Germline Mutation in MUS81 Resulting in Impaired Protein Stability is Associated with Familial Breast and Thyroid Cancer
Abstract
1. Introduction
2. Results
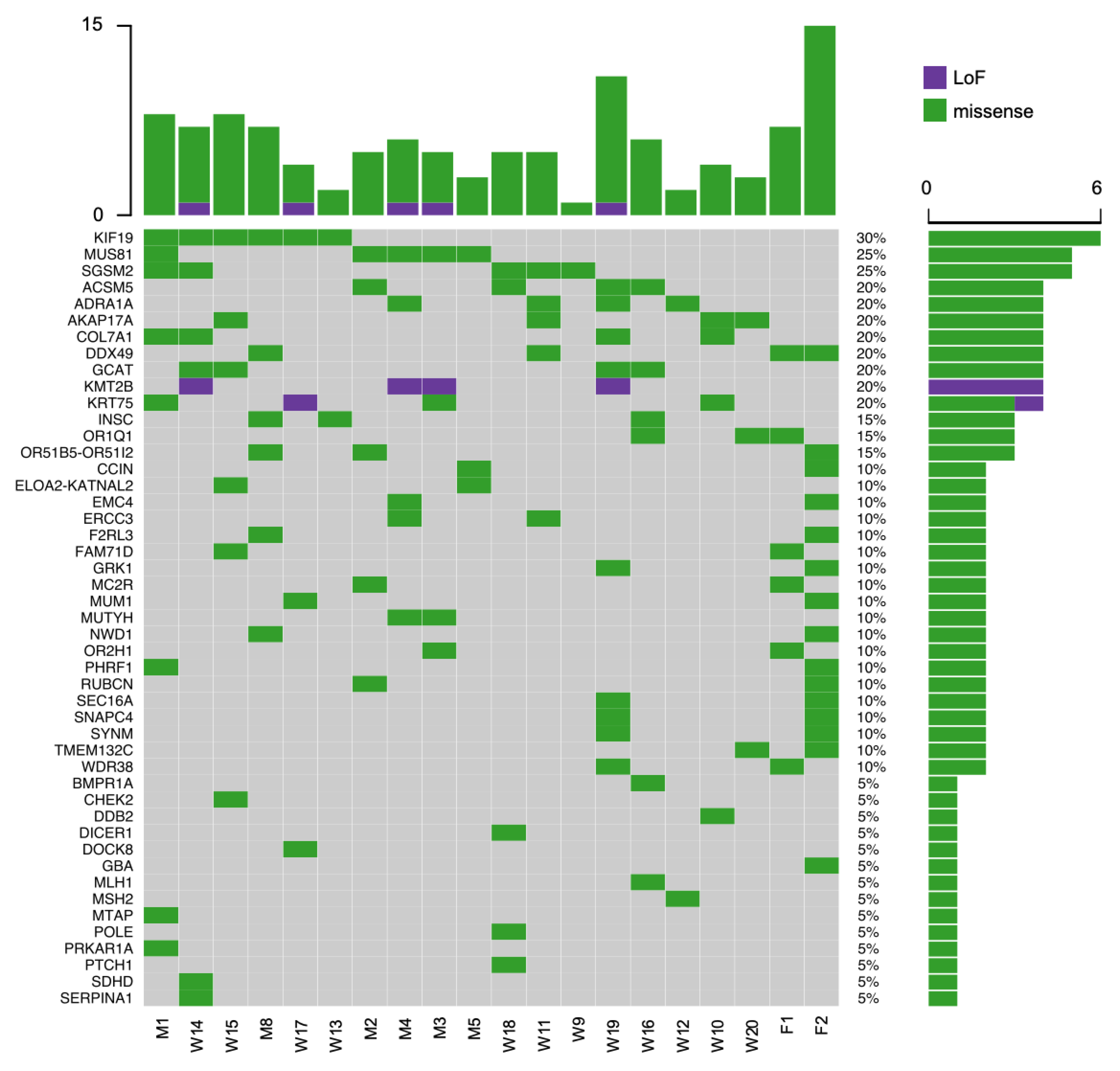
2.1. Variant Identification and Prioritization
2.2. MUS81 c.1292G>A Genotyping
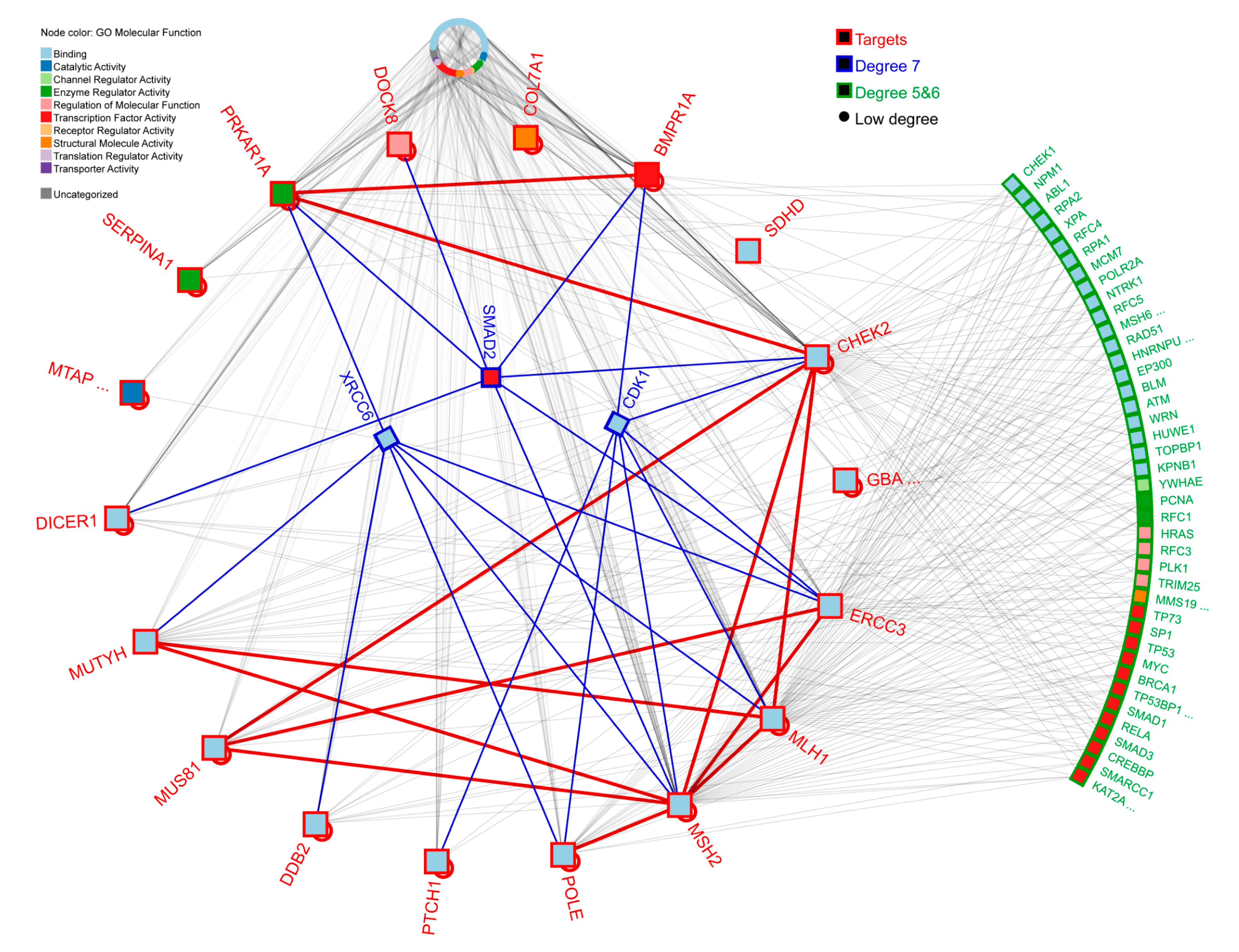
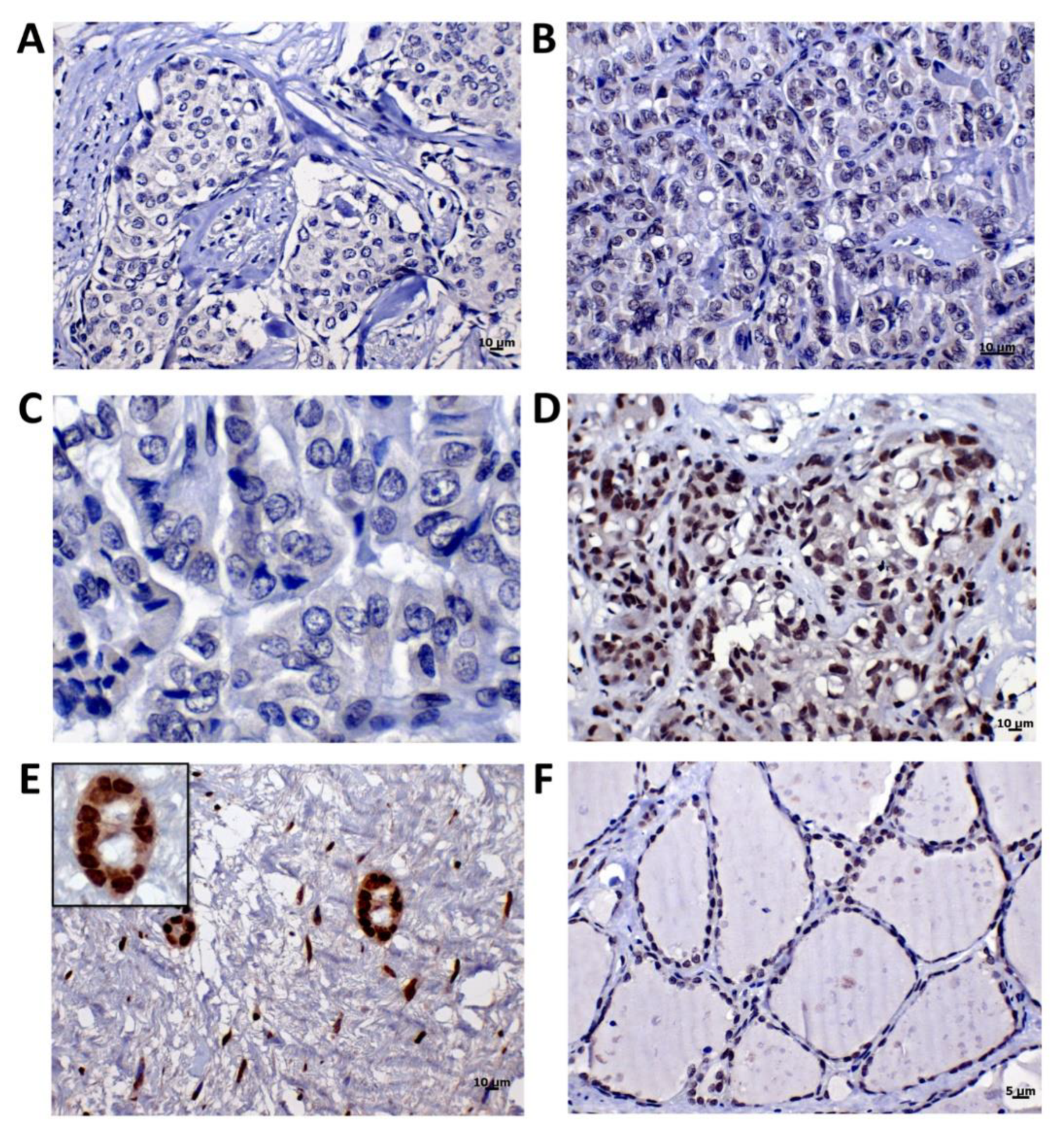
2.3. MUS81 Protein Expression
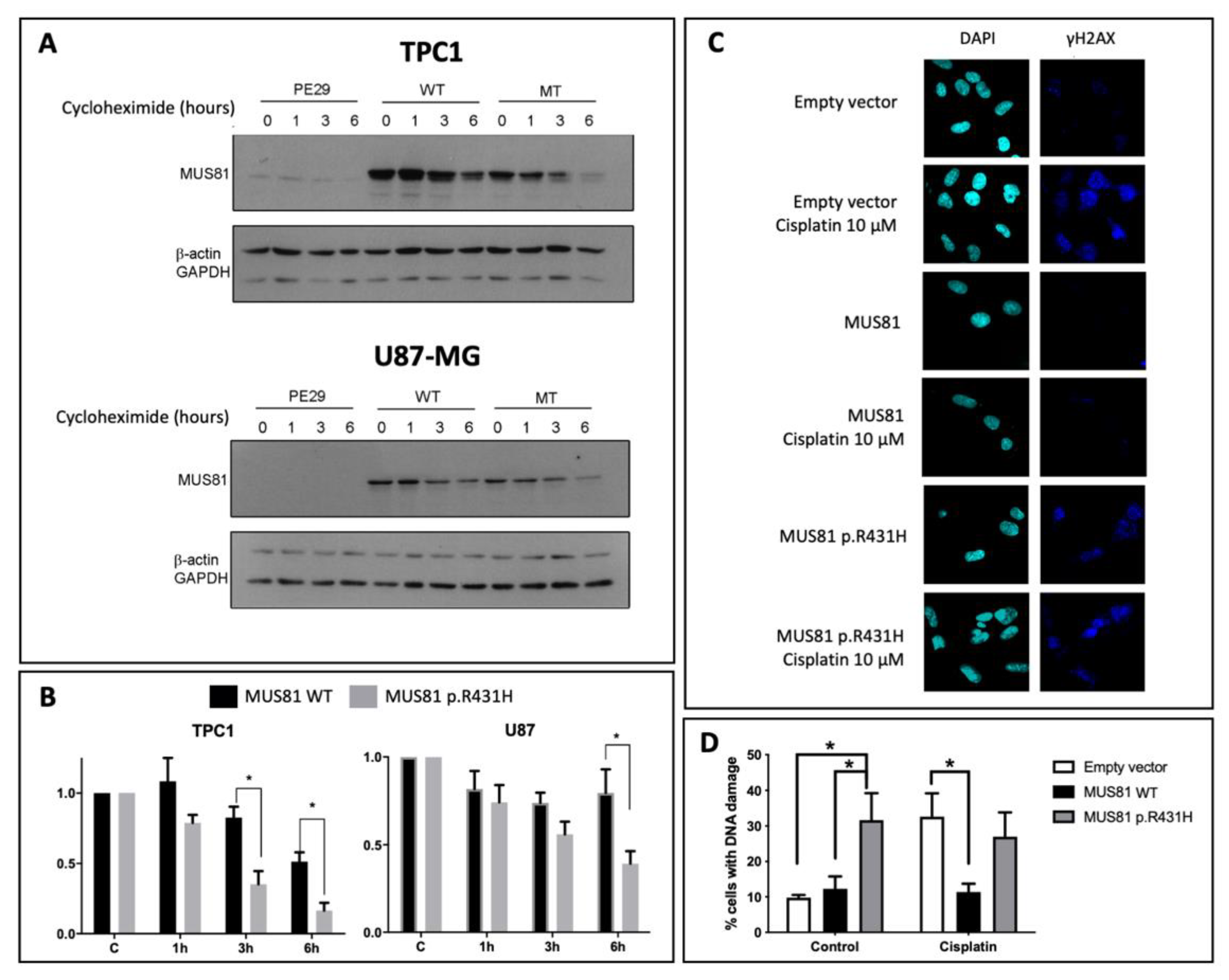
2.4. MUS81 p.R431H Presents Altered Stability
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. DNA Isolation and Library Construction
4.3. Whole-Exome Sequencing, Bioinformatics Analyses, and Variant Prioritization
4.4. Data Confirmation
4.5. Immunohistochemistry Analysis (IHC)
4.6. Functional Assessment of MUS81 c.1292G>A
4.7. Copy Number Alteration and DNA Methylation from Publicly Available Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, N.S.; Chen, X.X.; Wei, W.J.; Mo, M.; Chen, J.Y.; Ma, B.; Yang, S.W.; Xu, W.B.; Wu, J.; Ji, Q.H.; et al. Association between breast cancer and thyroid cancer: A study based on 13 978 patients with breast cancer. Cancer Med. 2018, 7, 6393–6400. