Tailoring Chemometric Models on Blood-Derived Cultures Secretome to Assess Personalized Cancer Risk Score
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Model and Subjects Details
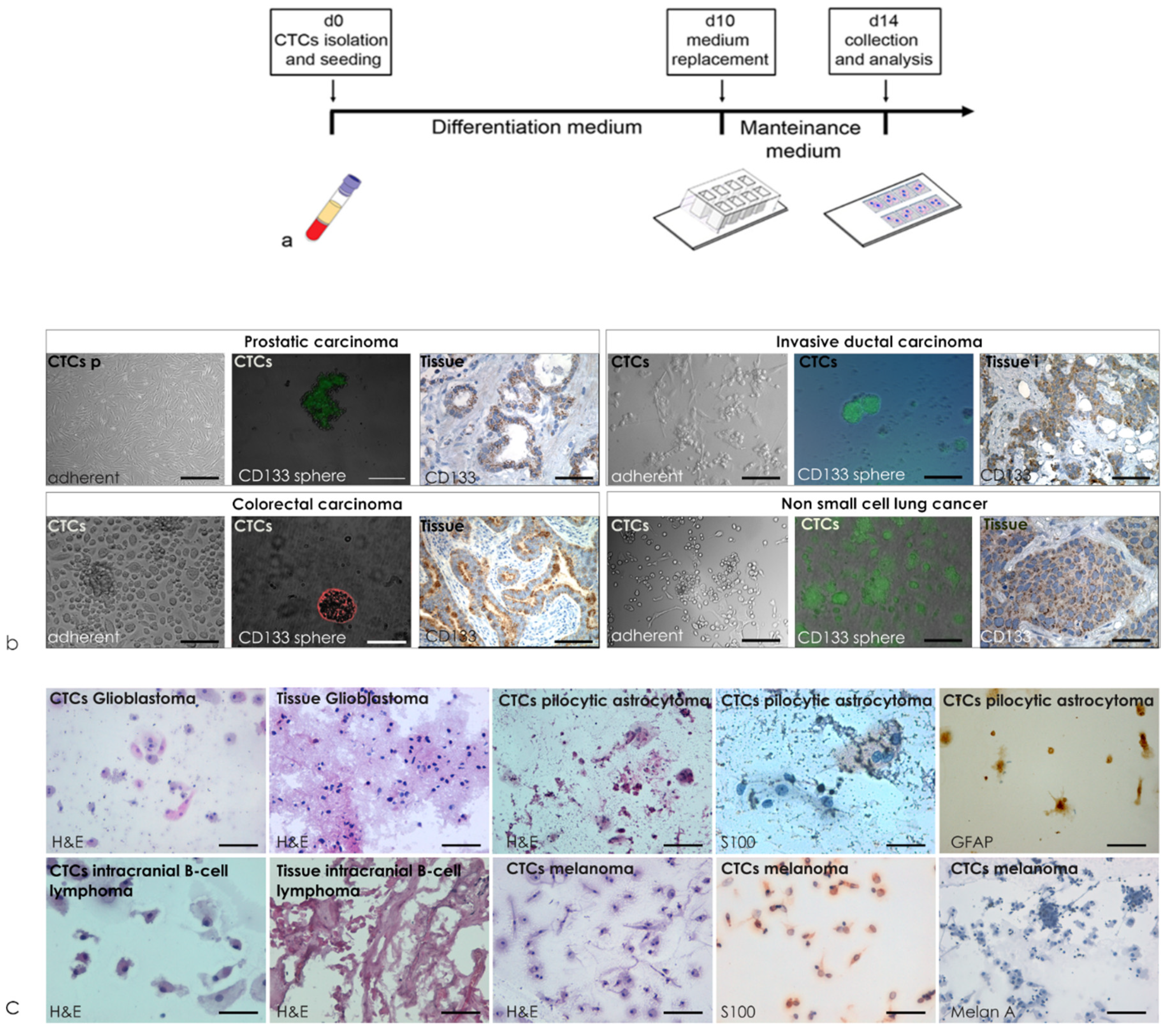
2.2. Liquid Biopsy Primary Culture
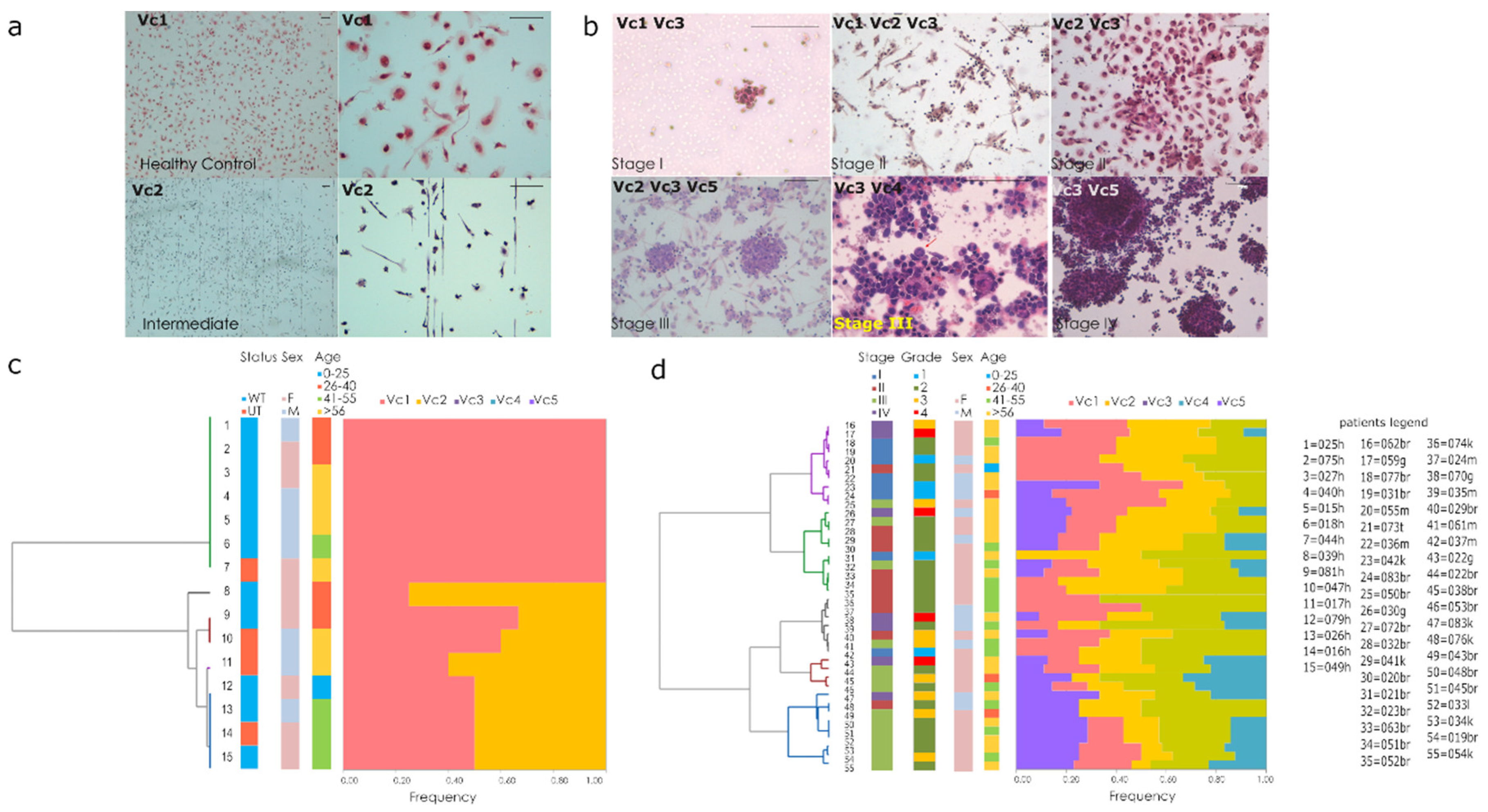
2.3. Cytopathology Characterization of Liquid Biopsy
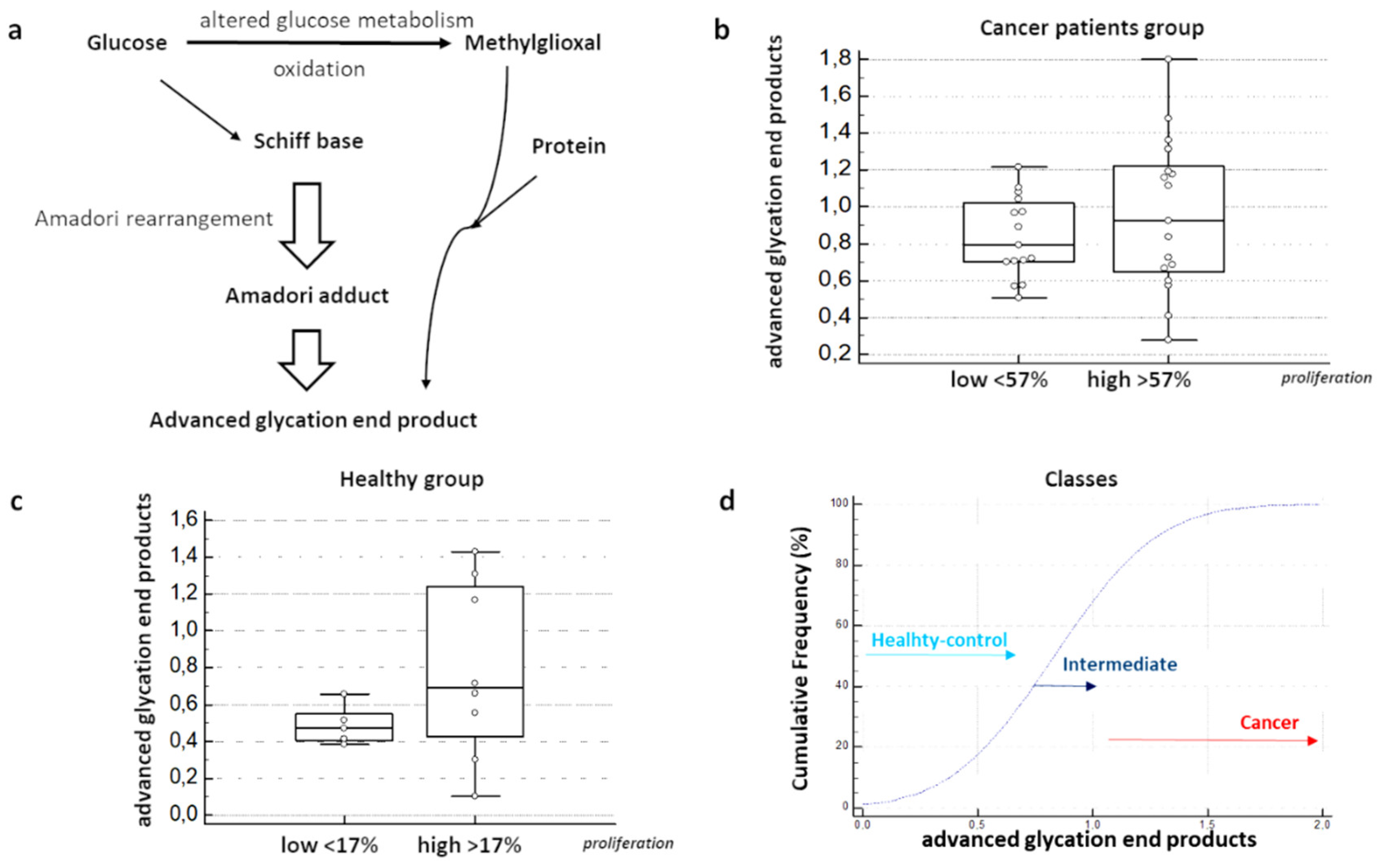
2.4. Secretome Collection and Characterization
2.5. Immunoblotting
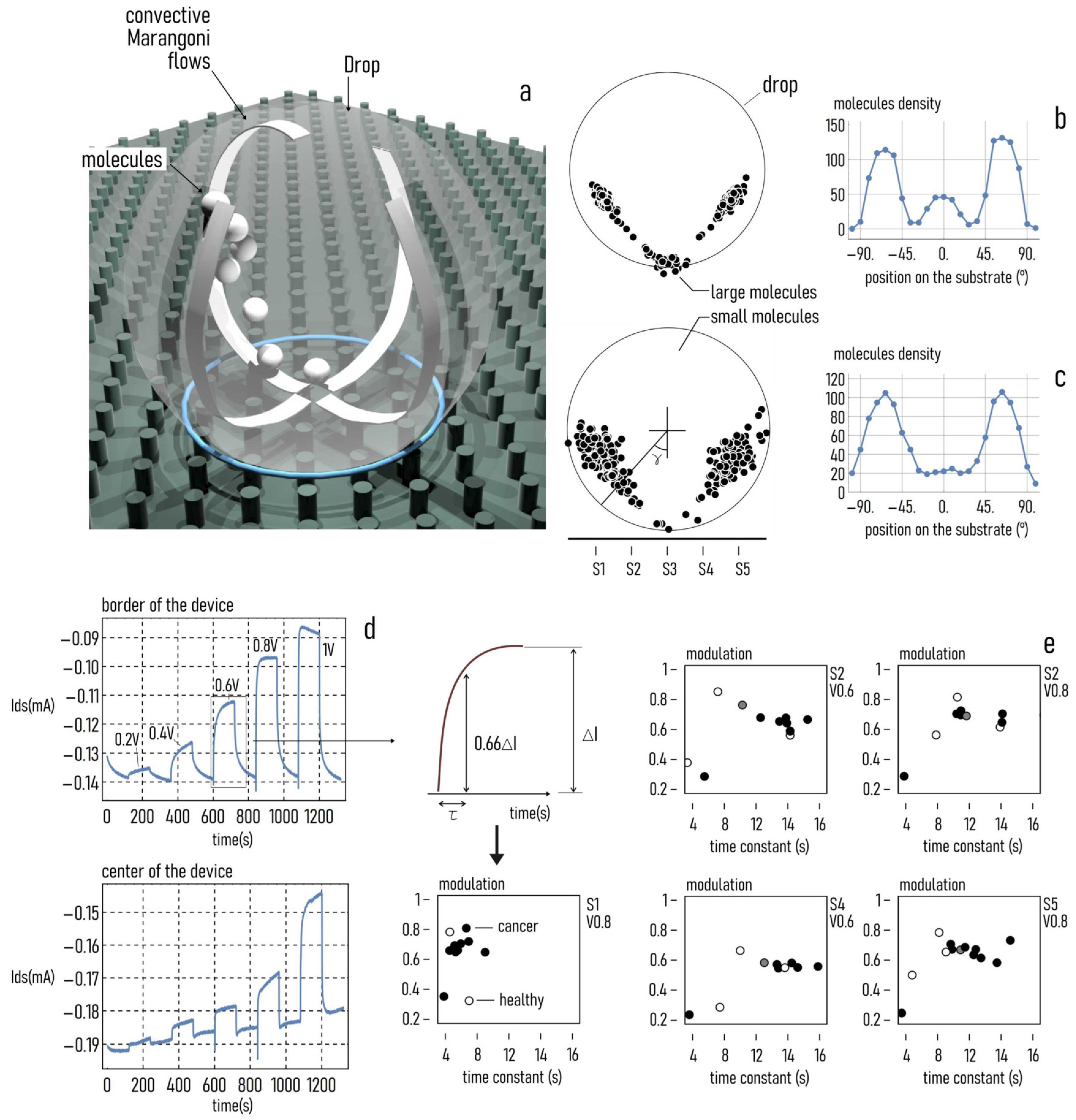
2.6. Raman Spectroscopy
2.7. Fabrication of the Device
2.7.1. Microfabrication of Gold Conductive Patterns on the Supporting Silicon Substrates (Layer A)
2.7.2. Microfabrication of Patterned Super-Hydrophobic Surfaces (Layer B)
2.7.3. Realizing Micro-Electrodes on the Top of the Pillars
2.7.4. The Conductive PEDOT:PSS Thin Film
2.8. SeOCET Operation
2.9. Statistical Analysis
3. Results
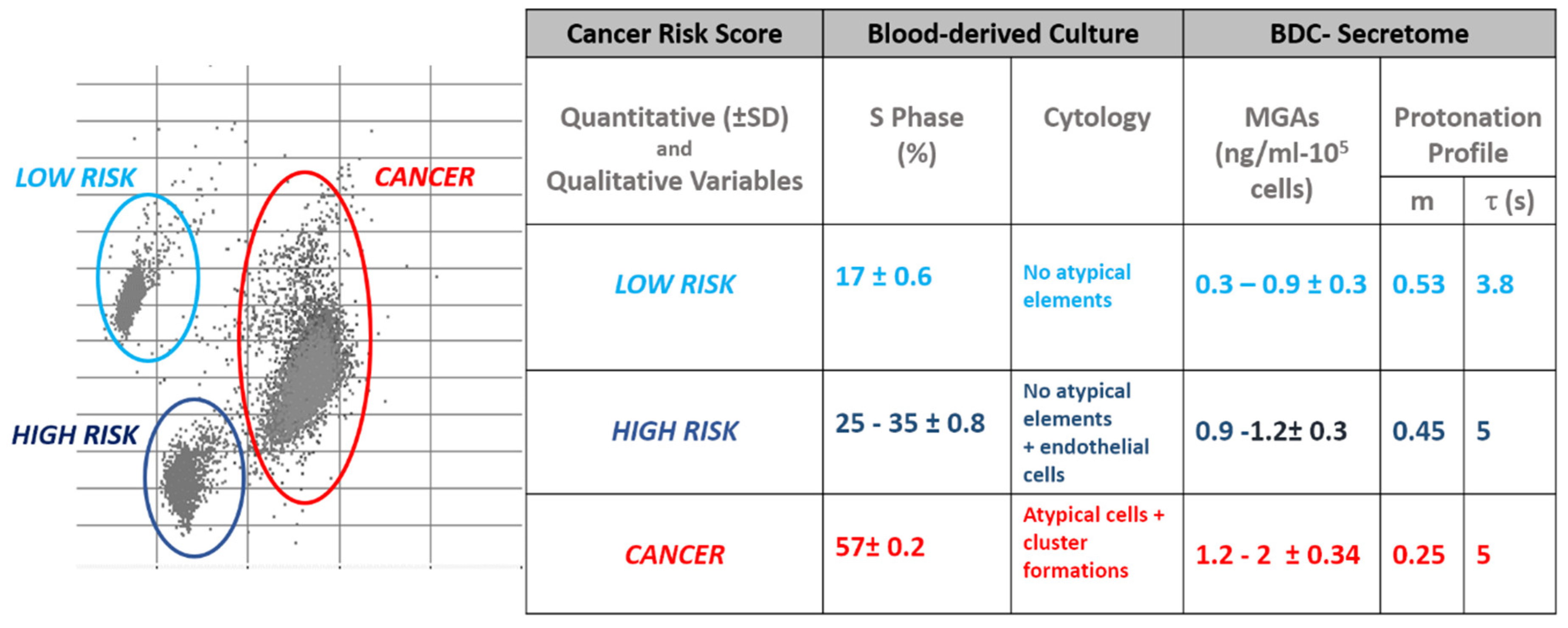
3.1. Protonation Profiles
3.2. Blood Derived Cultures of Enrolled Volunteers
3.3. Quantitative Evaluations of Proliferation Rate in BDCs
3.4. Qualitative Evaluations of Cytology Landscape in BDCs
3.5. Quantitative Evaluations of MAGs on BDC Secretome
3.6. Qualitative Evaluations by Using Spectroscopy RAMAN on BDC’s Secretome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malara, N.; Gentile, F.; Coppedè, N.; Coluccio, M.L.; Candeloro, P.; Perozziello, G.; Ferrara, L.; Giannetto, M.; Careri, M.; Castellini, A.; et