Utilisation of Colorectal Cancer Screening Tests in European Countries by Type of Screening Offer: Results from the European Health Interview Survey
Abstract
1. Introduction
2. Results
2.1. CRC Screening Offers in the EU Countries, Iceland, Norway and the UK
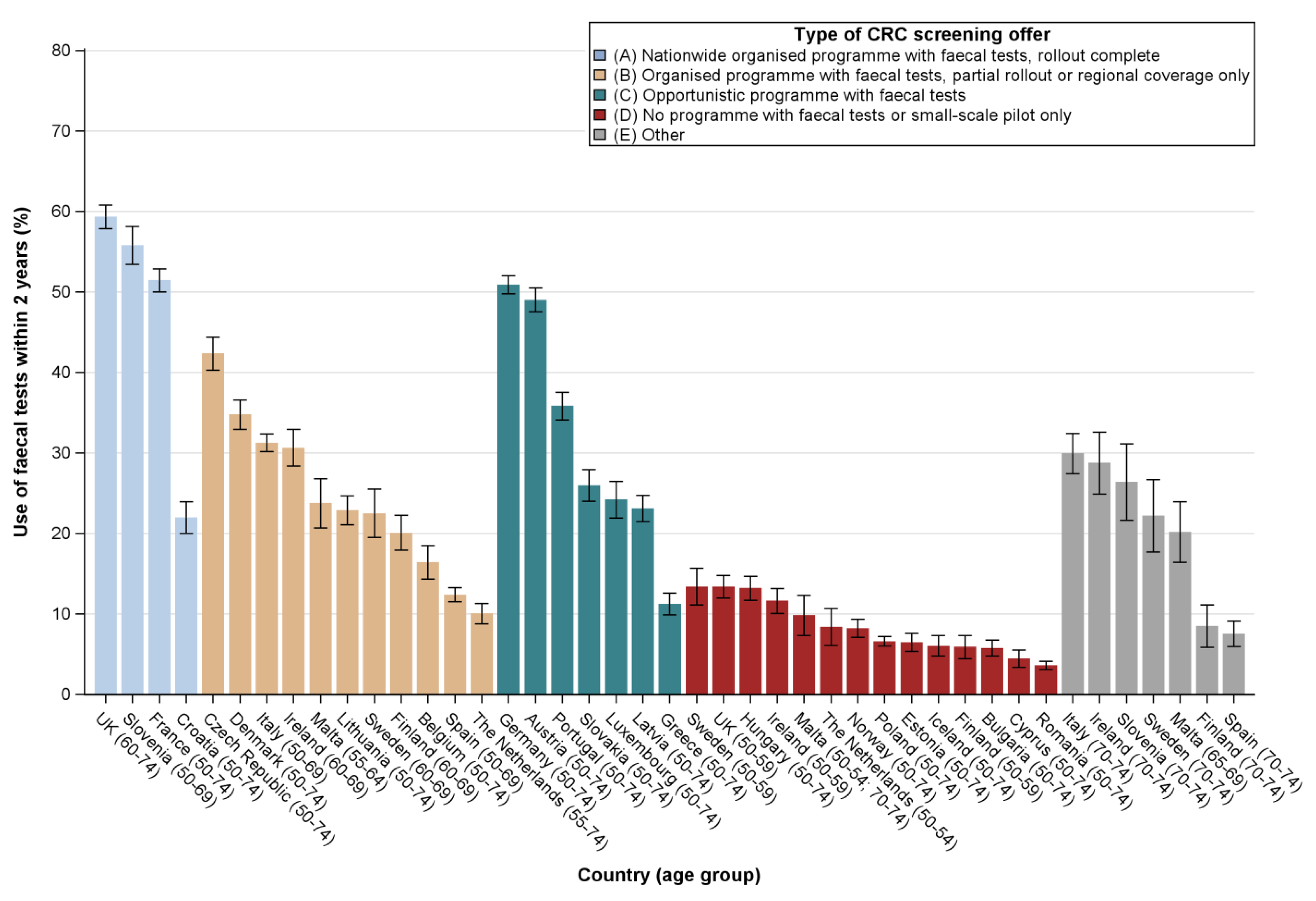
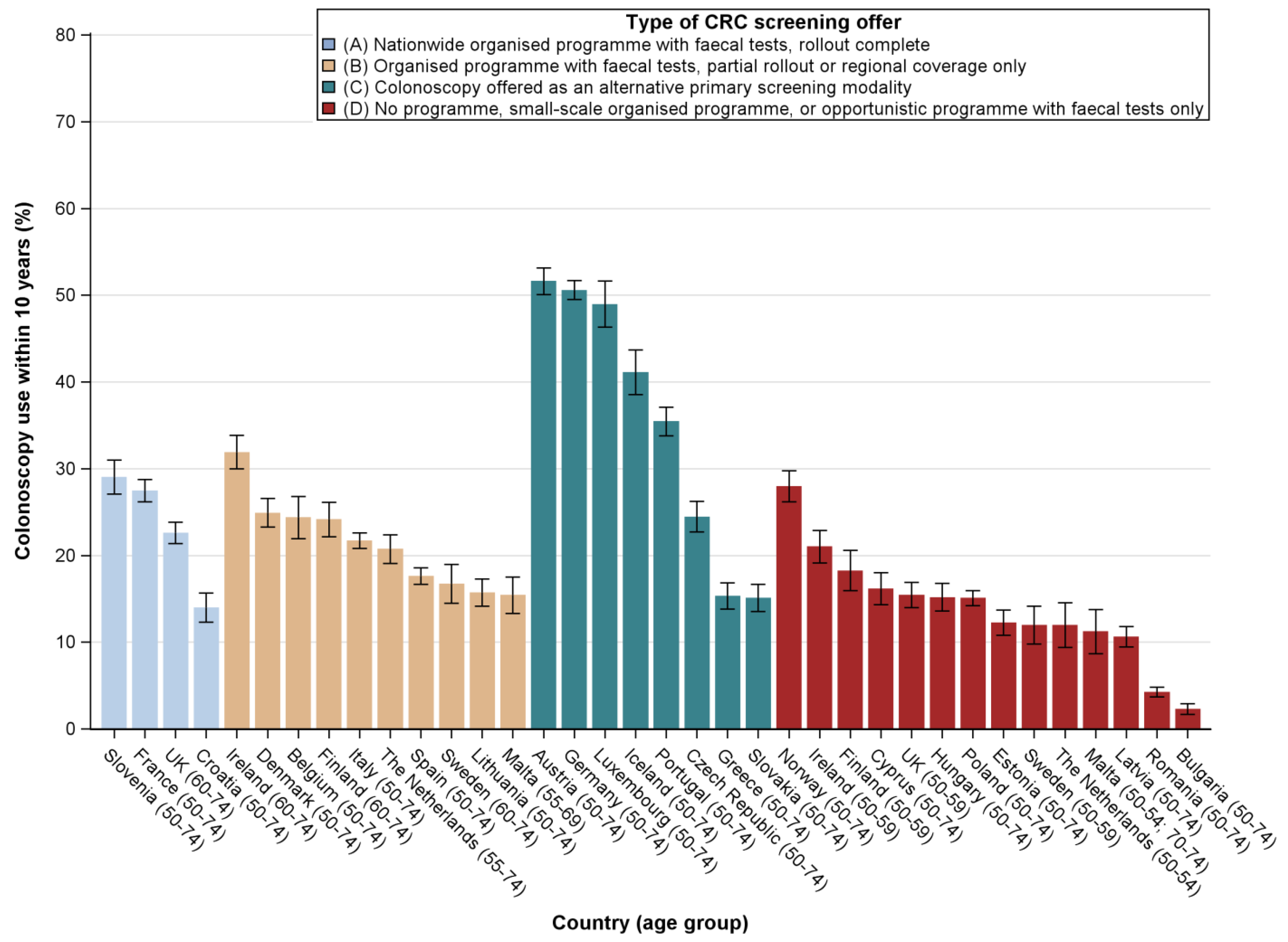
2.2. Utilisation Rates by Type of CRC Screening Offer
2.3. Determinants of Test Use by Type of CRC Screening Offer
3. Discussion
3.1. Principal Findings
3.2. Strengths and Weaknesses of the Study
3.3. Comparison with Other Studies and Interpretation of Results
4. Materials and Methods
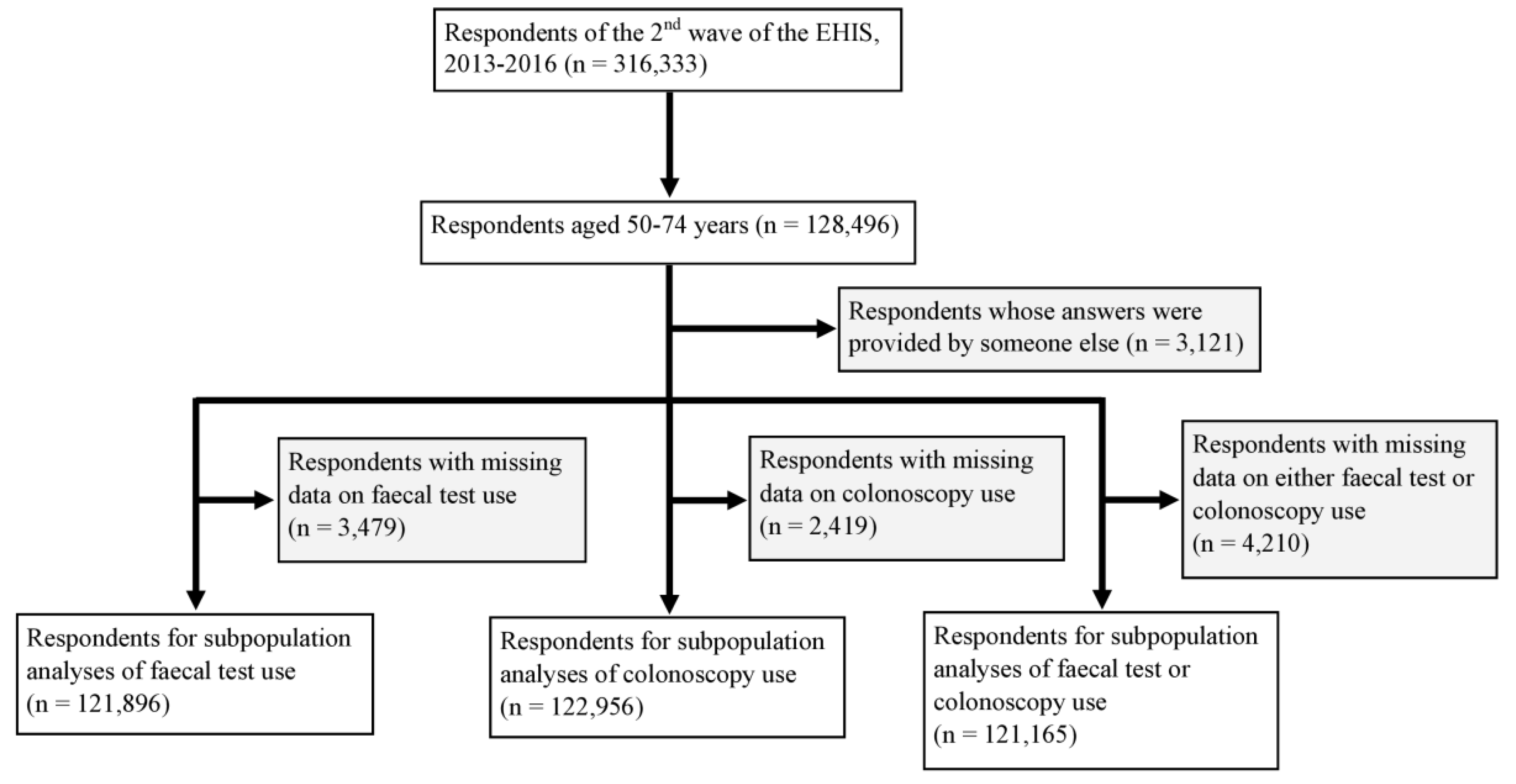
4.1. Study Design and Study Population
4.2. Data Collection
4.3. Measures
4.4. Derivation of the Healthy Lifestyle Score
4.5. Classification of Countries/Age Groups by Type of CRC Screening Offer
4.6. Statistical Analyses
4.7. Ethics Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Data Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Altenhofen, L.; Stock, C.; Hoffmeister, M. Natural history of colorectal adenomas: Birth cohort analysis among 3.6 million participants of screening colonoscopy. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1043–1051. [Google Scholar] [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Stock, C.; Hoffmeister, M.; Brenner, H. Public health impact of colonoscopy use on colorectal cancer mortality in Germany and the United States. Gastrointest. Endosc. 2018, 87, 213–221.e212. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, P.; Glasziou, P.; Watson, E.; Towler, B.; Irwig, L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am. J. Gastroenterol. 