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Liu, J.B.; Dougan, M.; Lee, J.S. Incidence of Second Malignancy in Patients with Papillary Thyroid Cancer from Surveillance, Epidemiology, and End Results 13 Dataset. J. Thyroid. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Garner, C.N.; Ganetzky, R.; Brainard, J.; Hammel, J.P.; Berber, E.; Siperstein, A.E.; Milas, M. Increased prevalence of breast cancer among patients with thyroid and parathyroid disease. Surgery 2007, 142, 806–813, discussion 813.e801–803. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.R.; Edirimanne, S.; Eslick, G.D. The association between breast cancer and thyroid cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 152, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.H.; Chabot, J.A.; Lee, J.A. Breast cancer in thyroid cancer survivors: An analysis of the Surveillance, Epidemiology, and End Results-9 database. Surgery 2016, 159, 23–30. [Google Scholar] [CrossRef]
- Galanti, M.R.; Ekbom, A.; Grimelius, L.; Yuen, J. Parental cancer and risk of papillary and follicular thyroid carcinoma. Br. J. Cancer 1997, 75, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Goldgar, D.E.; Easton, D.F.; Cannon-Albright, L.A.; Skolnick, M.H. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J. Natl. Cancer Inst. 1994, 86, 1600–1608. [Google Scholar] [CrossRef]