al. Superhydrophobic lab-on-chip measures secretome protonation state and provides a personalized risk assessment of sporadic tumour. NPJ Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Malara, N.; Trunzo, V.; Foresta, U.; Amodio, N.; De Vitis, S.; Roveda, L.; Fava, M.; Coluccio, M.L.; Macrì, R.; Di Vito, A.; et al. Ex-vivo characterization of circulating colon cancer cells distinguished in stem and differentiated subset provides useful biomarker for personalized metastatic risk assessment. J. Transl. Med. 2016, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Malara, N.; Innaro, N.; Mignogna, C.; Presta, I.; Pirrone, K.C.; Donato, A.; Gangemi, V.; Sacco, R.; Mollace, V.; Donato, G. La biopsia liquida nella diagnosi del carcinoma tiroideo indifferenziato. L’Endocrinologo 2018, 19, 270–272. [Google Scholar] [CrossRef]
- Simone, G.; Malara, N.; Trunzo, V.; Perozziello, G.; Neuzil, P.; Francardi, M.; Roveda, L.; Renne, M.; Prati, U.; Mollace, V.; et al. Protein-carbohydrate complex reveals circulating metastatic cells in a microfluidic assay. Small 2013, 9, 2152–2161. [Google Scholar] [CrossRef]
- Malara, N.; Coluccio, M.L.; Limongi, T.; Asande, M.; Trunzo, V.; Cojoc, G.; Raso, C.; Candeloro, P.; Perozziello, G.; Raimondo, R.; et al. Folic acid functionalized surface highlights 5-methylcytosine-genomic content within circulating tumor cells. Small 2014, 10, 4324–4331. [Google Scholar] [CrossRef]