2008, 103, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Scholefield, J.H.; Moss, S.M.; Mangham, C.M.; Whynes, D.K.; Hardcastle, J.D. Nottingham trial of faecal occult blood testing for colorectal cancer: A 20-year follow-up. Gut 2012, 61, 1036–1040. [Google Scholar] [CrossRef]
- Shaukat, A.; Mongin, S.J.; Geisser, M.S.; Lederle, F.A.; Bond, J.H.; Mandel, J.S.; Church, T.R. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 2013, 369, 1106–1114. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W., Jr.; García, F.A.R.; Gillman, M.W.; Harper, D.M.; Kemper, A.R.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 315, 2564–2575. [Google Scholar]
- Rex, D.K.; Boland, R.C.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Basu, P.; Ponti, A.; Anttila, A.; Ronco, G.; Senore, C.; Vale, D.B.; Segnan, N.; Tomatis, M.; Soerjomataram, I.; Zakelj, M.P.; et al. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int. J. Cancer 2018, 142, 44–56. [Google Scholar] [PubMed]
- The Council Of The European Union. Council Recommendations of 2 December 2003 on cancer screening (2003/878/EC). Off. J. Eur. Union 2003, 327, 34–38. [Google Scholar]
- European Health Interview Survey (EHIS). Available online: https://ec.europa.eu/eurostat/web/microdata/european-health-interview-survey (accessed on 6 January 2020).
- Vale, D.B.; Anttila, A.; Ponti, A.; Senore, C.; Sankaranaryanan, R.; Ronco, G.; Segnan, N.; Tomatis, M.; Zakelj, M.P.; Elfstrom, K.M.; et al. Invitation strategies and coverage in the population-based cancer screening programmes in the European Union. Eur. J. Cancer Prev. 2019, 28, 131–140. [Google Scholar] [PubMed]
