Eupatilin Promotes Cell Death by Calcium Influx through ER-Mitochondria Axis with SERPINB11 Inhibition in Epithelial Ovarian Cancer
Abstract
1. Introduction
2. Results
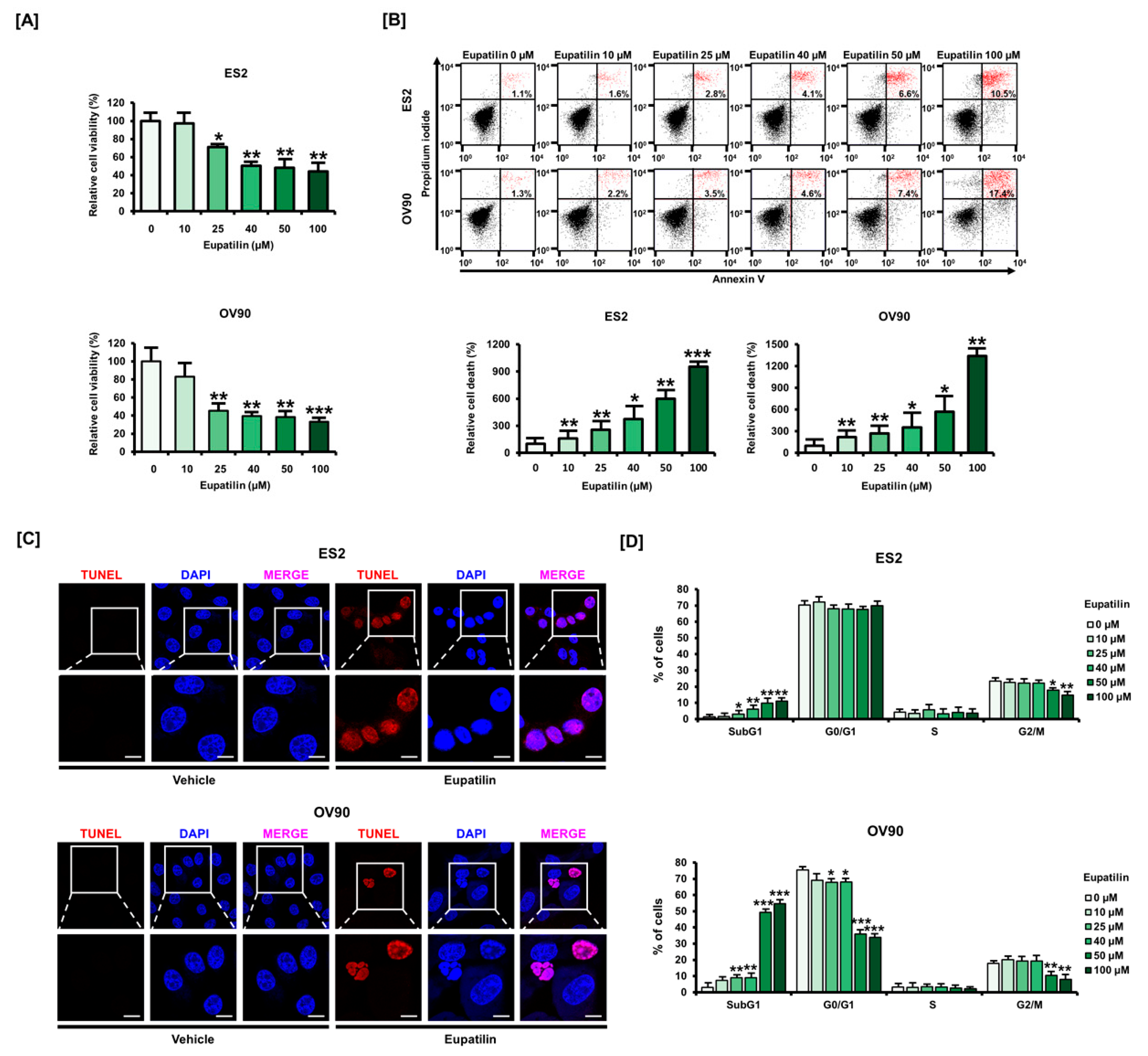
2.1. Eupatilin Induces Apoptosis in Ovarian Cancer (OC) Cell Lines
2.2. Effects of Eupatilin on ER Stress and Oxidative Stress on Ovarian Cancer Cells
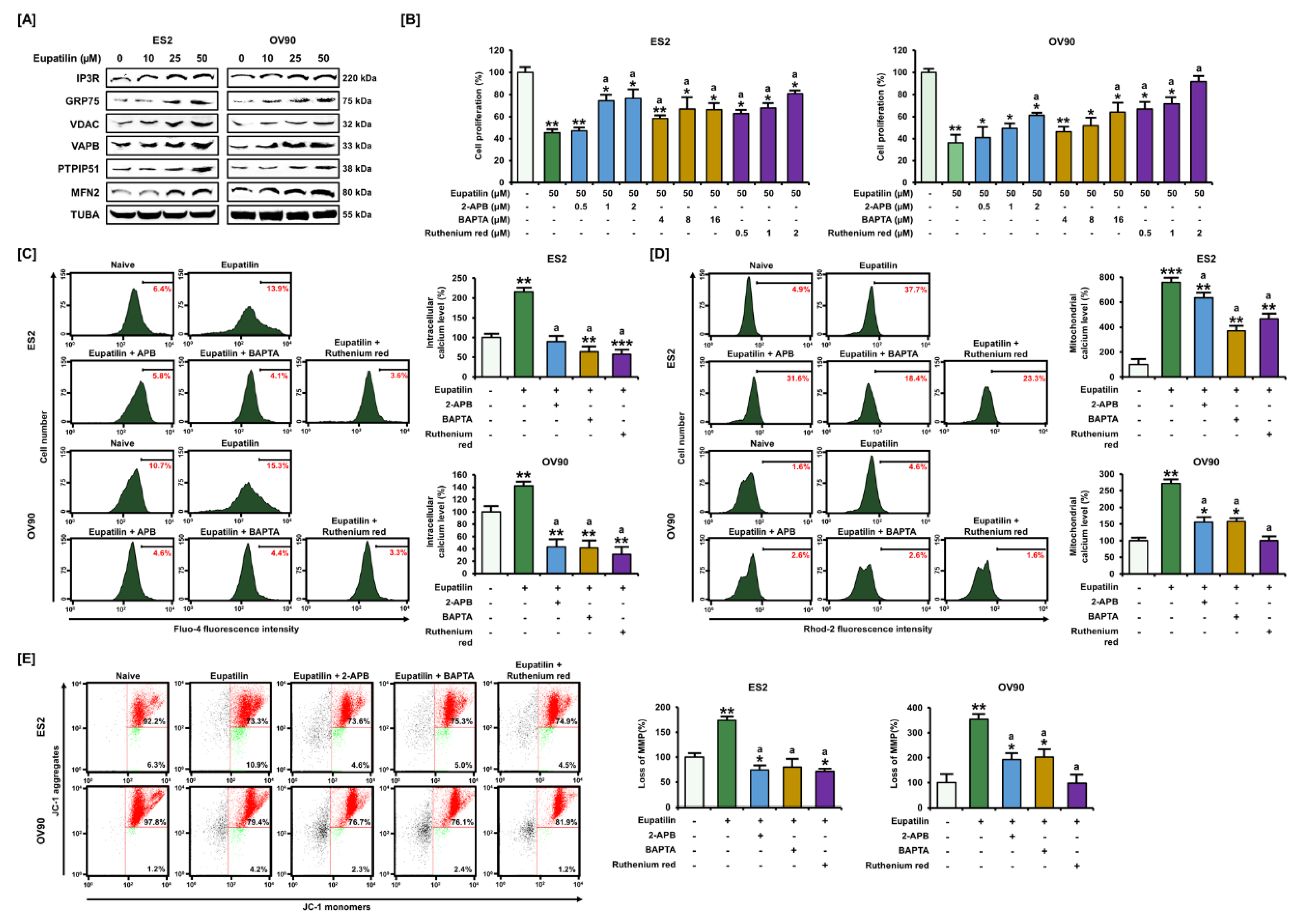
2.3. Regulation of Ca2+ Leading to Cell Death through the ER–Mitochondria Axis
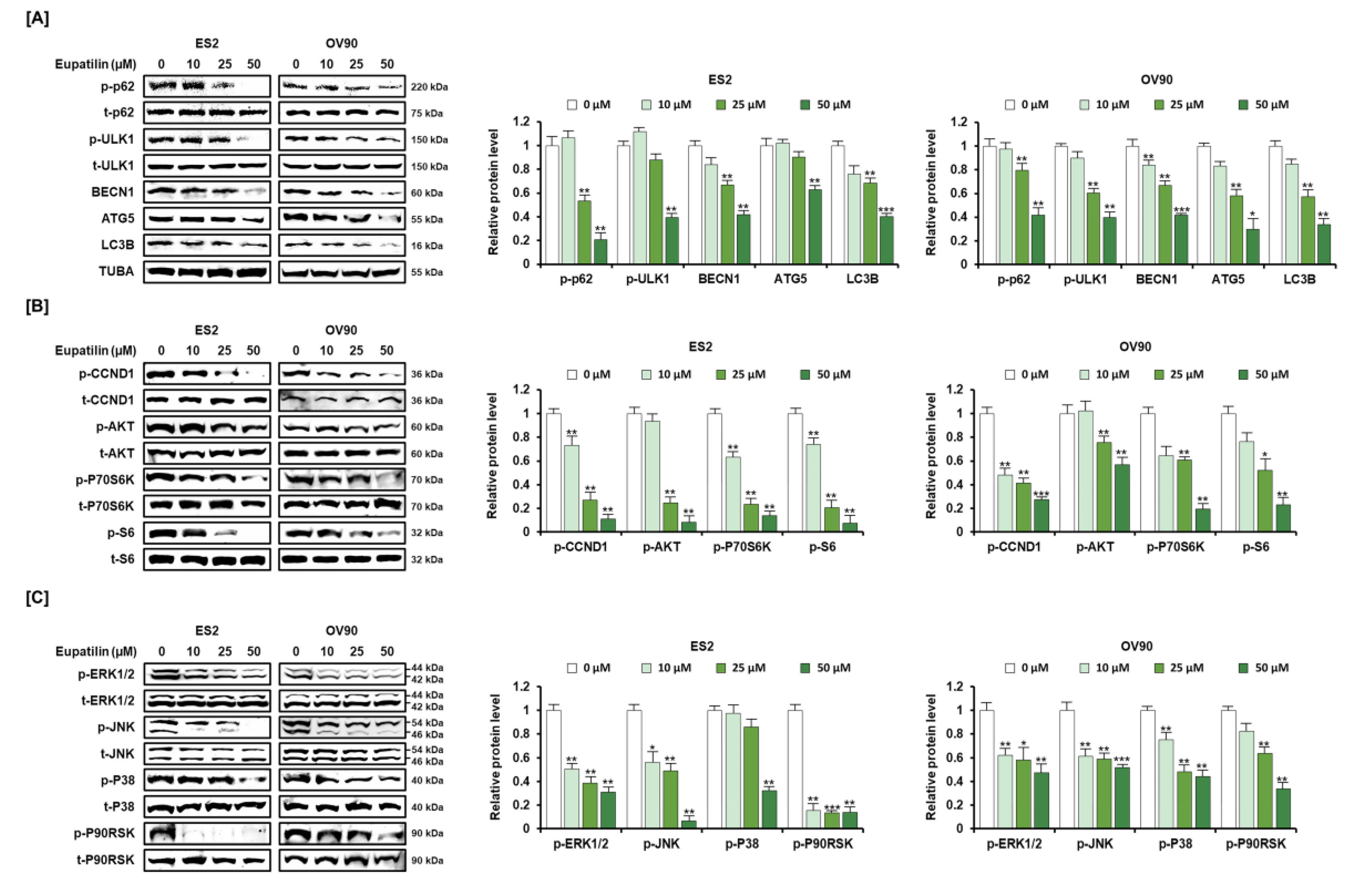
2.4. Anticancer Signaling Pathway by Eupatilin in ES2 and OV90 Cells
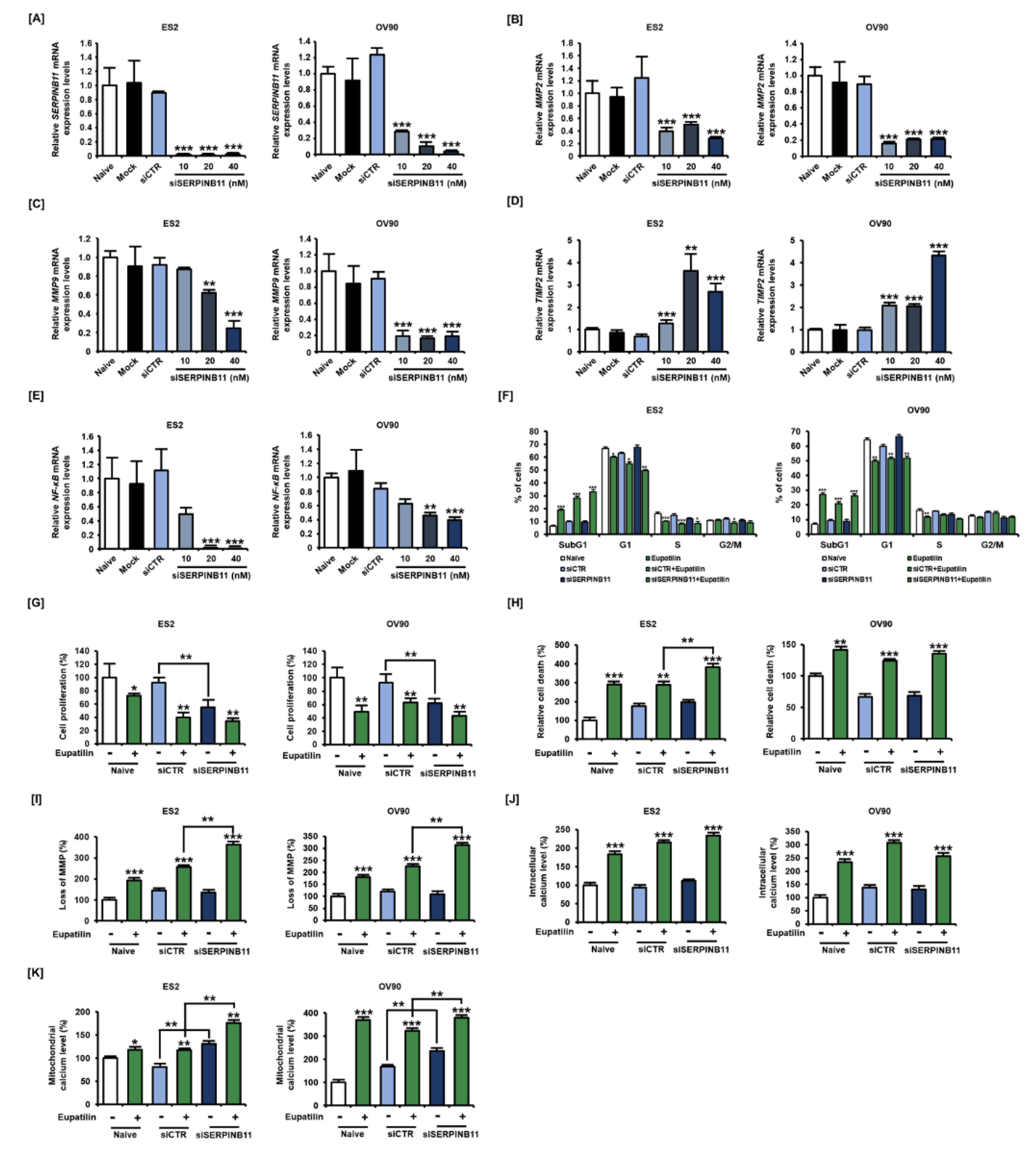
2.5. Inhibition of SERPINB11 by the Anticancer Effect of Eupatilin, with Apoptosis Induced in OC Cells
2.6. Effect of siSERPINB11 on Anticancer Activity within OC Cells
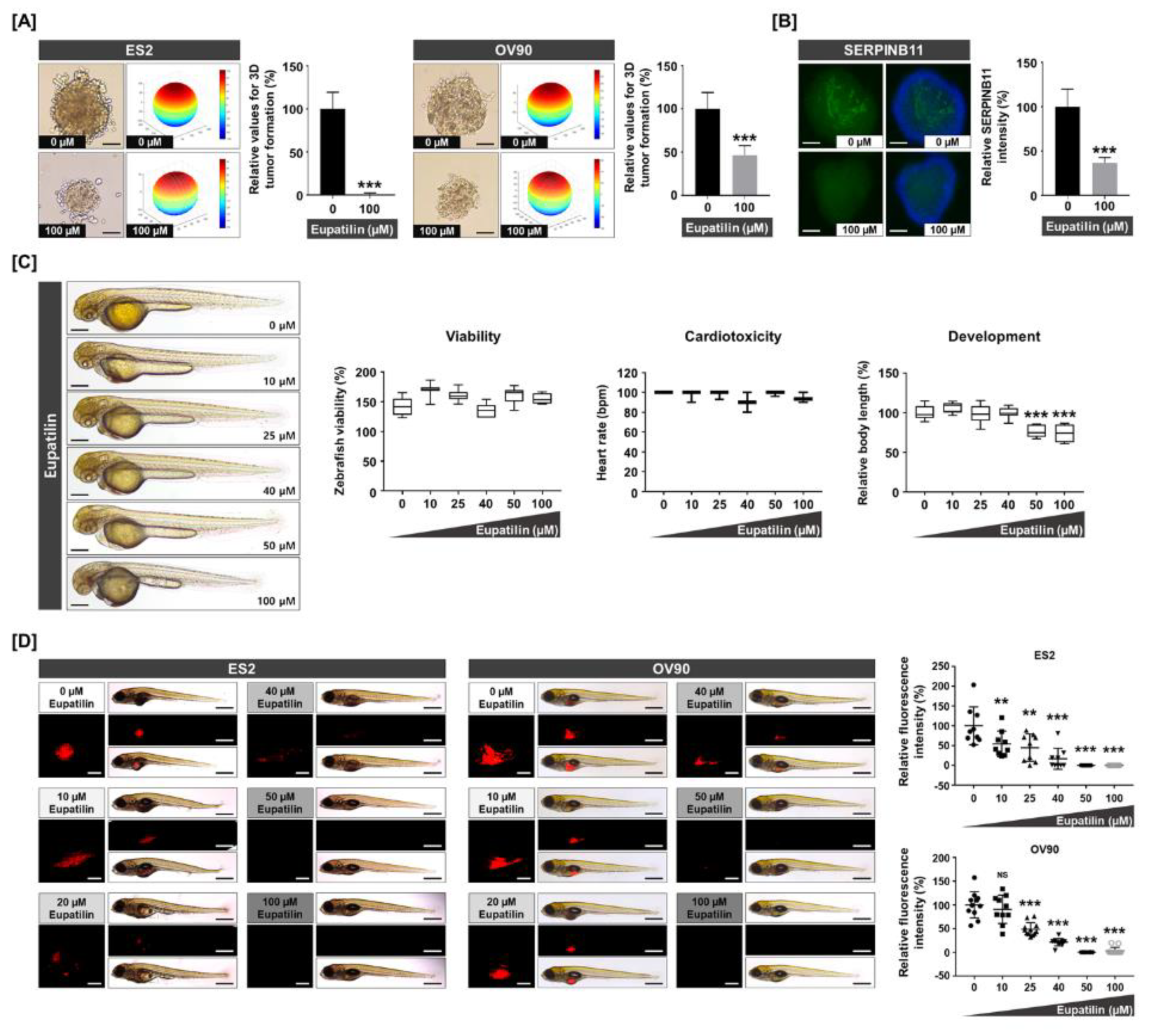
2.7. Expression of SERPINB11 on 3D Tumor Formation and Effects of Eupatilin in Vivo and in Vitro
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Proliferation Assay
4.4. Determination of Apoptosis by Annexin V and Propidium Iodide (PI) Staining
4.5. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
4.6. Cell Cycle Analysis
4.7. Western Blot Analysis
4.8. Cellular ROS Determination
4.9. Lipid Peroxidation
4.10. Measurement of Intracellular Free Ca2+ Concentration
4.11. Determination of Mitochondrial Free Ca2+ Levels
4.12. JC-1 Mitochondrial Membrane Potential Assay
4.13. RNA Extraction and Quantitative RT-PCR
4.14. Small Interference RNA (siRNA) Transfection
4.15. Spheroid Formation
4.16. Immunofluorescence Analysis
4.17. In Vivo Toxicity and Xenograft Analysis
4.18. Angiogenesis Analysis of Blood Vessel Development in Transgenic Zebrafish
