang, J.D.; Zhang, A.L.; Song, X.; Li, J.; Zheng, Y.Q.; Liu, L. DW-MRI ADC values can predict treatment response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Med. Oncol. 2012, 29, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Moon, W.K.; Cho, N.; Chang, J.M.; Im, S.A.; Park, I.A.; Kang, K.W.; Han, W.; Noh, D.Y. Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. Eur. Radiol. 2012, 22, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.M.; Lau, P.; Ramadan, S. Utilisation of MR spectroscopy and diffusion weighted imaging in predicting and monitoring of breast cancer response to chemotherapy. J. Med. Imaging Radiat. Oncol. 2015, 59, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, S.H.; Lee, H.J.; Gong, G.; Baek, S.; Chae, E.Y.; Choi, W.J.; Cha, J.H.; Kim, H.H. Tumor apparent diffusion coefficient as an imaging biomarker to predict tumor aggressiveness in patients with estrogen-receptor-positive breast cancer. NMR Biomed. 2016, 29, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.P.; Curi, C.; Osorio, C.; Marques, E.F.; Makdissi, F.B.; Pinker, K.; Bitencourt, A.G.V. Diffusion-Weighted Magnetic Resonance Imaging of Patients with Breast Cancer Following Neoadjuvant Chemotherapy Provides Early Prediction of Pathological Response—A Prospective Study. Sci. Rep. 2019, 9, 16372. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Zhang, Z.; Newitt, D.C.; Gibbs, J.E.; Chenevert, T.L.; Rosen, M.A.; Bolan, P.J.; Marques, H.S.; Romanoff, J.; Cimino, L.; et al. Diffusion-weighted MRI Findings Predict Pathologic Response in Neoadjuvant Treatment of Breast Cancer: The ACRIN 6698 Multicenter Trial. Radiology 2018, 289, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Bufi, E.; Belli, P.; Di Matteo, M.; Terribile, D.; Franceschini, G.; Nardone, L.; Petrone, G.; Bonomo, L. Effect of breast cancer phenotype on diagnostic performance of MRI in the prediction to response to neoadjuvant treatment. Eur. J. Radiol. 2014, 83, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Newitt, D.C.; Wilmes, L.J.; Jones, E.F.; Arasu, V.; Gibbs, J.; La Yun, B.; Li, E.; Partridge, S.C.; Kornak, J.; et al. Additive value of diffusion-weighted MRI in the I-SPY 2 TRIAL. J. Magn. Reson. Imaging JMRI 2019, 50, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Haukaas, H.T.; Euceda, R.L.; Giskeødegård, F.G.; Bathen, F.T. Metabolic Portraits of Breast Cancer by HR MAS MR Spectroscopy of Intact Tissue Samples. Metabolites 2017, 7. [Google Scholar] [CrossRef]
- Cao, M.D.; Sitter, B.; Bathen, T.F.; Bofin, A.; Lønning, P.E.; Lundgren, S.; Gribbestad, I.S. Predicting long-term survival and treatment response in breast cancer patients receiving neoadjuvant chemotherapy by MR metabolic profiling. NMR Biomed. 2012, 25, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Andreassen, T.; Jensen, L.; Bathen, T.F.; Sinha, I.; Gao, H.; Zhao, C.; Haldosen, L.A.; Cao, Y.; Girnita, L.; et al. Estrogen Receptor alpha Promotes Breast Cancer by Reprogramming Choline Metabolism. Cancer Res. 2016, 76, 5634–5646. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.G.; Sharma, U.; Parshad, R.; Seenu, V.; Mathur, S.R.; Jagannathan, N.R. Association of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status with total choline concentration and tumor volume in breast cancer patients: An MRI and in vivo proton MRS study. Magn. Reson. Med. 2012, 68, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) and Institute of Medicine (US) Committee on the Mathematics and Physics of Emerging Dynamic Biomedical Imaging. Positron Emission Tomography. In Mathematics and Physics of Emerging Biomedical Imaging; National Academies Press (USA): Washington, DC, USA, 1996; Chapter 6. [Google Scholar]
- Ter-Pogossian, M.M.; Raichle, M.E.; Sobel, B.E. Positron-Emission Tomography. Sci. Am. 1980, 243, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (USA); 2004–2013. About MICAD. Available online: https://www.ncbi.nlm.nih.gov/books/NBK5923/ (accessed on 9 June 2020).