- Haidinger, G.; Waldhoer, T.; Vutuc, C. Self-reported colonoscopy screening in Austria. Eur. J. Cancer Prev. 2008, 17, 354–357. [Google Scholar] [CrossRef]
- Young, G.P.; Rabeneck, L.; Winawer, S.J. The Global Paradigm Shift in Screening for Colorectal Cancer. Gastroenterology 2019, 156, 843–851.e842. [Google Scholar]
- Hoeck, S.; Pringels, S.; Kellen, E.; Van Herck, K.; Martens, P.; Van Limbergen, E.; Francart, J.; Van Hal, G. First results of the Flemish colorectal cancer screening program: Start-up- period late 2013. Acta Gastroenterol. Belg. 2016, 79, 421–428. [Google Scholar]
- Ponti, A.; Anttila, A.; Ronco, G.; Senore, C.; Basu, P.; Segnan, N.; Tomatis, M.; Zakelj, M.P.; Dillner, J.; Fernan, M.; et al. Cancer Screening in the European Union (2017) —Report on the Implementation of the Council Recommendation on Cancer Screening; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Katicic, M.; Antoljak, N.; Kujundzic, M.; Stamenic, V.; Poljak, D.S.; Kramaric, D.; Stimac, D.; Strnad Pesikan, M.; Samija, M.; Ebling, Z. Results of National Colorectal Cancer Screening Program in Croatia (2007-2011). World J. Gastroenterol. 2012, 18, 4300–4307. [Google Scholar] [CrossRef]
- Farazi, P.A. Cancer trends and risk factors in Cyprus. Ecancermedicalscience 2014, 8, 389. [Google Scholar]
- Dušek, L.; Zavoral, M.; Májek, O.; Suchánek, Š.; Mužík, J.; Pavlík, T.; Šnajdrová, L.; Gregor, J. Kolorektum.cz–Colorectal Cancer Screening Programme in the Czech Republic.; Masaryk University: Brno, Czech Republic, 2019; Available online: http://www.kolorektum.cz/index-en.php (accessed on 6 January 2020).