- Guadagno, E.; Presta, I.; Maisano, D.; Donato, A.; Pirrone, C.K.; Cardillo, G.; Corrado, S.D.; Mignogna, C.; Mancuso, T.; Donato, G.; et al. Role of macrophages in brain tumor growth and progression. Int. J. Mol. Sci. 2018, 19, 1005. [Google Scholar] [CrossRef]
- Malara, N.M.; Givigliano, F.; Trunzo, V.; Macrina, L.; Raso, C.; Amodio, N.; Aprigliano, S.; Minniti, A.M.; Russo, V.; Roveda, L.; et al. In vitro expansion of tumour cells derived from blood and tumour tissue is useful to redefine personalized treatment in non-small cell lung cancer patients. J. Biol. Regul. Homeost. Agents 2014, 28, 717–731. [Google Scholar]
- Malara, N.; Guzzi, G.; Mignogna, C.; Trunzo, V.; Camastra, C.; Torre, A.D.; Di Vito, A.; Lavecchia, A.M.; Gliozzi, M.; Ceccotti, C.; et al. Non-invasive real-time biopsy of intracranial lesions using short time expanded circulating tumor cells on glass slide: Report of two cases. BMC Neurol. 2016, 16, 127. [Google Scholar] [CrossRef]
- Malara, N.; Gentile, F.; Ferrara, L.; Villani, M.; Iannotta, S.; Zappettini, A.; Di Fabrizio, E.; Trunzo, V.; Mollace, V.; Coppedé, N. Tailoring super-hydrophobic properties of electrochemical biosensor for early cancer detection. MRS Adv. 2016, 1, 3545–3552. [Google Scholar] [CrossRef]
- Kalapos, M.P. On the promine/retine theory of cell division: Now and then. Biochim. Biophys. Acta Gen. Subj. 1999, 1426, 1–16. [Google Scholar] [CrossRef]