- Mankoff, D.A.; Eary, J.F.; Link, J.M.; Muzi, M.; Rajendran, J.G.; Spence, A.M.; Krohn, K.A. Tumor-specific positron emission tomography imaging in patients: [18F] fluorodeoxyglucose and beyond. Clin. Cancer Res. 2007, 13, 3460–3469. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Mankoff, D.; Espie, M.; Hindie, E. (1)(8)F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: Review of the literature and recommendations for use in clinical trials. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Liu, J.; Huang, G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res. Treat. 2012, 131, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Dose-Schwarz, J.; Tiling, R.; Avril-Sassen, S.; Mahner, S.; Lebeau, A.; Weber, C.; Schwaiger, M.; Janicke, F.; Untch, M.; Avril, N. Assessment of residual tumour by FDG-PET: Conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer. Br. J. Cancer 2010, 102, 35–41. [Google Scholar] [CrossRef]
- Avril, S.; Muzic, R.F., Jr.; Plecha, D.; Traughber, B.J.; Vinayak, S.; Avril, N. ¹⁸F-FDG PET/CT for Monitoring of Treatment Response in Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. 1), 34s–39s. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Zwarthoed, C.; Borra, A. Positron Emission Tomography (PET) in Oncology. Cancers 2014, 6, 1821–1889. [Google Scholar] [CrossRef]
- Dunnwald, L.K.; Gralow, J.R.; Ellis, G.K.; Livingston, R.B.; Linden, H.M.; Specht, J.M.; Doot, R.K.; Lawton, T.J.; Barlow, W.E.; Kurland, B.F.; et al. Tumor metabolism and blood flow changes by positron emission tomography: Relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J. Clin. Oncol. 2008, 26, 4449–4457. [Google Scholar] [CrossRef] [PubMed]
- Mankoff, D.A.; Dunnwald, L.K.; Gralow, J.R.; Ellis, G.K.; Schubert, E.K.; Tseng, J.; Lawton, T.J.; Linden, H.M.; Livingston, R.B. Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J. Nucl. Med. 2003, 44, 1806–1814. [Google Scholar] [PubMed]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Beriwal, S.; Dabbs, D.J.; Ozbek, U.; Soran, A.; Johnson, R.R.; Brufsky, A.M.; Lembersky, B.C.; Ahrendt, G.M. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: A single institutional experience with 359 cases. Cancer 2010, 116, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Lips, E.H.; Mulder, L.; de Ronde, J.J.; Mandjes, I.A.; Vincent, A.; Vrancken Peeters, M.T.; Nederlof, P.M.; Wesseling, J.; Rodenhuis, S. Neoadjuvant chemotherapy in ER+ HER2- breast cancer: Response prediction based on immunohistochemical and molecular characteristics. Breast Cancer Res. Treat. 2012, 131, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Semiglazov, V.F.; Semiglazov, V.V.; Dashyan, G.A.; Ziltsova, E.K.; Ivanov, V.G.; Bozhok, A.A.; Melnikova, O.A.; Paltuev, R.M.; Kletzel, A.; Berstein, L.M. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 2007, 110, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Hatt, M.; Hindie, E.; Giacchetti, S.; de Cremoux, P.; Lehmann-Che, J.; Martineau, A.; Marty, M.; Cuvier, C.; Cheze-Le Rest, C.; et al. Estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast tumors: Early prediction of chemosensitivity with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography during neoadjuvant chemotherapy. Cancer 2013, 119, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, D.O.; Kilbourn, M.R.; Landvatter, S.W.; Heiman, D.F.; Katzenellenbogen, J.A.; Welch, M.J. Preparation of four fluorine- 18-labeled estrogens and their selective uptakes in target tissues of immature rats. J. Nucl. Med. 1984, 25, 1212–1221. [Google Scholar]