- Suchanek, S.; Majek, O.; Vojtechova, G.; Minarikova, P.; Rotnaglova, B.; Seifert, B.; Minarik, M.; Kozeny, P.; Dusek, L.; Zavoral, M. Colorectal cancer prevention in the Czech Republic: Time trends in performance indicators and current situation after 10 years of screening. Eur. J. Cancer Prev. 2014, 23, 18–26. [Google Scholar] [CrossRef]
- Larsen, M.B.; Njor, S.; Ingeholm, P.; Andersen, B. Effectiveness of Colorectal Cancer Screening in Detecting Earlier-Stage Disease-A Nationwide Cohort Study in Denmark. Gastroenterology 2018, 155, 99–106. [Google Scholar] [PubMed]
- Njor, S.H.; Friis-Hansen, L.; Andersen, B.; Sondergaard, B.; Linnemann, D.; Jorgensen, J.C.R.; Roikjaer, O.; Rasmussen, M. Three years of colorectal cancer screening in Denmark. Cancer Epidemiol. 2018, 57, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Innos, K.; Reima, H.; Baburin, A.; Paapsi, K.; Aareleid, T.; Soplepmann, J. Subsite- and stage-specific colorectal cancer trends in Estonia prior to implementation of screening. Cancer Epidemiol. 2018, 52, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Malila, N.; Anttila, A.; Hakama, M. Colorectal cancer screening in Finland: Details of the national screening programme implemented in Autumn 2004. J. Med. Screen 2005, 12, 28–32. [Google Scholar] [CrossRef]
- Pitkaniemi, J.; Seppa, K.; Hakama, M.; Malminiemi, O.; Palva, T.; Vuoristo, M.S.; Jarvinen, H.; Paimela, H.; Pikkarainen, P.; Anttila, A.; et al. Effectiveness of screening for colorectal cancer with a faecal occult-blood test, in Finland. BMJ Open Gastroenterol. 2015, 2, e000034. [Google Scholar] [CrossRef]
- Finish Cancer Registry. Colorectal Cancer Screening. Available online: https://cancerregistry.fi/screening/colorectal-cancer-screening/ (accessed on 6 January 2020).
- Hamers, F.F.; Assogba, F.A.G.; Rogel, A. Implementation and organization of cancer screening in France. Int. J. Cancer 2018, 143, 3281. [Google Scholar] [CrossRef]
- Guo, F.; Chen, C.; Schottker, B.; Holleczek, B.; Hoffmeister, M.; Brenner, H. Changes in colorectal cancer screening use after introduction of alternative screening offer in Germany: Prospective cohort study. Int. J. Cancer 2020, 146, 2423–2432. [Google Scholar]
- Bundesausschuss, G. Richtlinie Für Organisierte Krebsfrüherkennungsprogramme [Directive On Organised Cancer Screening Programmes]. Available online: https://www.g-ba.de/richtlinien/104/ (accessed on 6 January 2020).
- McFerran, E.; Kee, F.; Coleman, H.G. Colorectal cancer screening: Surely FIT for us too. Frontline Gastroenterol. 2019, 10, 445–446. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Country Profiles, 2014: Greece. Available online: https://www.who.int/cancer/country-profiles/grc_en.pdf (accessed on 6 January 2020).