- Tamae, D.; Lim, P.; Wuenschell, G.E.; Termini, J. Mutagenesis and repair induced by the DNA advanced glycation end product N 2-1-(carboxyethyl)-2′-deoxyguanosine in human cells. Biochemistry 2011, 50, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J.; Dawczynski, J.; Franke, S.; Strobel, J.; Stein, G.; Haik, G.M. Methylglyoxal-Derived Hydroimidazolone Advanced Glycation End-Products of Human Lens Proteins. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5287–5292. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Ferrara, L.; Villani, M.; Bettelli, M.; Iannotta, S.; Zappettini, A.; Cesarelli, M.; Di Fabrizio, E.; Coppedè, N. Geometrical Patterning of Super-Hydrophobic Biosensing Transistors Enables Space and Time Resolved Analysis of Biological Mixtures. Sci. Rep. 2016, 6, 18992. [Google Scholar] [CrossRef]
- Coppedè, N.; Villani, M.; Gentile, F. Diffusion driven selectivity in organic electrochemical transistors. Sci. Rep. 2014, 4, 4297. [Google Scholar] [CrossRef] [PubMed]
- Candeloro, P.; Grande, E.; Raimondo, R.; Di Mascolo, D.; Gentile, F.; Coluccio, M.L.; Perozziello, G.; Malara, N.; Francardi, M.; Di Fabrizio, E. Raman database of amino acids solutions: A critical study of Extended Multiplicative Signal Correction. Analyst 2013, 138, 7331–7340. [Google Scholar] [CrossRef]