- Katzenellenbogen, J.A.; Welch, M.J.; Dehdashti, F. The development of estrogen and progestin radiopharmaceuticals for imaging breast cancer. Anticancer Res. 1997, 17, 1573–1576. [Google Scholar] [PubMed]
- Dehdashti, F.; Mortimer, J.E.; Siegel, B.A.; Griffeth, L.K.; Bonasera, T.J.; Fusselman, M.J.; Detert, D.D.; Cutler, P.D.; Katzenellenbogen, J.A.; Welch, M.J. Positron tomographic assessment of estrogen receptors in breast cancer: Comparison with FDG-PET and in vitro receptor assays. J. Nucl. Med. 1995, 36, 1766–1774. [Google Scholar]
- Peterson, L.M.; Mankoff, D.A.; Lawton, T.; Yagle, K.; Schubert, E.K.; Stekhova, S.; Gown, A.; Link, J.M.; Tewson, T.; Krohn, K.A. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J. Nucl. Med. 2008, 49, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Dehdashti, F.; Siegel, B.A.; Katzenellenbogen, J.A.; Fracasso, P.; Welch, M.J. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: Correlation with estrogen receptor status and response to systemic therapy. Clin. Cancer Res. 1996, 2, 933–939. [Google Scholar] [PubMed]
- Mortimer, J.E.; Dehdashti, F.; Siegel, B.A.; Trinkaus, K.; Katzenellenbogen, J.A.; Welch, M.J. Metabolic flare: Indicator of hormone responsiveness in advanced breast cancer. J. Clin. Oncol. 2001, 19, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Linden, H.M.; Stekhova, S.A.; Link, J.M.; Gralow, J.R.; Livingston, R.B.; Ellis, G.K.; Petra, P.H.; Peterson, L.M.; Schubert, E.K.; Dunnwald, L.K.; et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J. Clin. Oncol. 2006, 24, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Van Kruchten, M.; de Vries, E.G.E.; Brown, M.; de Vries, E.F.J.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; Schröder, C.P.; Hospers, G.A.P. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013, 14, e465–e475. [Google Scholar] [CrossRef]
- Fowler, A.M.; Clark, A.S.; Katzenellenbogen, J.A.; Linden, H.M.; Dehdashti, F. Imaging Diagnostic and Therapeutic Targets: Steroid Receptors in Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. 1), 75S–80S. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.J.; Clark, A.S.; Schubert, E.K.; Mankoff, D.A. 18F-Fluoroestradiol PET: Current Status and Potential Future Clinical Applications. J. Nucl. Med. 2016, 57, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, Z.; Zhang, Y.; Xue, J.; Wang, M.; Shi, W.; Zhu, B.; Hu, S.; Yao, Z.; Pan, H.; et al. The Preliminary Study of 16α-[18F]fluoroestradiol PET/CT in Assisting the Individualized Treatment Decisions of Breast Cancer Patients. PLoS ONE 2015, 10, e0116341. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, Y.; Xue, J.; Yao, Z.; Xu, J.; Cheng, J.; Shi, W.; Zhu, B.; Zhang, Y.; Zhang, Y. Can Positron Emission Tomography/Computed Tomography with the Dual Tracers Fluorine-18 Fluoroestradiol and Fluorodeoxyglucose Predict Neoadjuvant Chemotherapy Response of Breast Cancer?—A Pilot Study. PLoS ONE 2013, 8, e78192. [Google Scholar] [CrossRef] [PubMed]
- Sataloff, D.M.; Mason, B.A.; Prestipino, A.J.; Seinige, U.L.; Lieber, C.P.; Baloch, Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: A determinant of outcome. J. Am. Coll. Surg. 1995, 180, 297–306. [Google Scholar]