- Kívés, Z.H.; Vajda, R.; Endrei, D.; Boncz, I. Colorectal Cancer Screening Pilot Program Results in Hungary. Value Health 2018, 21, S48. [Google Scholar] [CrossRef][Green Version]
- Altobelli, E.; D’Aloisio, F.; Angeletti, P.M. Colorectal cancer screening in countries of European council outside of the EU-28. World J. Gastroenterol. 2016, 22, 4946–4957. [Google Scholar] [CrossRef] [PubMed]
- Senore, C.; Ederle, A.; Benazzato, L.; Arrigoni, A.; Silvani, M.; Fantin, A.; Fracchia, M.; Armaroli, P.; Segnan, N. Offering people a choice for colorectal cancer screening. Gut 2013, 62, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.S.; Martini, A.; Puliti, D.; Ciatto, S.; Castiglione, G.; Grazzini, G.; Zappa, M. Colorectal cancer mortality in two areas of Tuscany with different screening exposures. J. Natl. Cancer Inst. 2008, 100, 1818–1821. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Re, F.D.; Mantellini, P.; Naldoni, C.; De’Bianchi, P.S.; Senore, C.; Turrin, A.; Visioli, C.B.; Zappa, M. Screening for colorectal cancer in Italy: 2011-2012 survey. Epidemiol. Prev. 2015, 39, 93–107. [Google Scholar] [PubMed]
- Senore, C.; Basu, P.; Anttila, A.; Ponti, A.; Tomatis, M.; Vale, D.B.; Ronco, G.; Soerjomataram, I.; Primic-Zakelj, M.; Riggi, E.; et al. Performance of colorectal cancer screening in the European Union Member States: Data from the second European screening report. Gut 2019, 68, 1232–1244. [Google Scholar] [CrossRef]
- Poskus, T.; Strupas, K.; Mikalauskas, S.; Bitinaite, D.; Kavaliauskas, A.; Samalavicius, N.E.; Saladzinskas, Z. Initial results of the national colorectal cancer screening program in Lithuania. Eur. J. Cancer Prev. 2015, 24, 76–80. [Google Scholar] [CrossRef]
- Cancer Registry of Norway. Colorectal Cancer Screening, Bowel Cancer Screening in Norway – A Pilot Study. Available online: https://www.kreftregisteret.no/en/screening/Screening-for-colorectal-cancer/ (accessed on 6 January 2020).
- Kaminski, M.F.; Kraszewska, E.; Rupinski, M.; Laskowska, M.; Wieszczy, P.; Regula, J. Design of the Polish Colonoscopy Screening Program: A randomized health services study. Endoscopy 2015, 47, 1144–1150. [Google Scholar] [CrossRef]
- Directorate-General of Health, Portugal. Rastreio Oportunístico do Cancro do Cólon e Reto [Opportunistic Screening for Colorectal Cancer]. Lisbon, Portugal, 2014. Available online: https://www.nghd.pt/uploads/noc_rccr_act.pdf (accessed on 6 January 2020).
- Dinis-Ribeiro, M.; Areia, M.; Dias-Pereira, A. SPED Statement: Colorectal cancer screening in Portugal. Endoscopy 2019, 51, 803–804. [Google Scholar] [CrossRef]
- do Forno, S.E.; Poças, F.C.; dos Santos, M.E. O cancro colorretal e o rastreio: Conhecimentos e atitudes dos portuenses [Colorectal cancer and screening: Portuenses’ knowledge and attitudes]. GE Port. J. Gastroenterol. 2012, 19, 118–125. [Google Scholar]
- Araujo, F. Despacho no. 8254/2017 [Order no. 8254/2017]. In: Saúde—Gabinete do Secretário de Estado Adjunto e da Saúde. Available online: https://dre.pt/pesquisa/-/search/108189401/details/normal?l=1 (accessed on 6 January 2020).
- Mlakar, D.N.; Bric, T.K. Slovenian national colorectal cancer screening – Programme SVIT. Eur. J. Public Health 2018, 28. [Google Scholar]
- Tepes, B.; Bracko, M.; Mlakar, D.N.; Stefanovic, M.; Stabuc, B.; Grazio, S.F.; Zakotnik, J.M. Results of the FIT-based National Colorectal Cancer Screening Program in Slovenia. J. Clin. Gastroenterol. 2017, 51, e52–e59. [Google Scholar] [CrossRef] [PubMed]
- Trejo, D.S.; Villares, I.P.; Pinol, J.A.E.; Cabanell, J.I.; Espi, M.V.; Riquelme, F.P.; de la Vega Prieto, M.; de Aledo Linos, A.G.; Rubio, I.I.; Terroba, B.S.; et al. Implementation of colorectal cancer screening in Spain: Main results 2006-2011. Eur. J. Cancer Prev. 2017, 26, 17–26. [Google Scholar] [CrossRef]
- Idigoras, I.; Arrospide, A.; Portillo, I.; Arana-Arri, E.; Martinez-Indart, L.; Mar, J.; de Koning, H.J.; Lastra, R.; Soto-Gordoa, M.; van der Meulen, M.; et al. Evaluation of the colorectal cancer screening Programme in the Basque Country (Spain) and its effectiveness based on the Miscan-colon model. BMC Public Health 2017, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Binefa, G.; Garcia, M.; Mila, N.; Fernandez, E.; Rodriguez-Moranta, F.; Gonzalo, N.; Benito, L.; Clopes, A.; Guardiola, J.; Moreno, V. Colorectal Cancer Screening Programme in Spain: Results of Key Performance Indicators After Five Rounds (2000-2012). Sci. Rep. 2016, 6, 19532. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.; Kilpelainen, S.; Hultcrantz, R.; Tornberg, S. Five-year experience of organized colorectal cancer screening in a Swedish population - increased compliance with age, female gender, and subsequent screening round. J. Med. Screen. 2014, 21, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.; Lowbeer, C.; Elfstrom, K.M.; Sventelius, M.; Ohman, D.; Saraste, D.; Tornberg, S. Gender-specific cut-offs in colorectal cancer screening with FIT: Increased compliance and equal positivity rate. J. Med. Screen. 2019, 26, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Bowel Cancer Screening: Programme Overview 2019. Available online: https://www.gov.uk/guidance/bowel-cancer-screening-programme-overview (accessed on 6 January 2020).