- Gentile, F.; Coluccio, M.L.; Rondanina, E.; Santoriello, S.; Di Mascolo, D.; Accardo, A.; Francardi, M.; De Angelis, F.; Candeloro, P.; Di Fabrizio, E. Non periodic patterning of super-hydrophobic surfaces for the manipulation of few molecules. Microelectron. Eng. 2013, 111, 272–276. [Google Scholar] [CrossRef]
- Perozziello, G.; La Rocca, R.; Cojoc, G.; Liberale, C.; Malara, N.; Simone, G.; Candeloro, P.; Anichini, A.; Tirinato, L.; Gentile, F.; et al. Microfluidic devices modulate tumor cell line susceptibility to NK cell recognition. Small 2012, 8, 2886–2894. [Google Scholar] [CrossRef]
- Perozziello, G.; Simone, G.; Candeloro, P.; Gentile, F.; Malara, N.; Larocca, R.; Coluccio, M.; Pullano, S.; Tirinato, L.; Geschke, O.; et al. A Fluidic Motherboard for Multiplexed Simultaneous and Modular Detection in Microfluidic Systems for Biological Application. Micro Nanosyst. 2010, 2, 227–238. [Google Scholar] [CrossRef]
- Dingari, N.C.; Horowitz, G.L.; Kang, J.W.; Dasari, R.R.; Barman, I. Raman Spectroscopy Provides a Powerful Diagnostic Tool for Accurate Determination of Albumin Glycation. PLoS ONE 2012, 7, e32406. [Google Scholar] [CrossRef]
- Coluccio, M.L.; de Vitis, S.; Strumbo, G.; Candeloro, P.; Perozziello, G.; di Fabrizio, E.; Gentile, F. Inclusion of gold nanoparticles in meso-porous silicon for the SERS analysis of cell adhesion on nano-structured surfaces. Microelectron. Eng. 2016, 158, 102–106. [Google Scholar] [CrossRef]