4.19. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.D.; Kurman, R.J. Pathology of ovarian carcinoma. Hematol. Oncol. Clin. N. Am. 2003, 17, 909–925. [Google Scholar] [CrossRef]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, H.; Li, M.; Yang, Y.; Sun, L. Anticancer effect of eupatilin on glioma cells through inhibition of the Notch-1 signaling pathway. Mol. Med. Rep. 2016, 13, 1141–1146. [Google Scholar] [CrossRef]
- Park, B.B.; Yoon, J.; Kim, E.; Choi, J.; Won, Y.; Choi, J.; Lee, Y.Y. Inhibitory effects of eupatilin on tumor invasion of human gastric cancer MKN-1 cells. Tumour. Biol. 2013, 34, 875–885. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.G.; Yang, Y.I.; Kim, J.H.; Ahn, J.H.; Baek, N.I.; Lee, K.T.; Choi, J.H. Eupatilin, a dietary flavonoid, induces G2/M cell cycle arrest in human endometrial cancer cells. Food Chem. Toxicol. 2011, 49, 1737–1744. [Google Scholar] [CrossRef]
- Zhong, W.F.; Wang, X.H.; Pan, B.; Li, F.; Kuang, L.; Su, Z.X. Eupatilin induces human renal cancer cell apoptosis via ROS-mediated MAPK and PI3K/AKT signaling pathways. Oncol. Lett. 2016, 12, 2894–2899. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Zhu, L.; Chen, X.; Xu, Y.; Zhao, Y.; Shao, Y.; Li, F.; Jiang, Y.; Lu, J.; et al. Eupatilin inhibits the proliferation of human esophageal cancer TE1 cells by targeting the AktGSK3beta and MAPK/ERK signaling cascades. Oncol. Rep. 2018, 39, 2942–2950. [Google Scholar] [CrossRef]
- Silverman, G.A.; Bird, P.I.; Carrell, R.W.; Church, F.C.; Coughlin, P.B.; Gettins, P.G.; Irving, J.A.; Lomas, D.A.; Luke, C.J.; Moyer, R.W.; et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 2001, 276, 33293–33296. [Google Scholar] [CrossRef]
- Law, R.H.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An overview of the serpin superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farshchian, M.; Kivisaari, A.; Ala-Aho, R.; Riihila, P.; Kallajoki, M.; Grenman, R.; Peltonen, J.; Pihlajaniemi, T.; Heljasvaara, R.; Kahari, V.M. Serpin peptidase inhibitor clade A member 1 (SerpinA1) is a novel biomarker for progression of cutaneous squamous cell carcinoma. Am. J. Pathol. 