- Public Health England. Bowel Screening to Start at 50, 2018. Available online: https://www.gov.uk/government/news/bowel-screening-to-start-at-50 (accessed on 6 January 2020).
- National Services Scotland. Scottish Bowel Screening Programme Statistics For invitations between 1 November 2015 and 31 October 2017, 2018. Available online: https://www.isdscotland.org/Health-Topics/Cancer/Publications/2018-08-07/2018-08-07-Bowel-Screening-Publication-Report.pdf?95512026549 (accessed on 6 January 2020).
- Cancer Research UK. Introduction of the Faecal Immunochemical Test (FIT) 2019. Available online: https://www.cancerresearchuk.org/health-professional/screening/bowel-screening-evidence-and-resources/faecal-immunochemical-test-fit#FIT1 (accessed on 6 January 2020).
- Eurostat, European Health Interview Survey (EHIS wave 2). Methodological Manual; European Union: Luxembourg, 2013; Available online: https://ec.europa.eu/eurostat/documents/7870049/8920155/KS-FT-18-003-EN-N.pdf/eb85522d-bd6d-460d-b830-4b2b49ac9b03 (accessed on 6 January 2020).
- Cardoso, R.; Niedermaier, T.; Chen, C.; Hoffmeister, M.; Brenner, H. Colonoscopy and sigmoidoscopy use among the average-risk population for colorectal cancer: A systematic review and trend analysis. Cancer Prev. Res. (Phila) 2019, 12, 617–630. [Google Scholar] [CrossRef]
- Rauscher, G.H.; Johnson, T.P.; Cho, Y.I.; Walk, J.A. Accuracy of self-reported cancer-screening histories: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2008, 17, 748–757. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. Revised 2018. Available online: https://www.wcrf.org/sites/default/files/Colorectal-Cancer-2017-Report.pdf (accessed on 6 January 2020).
- Public Health England. Bowel scope screening—Bowel cancer screening. Available online: https://www.nhs.uk/conditions/bowel-cancer-screening/bowel-scope-screening/ (accessed on 6 January 2020).
- Stock, C.; Brenner, H. Utilization of lower gastrointestinal endoscopy and fecal occult blood test in 11 European countries: Evidence from the Survey of Health, Aging and Retirement in Europe (SHARE). Endoscopy 2010, 42, 546–556. [Google Scholar] [CrossRef]
- Strnad, M.; Sogoric, S. National program of early detection of cancer in Croatia. Acta Med. Croat. 2010, 64, 461–468. [Google Scholar]
- Dimitrakaki, C.; Boulamatsis, D.; Mariolis, A.; Kontodimopoulos, N.; Niakas, D.; Tountas, Y. Use of cancer screening services in Greece and associated social factors: Results from the nation-wide Hellas Health I Survey. Eur. J. Cancer Prev. 2009, 18, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.; Kaitelidou, D.; Karanikolos, M.; Maresso, A. Greece: Health system review. Health Syst. Transit. 2017, 19, 1–192. Available online: http://www.euro.who.int/__data/assets/pdf_file/0006/373695/hit-greece-eng.pdf?ua=1 (accessed on 6 January 2020). [PubMed]
- OECD/European Observatory on Health Systems and Policies. Greece: Country Health Profile 2017, State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2017; Available online: http://dx.doi.org/10.1787/9789264283404-en (accessed on 6 January 2020).
- OECD/European Observatory on Health Systems and Policies. Slovakia: Country Health Profile 2019, State of Health in the EU; OECD Publishing: Paris, France; European Observatory on Health Systems and Policies: Brussels, Belgium, 2019; Available online: https://ec.europa.eu/health/sites/health/files/state/docs/2019_chp_sk_english.pdf (accessed on 6 January 2020).
- Brenner, H.; Altenhofen, L.; Stock, C.; Hoffmeister, M. Prevention, Early detection, and Overdiagnosis of Colorectal Cancer Within 10 Years of Screening Colonoscopy in Germany. Clin. Gastroenterol. Hepatol. 2015, 13, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Santourian, A.; Kitromilidou, S. Quality Report of The Second Wave of the European Health Interview Survey; Publications Office of the European Union: Luxembourg, 2018; Available online: https://ec.europa.eu/eurostat/documents/7870049/8920155/KS-FT-18-003-EN-N.pdf/eb85522d-bd6d-460d-b830-4b2b49ac9b03 (accessed on 6 January 2020).
- Carr, P.R.; Weigl, K.; Jansen, L.; Walter, V.; Erben, V.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Healthy Lifestyle Factors Associated With Lower Risk of Colorectal Cancer Irrespective of Genetic Risk. Gastroenterology 2018, 155, 1805–1815.e1805. [Google Scholar] [CrossRef] [PubMed]
- Erben, V.; Carr, P.R.; Holleczek, B.; Stegmaier, C.; Hoffmeister, M.; Brenner, H. Strong associations of a healthy lifestyle with all stages of colorectal carcinogenesis: Results from a large cohort of participants of screening colonoscopy. Int. J. Cancer 2019, 144, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.who.int/dietphysicalactivity/global-PA-recs-2010.pdf (accessed on 6 January 2020).