- Managò, S.; Valente, C.; Mirabelli, P.; Circolo, D.; Basile, F.; Corda, D.; de Luca, A.C. A reliable Raman-spectroscopybased approach for diagnosis, classifcation and follow-up of B-cell acute lymphoblastic leukemia. Sci. Rep. 2016, 6, 24821. [Google Scholar] [CrossRef] [PubMed]
- Notingher, I.; Verrier, S.; Romanska, H.; Bishop, A.E.; Polak, J.M.; Hench, L.L. In situ characterisation of living cells by raman spectroscopy. Spectroscopy 2002, 16, 43–51. [Google Scholar] [CrossRef]
- Gong, J.-L.; Liang, Y.; Huang, Y.; Chen, J.-W.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Ag/SiO2 core-shell nanoparticle-based surface-enhanced Raman probes for immunoassay of cancer marker using silica-coated magnetic nanoparticles as separation tools. Biosens. Bioelectron. 2007, 22, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, I.; Bruno, L.; Maltese, L.; Russo, E.; Donato, A.; Donato, G. Immunohistochemical analysis of the ubiquitin-conjugating enzyme UbcH10 in lung cancer: A useful tool for diagnosis and therapy. J. Histochem. Cytochem. 2012, 60, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Albano, C.; Dunn, W.J.; Edlund, U.; Esbensen, K.; Geladi, P.; Hellberg, S.; Johansson, E.; Lindberg, W.; Sjöström, M. Multivariate Data Analysis in Chemistry. In Chemometrics; Kowalski, B.R., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1984; pp. 17–95. ISBN 978-94-017-1026-8. [Google Scholar]