2011, 179, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Yeo, H.Y.; Chang, H.J.; Kim, K.H.; Kim, S.Y.; Park, J.W.; Park, S.C.; Choi, H.S.; Kim, D.Y.; Oh, J.H. Serpin B5 is a CEA-interacting biomarker for colorectal cancer. Int. J. Cancer 2014, 134, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Kim, J.H.; Ahn, S.E.; Jeong, W.; Kim, J.; Bazer, F.W.; Han, J.Y.; Song, G. Avian SERPINB11 gene: A marker for ovarian endometrioid cancer in chickens. Exp. Biol. Med. 2012, 237, 150–159. [Google Scholar] [CrossRef]
- Xu, H.; Guan, N.; Ren, Y.L.; Wei, Q.J.; Tao, Y.H.; Yang, G.S.; Liu, X.Y.; Bu, D.F.; Zhang, Y.; Zhu, S.N. IP3R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC Nephrol. 2018, 19, 140. [Google Scholar] [CrossRef]
- Pedriali, G.; Rimessi, A.; Sbano, L.; Giorgi, C.; Wieckowski, M.R.; Previati, M.; Pinton, P. Regulation of endoplasmic reticulum-mitochondria Ca(2+) transfer and its importance for anti-cancer therapies. Front. Oncol. 2017, 7, 180. [Google Scholar] [CrossRef]
- Gomez-Suaga, P.; Paillusson, S.; Miller, C.C.J. ER-mitochondria signaling regulates autophagy. Autophagy 2017, 13, 1250–1251. [Google Scholar] [CrossRef]
- Mabuchi, S.; Kuroda, H.; Takahashi, R.; Sasano, T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015, 137, 173–179. [Google Scholar] [CrossRef]
- Miller, C.R.; Oliver, K.E.; Farley, J.H. MEK1/2 inhibitors in the treatment of gynecologic malignancies. Gynecol. Oncol. 2014, 133, 128–137. [Google Scholar] [CrossRef]
- Nagaraj, A.B.; Wang, Q.Q.; Joseph, P.; Zheng, C.; Chen, Y.; Kovalenko, O.; Singh, S.; Armstrong, A.; Resnick, K.; Zanotti, K.; et al. Using a novel computational drug-repositioning approach (DrugPredict) to rapidly identify potent drug candidates for cancer treatment. Oncogene 2018, 37, 403–414. [Google Scholar] [CrossRef]