- Chae, S.Y.; Kim, S.-B.; Ahn, S.H.; Kim, H.O.; Yoon, D.H.; Ahn, J.-H.; Jung, K.H.; Han, S.; Oh, S.J.; Lee, S.J.; et al. A Randomized Feasibility Study of 18F-Fluoroestradiol PET to Predict Pathologic Response to Neoadjuvant Therapy in Estrogen Receptor–Rich Postmenopausal Breast Cancer. J. Nucl. Med. 2017, 58, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.M.; Linden, H.M. Functional Estrogen Receptor Imaging Before Neoadjuvant Therapy for Primary Breast Cancer. J. Nucl. Med. 2017, 58, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Mankoff, D.A.; Shields, A.F.; Krohn, K.A. PET imaging of cellular proliferation. Radiol. Clin. N Am. 2005, 43, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Kostakoglu, L.; Duan, F.; Idowu, M.O.; Jolles, P.R.; Bear, H.D.; Muzi, M.; Cormack, J.; Pryma, D.A.; Specht, J.M.; Hovanessian Larsen, L.; et al. Phase II study of 3′-deoxy-3′-18F fluorothymidine PET/CT (FLT-PET) in the assessment of early response in locally advanced breast cancer (LABC): Preliminary results of ACRIN 6688. J. Clin. Oncol. 2014, 32, 526. [Google Scholar] [CrossRef]
- Roberts, T.K.; Peterson, L.; Kurland, B.; Novakova, A.; Shields, A.; Doot, R.K.; Schubert, E.K.; Gadi, V.K.; Specht, J.M.; Gralow, J.; et al. Use of serial 18F-Fluorothymidine (FLT) PET and Ki-67 to predict response to aromatase inhibitors (AI) in women with ER+ breast cancer. J. Clin. Oncol. 2016, 34, e12039. [Google Scholar] [CrossRef]
- Woolf, D.K.; Beresford, M.; Li, S.P.; Dowsett, M.; Sanghera, B.; Wong, W.L.; Sonoda, L.; Detre, S.; Amin, V.; Ah-See, M.L.; et al. Evaluation of FLT-PET-CT as an imaging biomarker of proliferation in primary breast cancer. Br. J. Cancer 2014, 110, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Contractor, K.B.; Kenny, L.M.; Stebbing, J.; Challapalli, A.; Al-Nahhas, A.; Palmieri, C.; Shousha, S.; Lewis, J.S.; Hogben, K.; De Nguyen, Q.; et al. Biological basis of [11C]choline-positron emission tomography in patients with breast cancer: Comparison with [18F]fluorothymidine positron emission tomography. Nucl. Med. Commun. 2011, 32, 997–1004. [Google Scholar] [CrossRef]
- Kenny, L.M.; Contractor, K.B.; Hinz, R.; Stebbing, J.; Palmieri, C.; Jiang, J.; Shousha, S.; Al-Nahhas, A.; Coombes, R.C.; Aboagye, E.O. Reproducibility of [11C]choline-positron emission tomography and effect of trastuzumab. Clin. Cancer Res. 2010, 16, 4236–4245. [Google Scholar] [CrossRef]
- Contractor, K.B.; Kenny, L.M.; Stebbing, J.; Al-Nahhas, A.; Palmieri, C.; Sinnett, D.; Lewis, J.S.; Hogben, K.; Osman, S.; Shousha, S.; et al. [11C]Choline Positron Emission Tomography in Estrogen Receptor–Positive Breast Cancer. Clin. Cancer Res. 2009, 15, 5503–5510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, H.; Tu, G.; Liu, Z.; Liu, M. Cancer-associated fibroblasts: A multifaceted driver of breast cancer progression. Cancer Lett. 2015, 361, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, T.A. Arousal of cancer-associated stromal fibroblasts: Palladin-activated fibroblasts promote tumor invasion. Cell Adh. Migr. 2012, 6, 488–494. [Google Scholar] [CrossRef][Green Version]
- Santi, A.; Kugeratski, F.G.; Zanivan, S. Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics 2018, 18, e1700167. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Amornsupak, K.; Insawang, T.; Thuwajit, P.; O-Charoenrat, P.; Eccles, S.A.; Thuwajit, C. Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer 2014, 14, 955. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Suzuki, T.; Tazawa, C.; Yamaguchi, Y.; Kitada, K.; Honma, S.; Moriya, T.; Hirakawa, H.; Evans, D.B.; Hayashi, S.; et al. Aromatase localization in human breast cancer tissues: Possible interactions between intratumoral stromal and parenchymal cells. Cancer Res. 2007, 67, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Takei, H.; Suemasu, K.; Kobayashi, Y.; Kurosumi, M.; Harada, N.; Hayashi, S. Tumor-stromal interaction through the estrogen-signaling pathway in human breast cancer. Cancer Res. 2005, 65, 4653–4662. [Google Scholar] [CrossRef][Green Version]