- An, J.H.; Hwangbo, Y.; Ahn, H.Y.; Keam, B.; Lee, K.E.; Han, W.; Park, d.J.; Park, I.A.; Noh, D.Y.; Youn, Y.K.; et al. A Possible Association between Thyroid Cancer and Breast Cancer. Thyroid 2015, 25, 1330–1338. [Google Scholar] [CrossRef]
- Bolf, E.L.; Sprague, B.L.; Carr, F.E. A Linkage between Thyroid and Breast Cancer: A Common Etiology? Cancer Epidemiol. Biomarkers Prev. 2018, 28, 643–649. [Google Scholar] [CrossRef]
- Liaw, D.; Marsh, D.J.; Li, J.; Dahia, P.L.; Wang, S.I.; Zheng, Z.; Bose, S.; Call, K.M.; Tsou, H.C.; Peacocke, M.; et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 1997, 16, 64–67. [Google Scholar] [CrossRef]
- Pilarski, R.; Burt, R.; Kohlman, W.; Pho, L.; Shannon, K.M.; Swisher, E. Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. J. Natl. Cancer Inst. 2013, 105, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Chompret, A.; Abel, A.; Stoppa-Lyonnet, D.; Brugiéres, L.; Pagés, S.; Feunteun, J.; Bonaïti-Pellié, C. Sensitivity and predictive value of criteria for p53 germline mutation screening. J. Med. Genet. 