- Brenner, H.; Tao, S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur. J. Cancer 2013, 49, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.A.; Bobo, J.K.; Church, T.R.; Rex, D.K.; Chovnick, G.; Thompson, T.D.; Zauber, A.G.; Lieberman, D.; Levin, T.R.; Joseph, D.A.; et al. A Comparison of Fecal Immunochemical and High-Sensitivity Guaiac Tests for Colorectal Cancer Screening. Am. J. Gastroenterol. 2017, 112, 1728–1735. [Google Scholar] [CrossRef]
- Buskermolen, M.; Cenin, D.R.; Helsingen, L.M.; Guyatt, G.; Vandvik, P.O.; Haug, U.; Bretthauer, M.; Lansdorp-Vogelaar, I. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A microsimulation modelling study. BMJ 2019, 367, l5383. [Google Scholar] [CrossRef]

| Country | Type of Programme | Are the Tests Paid for? | Screening Test a | Year of Programme Initiation and, If Applicable, Termination | Age Groups (years) | Screening Interval (years) | Is the Faecal Test Mailed? | Invitation Coverage in 2013 in the 50–75 Age Group (%) b | EHIS Data Collection (mm/yyyy) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Austria | Opportunistic | Yes | gFOBT | 1980 | 40+ | 1 | NAc | NA | 09/2013–06/2015 | [12,13,16,17] |
| Opportunistic | Yes | Colonoscopy | 2005 | 50+ | 7–10 | NA | NA | |||
| Austria (state of Burgenland) | Organised | Yes | FIT | 2003 | 40–80 | 1 | Yes | No data | ||
| Belgium (Wallonia/Brussels) | Organised | Yes | gFOBT | 2009–2016 | 50–74 | 2 | No | 99.0 | 01/2013–12/2013 | [12,13,16,18,19] |
| FIT | 2016 | |||||||||
| Belgium (Flemish region) | Organised | Yes | FIT | 2013 | 56–74 | 2 | Yes | 69.6 | ||
| Bulgaria | No programme | NA | NA | NA | NA | NA | NA | NA | 10/2014–01/2015 | [12,13,20] |
| Croatia | Organised | Yes d | gFOBT | 2008 | 50–74 | 2 | Yes | 100.6 | 04/2014–03/2015 | [12,13,16,21] |
| Cyprus (two rural areas of Larnaca) | Organised (pilot) | Yes | FIT | 2013 | 50–69 | 2 | No | No data | 09/2014–12/2014 | [12,13,16,22] |
| Czech Republic | Opportunistic | Yes | gFOBT | 2000–2009 | 50+ | 2 | NA | NA | 06/2014–01/2015 | [12,13,16,23,24] |
| Opportunistic | Yes | FIT | 2009 | 50–54 | 1 | NA | NA | |||
| Opportunistic | Yes | FIT/Colonoscopy | 2009 | 55+ | 2/10 | NA | NA | |||
| Organised | Yes | FIT | 2014 | 50+e | 2 | No | No data | |||
| Denmark | Organised | Yes | FIT | 2014 | 50–74 | 2 | Yes | No data | 10/2015–12/2015 | [12,13,16,25,26] |
| Estonia | Organised (pilot) | Yes | FIT | 2016 | 60–69 | 2 | No | No data | 04/2014–12/2014 | [12,13,16,27] |
| Finland f | Organised | Yes | gFOBT | 2004–2016 | 60–69 | 2 | Yes | 10.5 | 11/2014–01/2015 | [12,13,16,28,29,30] |
| Organised | Yes | FIT | 2019 | 60–66 | 2 | Yes | No data | |||
| France | Organised | Yes | gFOBT | 2002–2015 | 50–74 | 2 | No | 99.1 | 01/2014–02/2015 | [12,13,16,31] |
| FIT | 2015 | |||||||||
| Germany | Opportunistic | Yes | gFOBT | 1977–2002 | 45+ | 1 | NA | NA | 11/2014–07/2015 | [12,32,33] |
| Opportunistic | Yes | gFOBT | 2002–2017 | 50–54 | 1 | NA | NA | |||
| FIT | 2017 | |||||||||
| Opportunistic | Yes | gFOBT/colonoscopy | 2002–2017 | 55+ | 2/10 | NA | NA | |||
| FIT/colonoscopy | 2017 | |||||||||
| Organised | Yes | FIT | 2019 | 50–54/55+ | 1/2 | No | NA | |||
| Organised | Yes | Colonoscopy | 2019 | 50+ (men);55+ (women) | 10 (up to 2 colonoscopies) | NA | NA | |||
| Greece | Opportunistic | No information | gFOBT | No information | No information | No information | NA | NA | 11/2014–03/2015 | [12,34,35] |
| Opportunistic | No information | Colonoscopy | No information | 50–80 | 5 | NA | NA | |||
| Hungary | Organised (pilot) | Yes | FIT | 2007 | 50–70 | 2 | No | 1.5 | 10/2014–12/2014 | [12,13,16,36] |
| Iceland g | Opportunistic | Yes | Colonoscopy | No information | No information | No information | NA | NA | 09/2015–12/2015 | [12,37] |
| Ireland | Organised | Yes | FIT | 2012 | 60–69 | 2 | Yes | 10.9 | 10/2014–04/2016 | [12,13,16] |