- Martinez-Outschoorn, U.E.; Goldberg, A.F.; Lin, Z.; Ko, Y.-H.; Flomenberg, N.; Wang, C.; Pavlides, S.; Pestell, R.G.; Howell, A.; Sotgia, F.; et al. Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol. Ther. 2011, 12, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, M.J.; Raj, B.K.; Calvo, B.; Garin-Chesa, P.; Sanz-Moncasi, M.P.; Healey, J.H.; Old, L.J.; Rettig, W.J. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl. Acad. Sci. USA 1994, 91, 5657–5661. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Chang, L.; Pure, E. Fibroblast Activation Protein in Remodeling Tissues. Curr. Mol. Med. 2012, 12, 1220–1243. [Google Scholar] [CrossRef] [PubMed]
- Pure, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Ryabtsova, O.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med. Chem. Lett. 2013, 4, 491–496. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Rohrich, M.; Winter, H.; et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jager, D.; Flechsig, P.; Altmann, A.; et al. (68)Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Nicolasjilwan, M.; Hu, Y.; Yan, C.; Meerzaman, D.; Holder, C.A.; Gutman, D.; Jain, R.; Colen, R.; Rubin, D.L.; Zinn, P.O.; et al. Addition of MR imaging features and genetic biomarkers strengthens glioblastoma survival prediction in TCGA patients. J. Neuroradiol. 2015, 42, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Sirlin, C.B.; Ooi, C.; Adler, A.S.; Gollub, J.; Chen, X.; Chan, B.K.; Matcuk, G.R.; Barry, C.T.; Chang, H.Y.; et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat. Biotechnol. 2007, 25, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Leijenaar, R.T.; Grossmann, P.; Rios Velazquez, E.; Bussink, J.; Rietveld, D.; Rietbergen, M.M.; Haibe-Kains, B.; Lambin, P.; Aerts, H.J. Radiomic feature clusters and prognostic signatures specific for Lung and Head & Neck cancer. Sci. Rep. 2015, 5, 11044. [Google Scholar] [CrossRef] [PubMed]
- Jahani, N.; Cohen, E.; Hsieh, M.-K.; Weinstein, S.P.; Pantalone, L.; Hylton, N.; Newitt, D.; Davatzikos, C.; Kontos, D. Prediction of Treatment Response to Neoadjuvant Chemotherapy for Breast Cancer via Early Changes in Tumor Heterogeneity Captured by DCE-MRI Registration. Sci. Rep. 2019, 9, 12114. [Google Scholar] [CrossRef]
- Jones, E.F.; Ray, K.M.; Li, W.; Chien, A.J.; Mukhtar, R.A.; Esserman, L.J.; Franc, B.L.; Seo, Y.; Pampaloni, M.H.; Joe, B.N.; et al. Initial experience of dedicated breast PET imaging of ER+ breast cancers using [F-18]fluoroestradiol. NPJ Breast Cancer 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.F.; Ray, K.M.; Li, W.; Seo, Y.; Franc, B.L.; Chien, A.J.; Esserman, L.J.; Pampaloni, M.H.; Joe, B.N.; Hylton, N.M. Dedicated Breast Positron Emission Tomography for the Evaluation of Early Response to Neoadjuvant Chemotherapy in Breast Cancer. Clin. Breast Cancer 2017, 17, e155–e159. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, R.L.; Newitt, D.C.; Wilmes, L.J.; Jones, E.F.; Wisner, D.J.; Kornak, J.; Proctor, E.; Joe, B.N.; Hylton, N.M. High resolution in vivo characterization of apparent diffusion coefficient at the tumor-stromal boundary of breast carcinomas: A pilot study to assess treatment response using proximity-dependent diffusion-weighted imaging. J. Magn. Reson. Imaging JMRI 2014, 39, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, L.J.; McLaughlin, R.L.; Newitt, D.C.; Singer, L.; Sinha, S.P.; Proctor, E.; Wisner, D.J.; Saritas, E.U.; Kornak, J.; Shankaranarayanan, A.; et al. High-resolution diffusion-weighted imaging for monitoring breast cancer treatment response. Acad. Radiol. 