2001, 38, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Achatz, M.I.; Olivier, M.; Le Calvez, F.; Martel-Planche, G.; Lopes, A.; Rossi, B.M.; Ashton-Prolla, P.; Giugliani, R.; Palmero, E.I.; Vargas, F.R.; et al. The TP53 mutation, R337H, is associated with Li-Fraumeni and Li-Fraumeni-like syndromes in Brazilian families. Cancer Lett. 2007, 245, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Kiyotani, K.; Yew, P.Y.; Kato, T.; Tamura, K.; Yap, K.L.; Nielsen, S.M.; Mester, J.L.; Eng, C.; Nakamura, Y.; et al. Germline PARP4 mutations in patients with primary thyroid and breast cancers. Endocr. Relat. Cancer 2015, 23, 171–179. [Google Scholar] [CrossRef]
- Siolek, M.; Cybulski, C.; Gasior-Perczak, D.; Kowalik, A.; Kozak-Klonowska, B.; Kowalska, A.; Chlopek, M.; Kluzniak, W.; Wokolorczyk, D.; Palyga, I.; et al. CHEK2 mutations and the risk of papillary thyroid cancer. Int. J. Cancer 2015, 137, 548–552. [Google Scholar] [CrossRef]
- Ni, Y.; He, X.; Chen, J.; Moline, J.; Mester, J.; Orloff, M.S.; Ringel, M.D.; Eng, C. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum. Mol. Genet. 2012, 21, 300–310. [Google Scholar] [CrossRef]
- Bennett, K.L.; Mester, J.; Eng, C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010, 304, 2724–2731. [Google Scholar] [CrossRef]
- Consorti, F.; Di Tanna, G.L.; Milazzo, F.; Antonaci, A. Nulliparity enhances the risk of second primary malignancy of the breast in a cohort of women treated for thyroid cancer. World J. Surg. Oncol. 2011, 9, 88. [Google Scholar] [CrossRef]