- Ryoo, S.B.; Oh, H.K.; Yu, S.A.; Moon, S.H.; Choe, E.K.; Oh, T.Y.; Park, K.J. The effects of eupatilin (stillen(R)) on motility of human lower gastrointestinal tracts. Korean J. Physiol. Pharmacol. 2014, 18, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.H.; Hong, S.Y.; Zheng, Y.; Noh, S.H. Eupatilin Inhibits Gastric Cancer Cell Growth by Blocking STAT3-Mediated VEGF Expression. J. Gastric Cancer 2011, 11, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, D.H.; Na, H.K.; Oh, T.Y.; Shin, C.Y.; Surh, Y.J. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human gastric cancer (AGS) cells. J. Environ. Pathol. Toxicol. Oncol. 2005, 24, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Oh, H.M.; Wee, H.; Choi, C.S.; Choi, S.C.; Kim, K.H.; Han, W.C.; Oh, T.Y.; Kim, S.H.; Jun, C.D. Eupatilin exhibits a novel anti-tumor activity through the induction of cell cycle arrest and differentiation of gastric carcinoma AGS cells. Differentiation 2009, 77, 412–423. [Google Scholar] [CrossRef]
- Fei, X.; Wang, J.; Chen, C.; Ding, B.; Fu, X.; Chen, W.; Wang, C.; Xu, R. Eupatilin inhibits glioma proliferation, migration, and invasion by arresting cell cycle at G1/S phase and disrupting the cytoskeletal structure. Cancer Manag. Res. 2019, 11, 4781–4796. [Google Scholar] [CrossRef]
- Lou, Y.; Wu, J.; Liang, J.; Yang, C.; Wang, K.; Wang, J.; Guo, X. Eupatilin protects chondrocytes from apoptosis via activating sestrin2-dependent autophagy. Int. Immunopharmacol. 2019, 75, 105748. [Google Scholar] [CrossRef]
- Li, F.; Tao, Y.; Qiao, Y.; Li, K.; Jiang, Y.; Cao, C.; Ren, S.; Chang, X.; Wang, X.; Wang, Y.; et al. Eupatilin inhibits EGF-induced JB6 cell transformation by targeting PI3K. Int. J. Oncol. 2016, 49, 1148–1154. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, Z.; Chen, N.; Zhong, K.; Lin, Y.; Jiang, H.; Wan, P.; Lu, S.; Yang, L.; Liu, S. Eupatilin inhibits renal cancer growth by downregulating microRNA-21 through the activation of YAP1. BioMed Res. Int. 2019, 2019, 5016483. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, B.; Zhang, X.; Peng, X. Eupatilin regulates proliferation and cell cycle of cervical cancer by regulating hedgehog signalling pathway. Cell Biochem. Funct. 2020, 10.1002/cbf.3493. [Google Scholar] [CrossRef]