2013, 20, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.; Yip, C.; Siddique, M.; Goh, V.; Chicklore, S.; Roy, A.; Marsden, P.; Ahmad, S.; Landau, D. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J. Nucl. Med. 2013, 54, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Coroller, T.P.; Grossmann, P.; Hou, Y.; Rios Velazquez, E.; Leijenaar, R.T.; Hermann, G.; Lambin, P.; Haibe-Kains, B.; Mak, R.H.; Aerts, H.J. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother. Oncol. 2015, 114, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Grossmann, P.; Rietveld, D.; Rietbergen, M.M.; Lambin, P.; Aerts, H.J.W.L. Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer. Front. Oncol. 2015, 5. [Google Scholar] [CrossRef]
- Greenspan, H.; Ginneken, B.v.; Summers, R.M. Guest Editorial Deep Learning in Medical Imaging: Overview and Future Promise of an Exciting New Technique. IEEE Trans. Med. Imaging 2016, 35, 1153–1159. [Google Scholar] [CrossRef]
- Shen, L.; Margolies, L.R.; Rothstein, J.H.; Fluder, E.; McBride, R.; Sieh, W. Deep Learning to Improve Breast Cancer Detection on Screening Mammography. Sci. Rep. 2019, 9, 12495. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; Yala, A.; Schuster, T.; Dontchos, B.; Bahl, M.; Swanson, K.; Barzilay, R. Mammographic Breast Density Assessment Using Deep Learning: Clinical Implementation. Radiology 2019, 290, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Yala, A.; Lehman, C.; Schuster, T.; Portnoi, T.; Barzilay, R. A Deep Learning Mammography-based Model for Improved Breast Cancer Risk Prediction. Radiology 2019, 292, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Cai, H.; Tan, W.; Jin, C.; Li, L. Discrimination of Breast Cancer with Microcalcifications on Mammography by Deep Learning. Sci. Rep. 2016, 6, 27327. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, E.F.; Hathi, D.K.; Freimanis, R.; Mukhtar, R.A.; Chien, A.J.; Esserman, L.J.; van’t Veer, L.J.; Joe, B.N.; Hylton, N.M. Current Landscape of Breast Cancer Imaging and Potential Quantitative Imaging Markers of Response in ER-Positive Breast Cancers Treated with Neoadjuvant Therapy. Cancers 2020, 12, 1511. https://doi.org/10.3390/cancers12061511
Jones EF, Hathi DK, Freimanis R, Mukhtar RA, Chien AJ, Esserman LJ, van’t Veer LJ, Joe BN, Hylton NM. Current Landscape of Breast Cancer Imaging and Potential Quantitative Imaging Markers of Response in ER-Positive Breast Cancers Treated with Neoadjuvant Therapy. Cancers. 2020; 12(6):1511. https://doi.org/10.3390/cancers12061511
Chicago/Turabian StyleJones, Ella F., Deep K. Hathi, Rita Freimanis, Rita A. Mukhtar, A. Jo Chien, Laura J. Esserman, Laura J. van’t Veer, Bonnie N. Joe, and Nola M. Hylton. 2020. "Current Landscape of Breast Cancer Imaging and Potential Quantitative Imaging Markers of Response in ER-Positive Breast Cancers Treated with Neoadjuvant Therapy" Cancers 12, no. 6: 1511. https://doi.org/10.3390/cancers12061511
APA StyleJones, E. F., Hathi, D. K., Freimanis, R., Mukhtar, R. A., Chien, A. J., Esserman, L. J., van’t Veer, L. J., Joe, B. N., & Hylton, N. M. (2020). Current Landscape of Breast Cancer Imaging and Potential Quantitative Imaging Markers of Response in ER-Positive Breast Cancers Treated with Neoadjuvant Therapy. Cancers, 12(6), 1511. https://doi.org/10.3390/cancers12061511