- Chaker, L.; Visser, T.J. Thyroid function: Thyroid dysfunction and breast cancer risk—An unfinished story. Nat. Rev. Endocrinol. 2016, 12, 313–314. [Google Scholar] [CrossRef]
- Schonfeld, S.J.; Morton, L.M.; De González, A.B.; Curtis, R.E.; Kitahara, C.M. Risk of second primary papillary thyroid cancer among adult cancer survivors in the United States, 2000-2015. Cancer Epidemiol. 2020, 64, 101664. [Google Scholar] [CrossRef]
- Kalia, S.S.; Adelman, K.; Bale, S.J.; Chung, W.K.; Eng, C.; Evans, J.P.; Herman, G.E.; Hufnagel, S.B.; Klein, T.E.; Korf, B.R.; et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): A policy statement of the American College of Medical Genetics and Genomics. Genet. Med. 2017, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N. Realizing the promise of cancer predisposition genes. Nature 2014, 505, 302–308. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [PubMed]
- Shyr, C.; Tarailo-Graovac, M.; Gottlieb, M.; Lee, J.; Van Karnebeek, C.; Wasserman, W.W. FLAGS, frequently mutated genes in public exomes. BMC Med. Genom. 2014, 7, 64. [Google Scholar] [CrossRef]
- Fu, H.; Martin, M.M.; Regairaz, M.; Huang, L.; You, Y.; Lin, C.-M.; Ryan, M.; Kim, R.; Shimura, T.; Pommier, Y.; et al. The DNA repair endonuclease Mus81 facilitates fast DNA replication in the absence of exogenous damage. Nat. Commun. 2015, 6, 6746. [Google Scholar] [CrossRef] [PubMed]
- Ghamrasni, S.E.; Cardoso, R.; Li, L.; Guturi, K.K.; Bjerregaard, V.A.; Liu, Y.; Venkatesan, S.; Hande, M.P.; Henderson, J.T.; Sanchez, O.; et al. Rad54 and Mus81 cooperation promotes DNA damage repair and restrains chromosome missegregation. Oncogene 2016, 35, 4836–4845. [Google Scholar] [CrossRef]
- Kotlyar, M.; Pastrello, C.; Malik, Z.; Jurisica, I. IID 2018 update: Context-specific physical protein-protein interactions in human, model organisms and domesticated species. Nucleic Acids Res. 2019, 47, D581–D589. [Google Scholar] [CrossRef]
- Rahmati, S.; Abovsky, M.; Pastrello, C.; Kotlyar, M.; Lu, R.; Cumbaa, C.A.; Rahman, P.; Chandran, V.; Jurisica, I. pathDIP 4: An extended pathway annotations and enrichment analysis resource for human, model organisms and domesticated species. Nucleic Acids Res. 2020, 48, D479–D488. [Google Scholar] [CrossRef]
- The Human Protein ATLAS. Available online: https://www.proteinatlas.org/ (accessed on 20 January 2020).