- Kim, Y.D.; Choi, S.C.; Oh, T.Y.; Chun, J.S.; Jun, C.D. Eupatilin inhibits T-cell activation by modulation of intracellular calcium flux and NF-kappaB and NF-AT activity. J. Cell. Biochem. 2009, 108, 225–236. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.G.; Min, K.; Kwon, T.K.; Kim, H.J.; Nam, J.O. Eupatilin inhibits adipogenesis through suppression of PPARgamma activity in 3T3-L1 cells. Biomed. Pharmacother. 2018, 103, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Shin, C.Y.; Oh, T.Y.; Sohn, U.D. The protective effect of eupatilin on indomethacin-induced cell damage in cultured feline ileal smooth muscle cells: Involvement of HO-1 and ERK. J. Ethnopharmacol. 2008, 118, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, D.; Jang, H.J.; Jang, D.S.; Kwon, H.C.; Kim, K.H.; Kim, S.N.; Hwang, G.S.; Kang, K.S.; Eom, D.W. Protective effect of artemisia asiatica extract and its active compound eupatilin against cisplatin-induced renal damage. Evid. Based Complement. Alternat. Med. 2015, 2015, 483980. [Google Scholar] [CrossRef] [PubMed]
- Shiiba, M.; Nomura, H.; Shinozuka, K.; Saito, K.; Kouzu, Y.; Kasamatsu, A.; Sakamoto, Y.; Murano, A.; Ono, K.; Ogawara, K.; et al. Down-regulated expression of SERPIN genes located on chromosome 18q21 in oral squamous cell carcinomas. Oncol. Rep. 2010, 24, 241–249. [Google Scholar] [CrossRef]
- Heit, C.; Jackson, B.C.; McAndrews, M.; Wright, M.W.; Thompson, D.C.; Silverman, G.A.; Nebert, D.W.; Vasiliou, V. Update of the human and mouse SERPIN gene superfamily. Hum. Genom. 2013, 7, 22. [Google Scholar] [CrossRef]