- Cieza, A.; Geyh, S.; Chatterji, S.; Kostanjsek, N.; Üstün, B.T.; Stucki, G. Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Med. Res. Methodol. 2006, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 96. [Google Scholar] [CrossRef]
- Maciosek, M.; Coffield, A.; Edwards, N.; Flottemesch, T.; Goodman, M.; Solberg, L. Priorities among effective clinical preventive services—Results of a systematic review and analysis. Am. J. Prev. Med. 2006, 31, 52–61. [Google Scholar] [CrossRef]
- May, P.; Garrido, M.M.; Cassel, J.B.; Kelley, A.S.; Meier, D.E.; Normand, C.; Stefanis, L.; Smith, T.J.; Morrison, R.S. Palliative Care Teams’ Cost-Saving Effect is Larger for Cancer Patients with Higher Numbers of Comorbidities. Health Affairs 2015, 35, 44–53. [Google Scholar] [CrossRef]

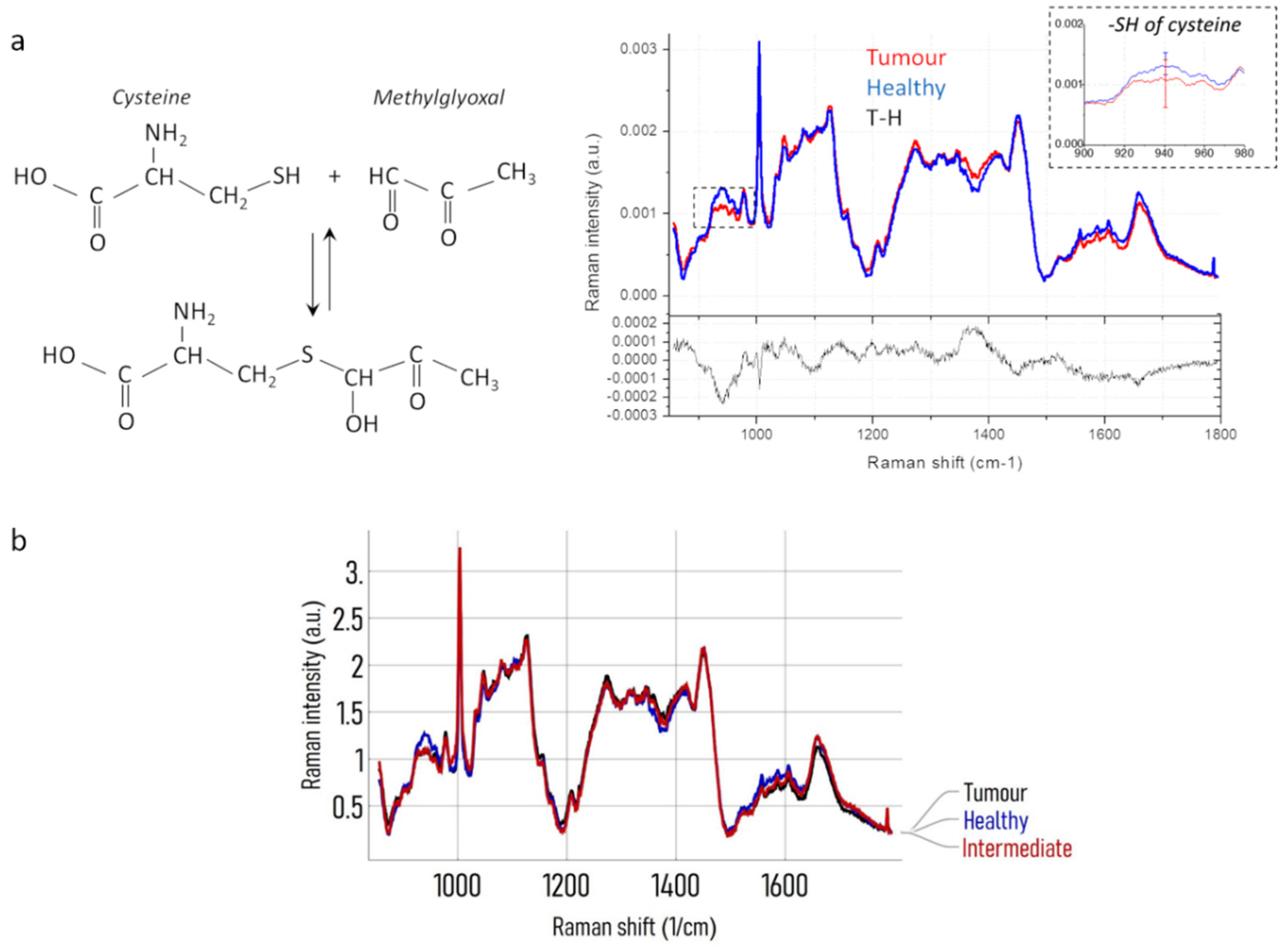
| Raman band (cm–1) | Assignment | Reference |
|---|---|---|
| 940 | SH of cysteine, ν(CCN)symm, ν(CC) glucose | [19] |
| 976 | Phe, C–C | [19] |
| 1004 | O–P–O sym str., BK | [19] |
| 1126 | C–N str., C–C | [19] |
| 1156 | Amide III, =CH def | [19] |
| 1208 | Amide III, =CH def | [19] |
| 1249 (shoulder) | G, amide III, CH twisting vibration | [19,20] |
| 1314 | A, G, CH vibration | [19] |
| 1346 | CH bending vibration | [21,22] |
| 1405 | CH2 def | [19] |
| 1447 | A, CH def | [22] |
| 1520 (small) | C=C ring, methyl cytosine | [20] |
| 1555 | Phe, Tyr, C=C | [19] |
| 1605 | Amide I, C=C | [19] |