| Italy h | Organised | Yes | gFOBT | 1982–1996 | 50–69 | 2 | No | 52.4 | 10/2015–12/2015 | [12,13,16,38,39,40] |
| FIT | 1996 | |||||||||
| Italy i (Piedmont and Veneto regions) | Organised | Yes | Sigmoidoscopy | 2003/2004 | 58–60 | Once only | NA | No dataj | ||
| Latvia | Opportunistic | Yes | gFOBT | 2005 | 50–74 | 1 | NA | NA | 09/2014–02/2015 | [12,41] |
| Lithuania | Organised | Yes | FIT | 2009 | 50–74 | 2 | No | No data | 09/2014–11/2014 | [12,13,16,42] |
| Luxembourg | Opportunistic | Yes | gFOBT/colonoscopy | 2005 | 50+ | No information | NA | NA | 02/2014–12/2014 | [12,13,16] |
| Organised | Yes | FIT | 2016 | 55–74 | 2 | Yes | NA | |||
| Malta | Organised | Yes | FIT | 2013 | 55–66 | 2 | Yes | 28.5 | 11/2014–08/2015 | [12,13,16] |
| The Netherlands | Organised | Yes | FIT | 2014 | 55–75 | 2 | Yes | 20.2 | 01/2014–12/2014 | [12,13,16] |
| Norway k | No programme | NA | NA | NA | NA | NA | NA | NA | 08/2015–12/2015 | [43] |
| Poland | Organisedl | Yes | Colonoscopy | 2012 | 55–64 | Once only | NA | 12.5 m | 09/2014–12/2014 | [12,13,16,44] |
| Portugal | Opportunistic | No information | FIT/Colonoscopy n | No information | 50–74 | 1/10 | NA | NA | 09/2014–12/2014 | [13,16,45,46,47,48] |
| Portugal (Alentejo and Central regions) | Organised (pilot) o | Yes | gFOBT | 2009–2018 | 50–70 | 2 | No information | 1.6 | ||
| FIT | 2018 | |||||||||
| Romania | No programme | NA | NA | NA | NA | NA | NA | NA | 09/2014–11/2014 | [12,13,16,20] |
| Slovakia | Opportunistic | No information | gFOBT/colonoscopy | No information | No information | No information | NA | NA | 07/2014–12/2014 | [12] |
| Slovenia | Organised | Yes | FIT | 2009–2015 | 50–69 | 2 | Yes | 80.0 | 08/2014–12/2014 | [12,13,16,49,50] |
| 2015 | 50–74 | |||||||||
| Spain p | Organised | Yes | gFOBT | 2000–2009/2010 | 50–69 | 2 | Yes | 14.2 | 01/2014–01/2015 | [12,13,16,51,52,53] |
| Sweden (Stockholm and Gotland) | Organised | Yes | gFOBT | 2008–2015 | 60–69 | 2 | Yes | 8.5 | 09/2014–01/2015 | [12,13,16,54,55] |
| FIT | 2015 | |||||||||
| UK (England) | Organised | Yes | gFOBT | 2006–2019 | 60–74 q | 2 | Yes | 54.0 | 04/2013–03/2015 | [12,13,16,56,57] |
| FIT | 2019 | |||||||||
| Organised | Yes | Sigmoidoscopy | 2013 | 55 | Once only | NA | No data | |||
| UK (Northern Ireland) | Organised | Yes | gFOBT | 2010 | 60–74 | 2 | Yes | 51.0 | 04/2014–09/2014 | [12,13,16,34] |
| UK (Scotland) | Organised | Yes | gFOBT | 2007 | 50–74 | 2 | Yes | 110.3 | 04/2013–03/2015 | [12,13,16,58] |
| FIT | 2017 | |||||||||
| UK (Wales) | Organised | Yes | gFOBT | 2008 | 60–74 | 2 | Yes | 50.1 | 04/2013–03/2015 | [12,13,16,59] |
| FIT | 2019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, R.; Guo, F.; Heisser, T.; Hoffmeister, M.; Brenner, H. Utilisation of Colorectal Cancer Screening Tests in European Countries by Type of Screening Offer: Results from the European Health Interview Survey. Cancers 2020, 12, 1409. https://doi.org/10.3390/cancers12061409
Cardoso R, Guo F, Heisser T, Hoffmeister M, Brenner H. Utilisation of Colorectal Cancer Screening Tests in European Countries by Type of Screening Offer: Results from the European Health Interview Survey. Cancers. 2020; 12(6):1409. https://doi.org/10.3390/cancers12061409
Chicago/Turabian StyleCardoso, Rafael, Feng Guo, Thomas Heisser, Michael Hoffmeister, and Hermann Brenner. 2020. "Utilisation of Colorectal Cancer Screening Tests in European Countries by Type of Screening Offer: Results from the European Health Interview Survey" Cancers 12, no. 6: 1409. https://doi.org/10.3390/cancers12061409
APA StyleCardoso, R., Guo, F., Heisser, T., Hoffmeister, M., & Brenner, H. (2020). Utilisation of Colorectal Cancer Screening Tests in European Countries by Type of Screening Offer: Results from the European Health Interview Survey. Cancers, 12(6), 1409. https://doi.org/10.3390/cancers12061409