- Baldzizhar, R.; Fedorchuk, C.; Jha, M.; Rathinam, C.; Henegariu, O.; Czyzyk, J. Anti-serpin antibody-mediated regulation of proteases in autoimmune diabetes. J. Biol. Chem. 2013, 288, 1612–1619. [Google Scholar] [CrossRef]
- Schick, C.; Pemberton, P.A.; Shi, G.P.; Kamachi, Y.; Cataltepe, S.; Bartuski, A.J.; Gornstein, E.R.; Bromme, D.; Chapman, H.A.; Silverman, G.A. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: A kinetic analysis. Biochemistry 1998, 37, 5258–5266. [Google Scholar] [CrossRef]
- Zou, Z.; Anisowicz, A.; Hendrix, M.J.; Thor, A.; Neveu, M.; Sheng, S.; Rafidi, K.; Seftor, E.; Sager, R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 1994, 263, 526–529. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, H.; Ma, W.; Sun, M.; Pu, J.; Yu, J.; Zhang, N.; Cao, X.; Pei, X.; Wang, Y. Expression and localization of SerpinB11 in mouse uteri during peri-implantation and the estrous cycle. Cell Tissue Res. 2014, 357, 373–380. [Google Scholar] [CrossRef]
- Chanda, B.; Mathew, M.K. Functional reconstitution of bacterially expressed human potassium channels in proteoliposomes: Membrane potential measurements with JC-1 to assay ion channel activity. Bba-Biomembranes 1999, 1416, 92–100. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kania, E.; Roest, G.; Vervliet, T.; Parys, J.B.; Bultynck, G. IP3 Receptor-Mediated Calcium Signaling and Its Role in Autophagy in Cancer. Front. Oncol. 2017, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, D.H.; Jeon, Y.; Kim, Y.J.; Lee, J.; Shin, M.S.; Kang, K.S.; Hwang, G.S.; Kim, H.Y.; Yamabe, N. Eupatilin inhibits angiogenesis-mediated human hepatocellular metastasis by reducing MMP-2 and VEGF signaling. Bioorg. Med. Chem. Lett. 2018, 28, 3150–3154. [Google Scholar] [CrossRef] [PubMed]



| Primary Antibodies | Dilution | Supplier | Catalog Number |
|---|---|---|---|
| Phospho-AKT (SER473) | 1:1000 | Cell Signaling | 4060 |
| AKT | 1:1000 | Cell Signaling | 9272 |