| 1655 | [19] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluccio, M.L.; Gentile, F.; Presta, I.; Donato, G.; Coppedè, N.; Valprapuram, I.; Mignogna, C.; Lavecchia, A.; Figuccia, F.; Garo, V.M.; et al. Tailoring Chemometric Models on Blood-Derived Cultures Secretome to Assess Personalized Cancer Risk Score. Cancers 2020, 12, 1362. https://doi.org/10.3390/cancers12061362
Coluccio ML, Gentile F, Presta I, Donato G, Coppedè N, Valprapuram I, Mignogna C, Lavecchia A, Figuccia F, Garo VM, et al. Tailoring Chemometric Models on Blood-Derived Cultures Secretome to Assess Personalized Cancer Risk Score. Cancers. 2020; 12(6):1362. https://doi.org/10.3390/cancers12061362
Chicago/Turabian StyleColuccio, Maria Laura, Francesco Gentile, Ivan Presta, Giuseppe Donato, Nicola Coppedè, Immanuel Valprapuram, Chiara Mignogna, Annamaria Lavecchia, Federica Figuccia, Virginia M. Garo, and et al. 2020. "Tailoring Chemometric Models on Blood-Derived Cultures Secretome to Assess Personalized Cancer Risk Score" Cancers 12, no. 6: 1362. https://doi.org/10.3390/cancers12061362
APA StyleColuccio, M. L., Gentile, F., Presta, I., Donato, G., Coppedè, N., Valprapuram, I., Mignogna, C., Lavecchia, A., Figuccia, F., Garo, V. M., Fabrizio, E. D., Candeloro, P., Viglietto, G., & Malara, N. (2020). Tailoring Chemometric Models on Blood-Derived Cultures Secretome to Assess Personalized Cancer Risk Score. Cancers, 12(6), 1362. https://doi.org/10.3390/cancers12061362