| Phospho-ERK1/2 (Thr202/Tyr204) | 1:1000 | Cell Signaling | 9101 |
| ERK1/2 | 1:1000 | Cell Signaling | 4695 |
| Phospho-JNK (Thr183/Tyr185) | 1:1000 | Cell Signaling | 4668 |
| JNK | 1:1000 | Cell Signaling | 9252 |
| Phospho-P38 (Thr180/Tyr182) | 1:1000 | Cell Signaling | 4511 |
| P38 | 1:1000 | Cell Signaling | 9212 |
| Phospho-P70S6K (Thr421/Ser424) | 1:1000 | Cell Signaling | 9204 |
| P70S6K | 1:1000 | Cell Signaling | 9202 |
| Phospho-S6 (Ser235/236) | 1:1000 | Cell Signaling | 2211 |
| S6 | 1:1000 | Cell Signaling | 2217 |
| Phospho-Cyclin D1 (Thr286) | 1:1000 | Cell Signaling | 3300 |
| Cyclin D1 | 1:1000 | Cell Signaling | 2922 |
| Phospho-P90RSK (Thr573) | 1:1000 | Cell Signaling | 9346 |
| P90RSK | 1:1000 | Cell Signaling | 9355 |
| IRE1α | 1:1000 | Cell Signaling | 3294 |
| Phospho-eIF2α (Ser51) | 1:1000 | Cell Signaling | 3398 |
| eIF2α | 1:1000 | Cell Signaling | 5324 |
| Phospho-PERK (Thr981) | 1:1000 | Santa Cruz | sc-32577 |
| PERK | 1:1000 | Santa Cruz | sc-13073 |
| ATF6α | 1:1000 | Santa Cruz | sc-166659 |
| GADD153 | 1:1000 | Santa Cruz | sc-7351 |
| GRP78 | 1:1000 | Santa Cruz | sc-13968 |
| SERPINB11 | 1:1000 | Santa Cruz | sc-85140 |
| Cleaved caspase-3 | 1:1000 | Cell Signaling | 9664 |
| Cleaved caspase-9 | 1:1000 | Cell Signaling | 9501 |
| Cytochrome c | 1:1000 | Cell Signaling | 11940 |
| TUBA | 1:2000 | Santa Cruz | sc-5286 |
| Gene | Primer Sequence (5′ →β 3′) | Size (bp) | ||
|---|---|---|---|---|
| Human | COX2 (NM_000963.4) | Forward | TTCTCCTGCCTACTGGAAGC | 106 |
| Reverse | ACAGCCCTTCACGTTATTGC | |||
| MMP2 (NM_001127891.2) | Forward | GGCATTCAGGAGCTCTATGG | 137 | |
| Reverse | ATCTCACCACGGATCTGAGC | |||
| MMP9 (NM_004994.3) | Forward | TTGACAGCGACAAGAAGTGG | 145 | |
| Reverse | TCAGTGAAGCGGTACATAGGG | |||
| NF-κB (NM_001165412.1) | Forward | TCTGTGTTTGTCCAGCTTCG | 115 | |
| Reverse | GCTTCTGACGTTTCCTCTGC | |||
| SERPINB11 (NM_001291278.1) | Forward | CATTCCGAGTTTGGTGTCG | 102 | |
| Reverse | AAATGCCATCGTCTTTGTCC | |||
| TIMP2 (NM_003255.5) | Forward | AGAAGAGCCTGAACCACAGG | 119 | |
| Reverse | CTCTGTGACCCAGTCCATCC | |||
| Zebrafish | flt1(BC139515.1) | Forward | CTGGTTATTCGGGATGTTGC | 121 |
| Reverse | TTTGGGGCTTCACATTTACC | |||
| flt4 (AY833404.1) | Forward | TCACAACTGGATGGATTTGG | 100 | |
| Reverse | GCCGACAGTCTTTTCTTTGC | |||
| kdrl (NM_131472.1) | Forward | CCTGATCCACAACTGCTTCC | 142 | |
| Reverse | CACACGACTCAATGCGTACC | |||
| vegfa (AF016244.1) | Forward | ATTCATACCCAGCAGCTTCG | 137 | |
| Reverse | GCAGACAGATGGAGGAGAGC | |||
| vegfc (AF466147.1) | Forward | GATGTGGGGAAAGAGTTTGG | 112 | |
| Reverse | TGATGTTCCTGCACTGAAGC | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-Y.; Bae, H.; Yang, C.; Park, S.; Youn, B.-S.; Kim, H.-S.; Song, G.; Lim, W. Eupatilin Promotes Cell Death by Calcium Influx through ER-Mitochondria Axis with SERPINB11 Inhibition in Epithelial Ovarian Cancer. Cancers 2020, 12, 1459. https://doi.org/10.3390/cancers12061459
Lee J-Y, Bae H, Yang C, Park S, Youn B-S, Kim H-S, Song G, Lim W. Eupatilin Promotes Cell Death by Calcium Influx through ER-Mitochondria Axis with SERPINB11 Inhibition in Epithelial Ovarian Cancer. Cancers. 2020; 12(6):1459. https://doi.org/10.3390/cancers12061459
Chicago/Turabian StyleLee, Jin-Young, Hyocheol Bae, Changwon Yang, Sunwoo Park, Byung-Soo Youn, Han-Soo Kim, Gwonhwa Song, and Whasun Lim. 2020. "Eupatilin Promotes Cell Death by Calcium Influx through ER-Mitochondria Axis with SERPINB11 Inhibition in Epithelial Ovarian Cancer" Cancers 12, no. 6: 1459. https://doi.org/10.3390/cancers12061459
APA StyleLee, J.-Y., Bae, H., Yang, C., Park, S., Youn, B.-S., Kim, H.-S., Song, G., & Lim, W. (2020). Eupatilin Promotes Cell Death by Calcium Influx through ER-Mitochondria Axis with SERPINB11 Inhibition in Epithelial Ovarian Cancer. Cancers, 12(6), 1459. https://doi.org/10.3390/cancers12061459