- Wu, F.; Liu, S.Y.; Tao, Y.M.; Ou, D.P.; Fang, F.; Yang, L.Y. Decreased expression of methyl methansulfonate and ultraviolet-sensitive gene clone 81 (Mus81) is correlated with a poor prognosis in patients with hepatocellular carcinoma. Cancer 2008, 112, 2002–2010. [Google Scholar] [CrossRef]
- Ron, E.; Curtis, R.; Hoffman, D.A.; Flannery, J.T. Multiple primary breast and thyroid cancer. Br. J. Cancer 1984, 49, 87–92. [Google Scholar] [CrossRef]
- Ngeow, J.; Sesock, K.; Eng, C. Clinical Implications for Germline PTEN Spectrum Disorders. Endocrinol. Metab. Clin. N. Am. 2017, 46, 503–517. [Google Scholar] [CrossRef]
- Komine, K.; Shimodaira, H.; Takao, M.; Soeda, H.; Zhang, X.; Takahashi, M.; Ishioka, C. Functional Complementation Assay for 47 MUTYH Variants in a MutY-Disrupted Escherichia coli Strain. Hum. Mutat. 2015, 36, 704–711. [Google Scholar] [CrossRef]
- Al-Tassan, N.; Chmiel, N.H.; Maynard, J.; Fleming, N.; Livingston, A.L.; Williams, G.T.; Hodges, A.K.; Davies, D.R.; David, S.S.; Sampson, J.R.; et al. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat. Genet. 2002, 30, 227–232. [Google Scholar] [CrossRef]
- Aretz, S.; Tricarico, R.; Papi, L.; Spier, I.; Pin, E.; Horpaopan, S.; Cordisco, E.L.; Pedroni, M.; Stienen, D.; Gentile, A.; et al. MUTYH-associated polyposis (MAP): Evidence for the origin of the common European mutations p.Tyr179Cys and p.Gly396Asp by founder events. Eur. J. Hum. Genet. 2014, 22, 923–929. [Google Scholar] [CrossRef]
- Correa, R.; Salpea, P.; Stratakis, C.A. Carney Complex: An update. Eur. J. Endocrinol. 2015. [Google Scholar] [CrossRef]
- Christiano, A.M.; Greenspan, D.S.; Hoffman, G.G.; Zhang, X.; Tamai, Y.; Lin, A.N.; Dietz, H.C.; Hovnanian, A.; Uitto, J. A missense mutation in type VII collagen in two affected siblings with recessive dystrophic epidermolysis bullosa. Nat. Genet. 1993, 4, 62–66. [Google Scholar] [CrossRef]
- Camacho-Vanegas, O.; Camacho, S.C.; Till, J.; Miranda-Lorenzo, I.; Terzo, E.; Ramirez, M.C.; Schramm, V.; Cordovano, G.; Watts, G.; Mehta, S.; et al. Primate genome gain and loss: A bone dysplasia, muscular dystrophy, and bone cancer syndrome resulting from mutated retroviral-derived MTAP transcripts. Am. J. Hum. Genet. 2012, 90, 614–627. [Google Scholar] [CrossRef]
- Miki, H.; Setou, M.; Kaneshiro, K.; Hirokawa, N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc. Natl. Acad. Sci. USA 2001, 98, 7004–7011. [Google Scholar] [CrossRef]
- Sarquis, M.; Moraes, D.C.; Bastos-Rodrigues, L.; Azevedo, P.G.; Ramos, A.V.; Reis, F.V.; Dande, P.V.; Paim, I.; Friedman, E.; De Marco, L. Germline Mutations in Familial Papillary Thyroid Cancer. Endocr. Pathol. 2020, 31, 14–20. [Google Scholar] [CrossRef]
- Constantinou, A.; Chen, X.B.; McGowan, C.H.; West, S.C. Holliday junction resolution in human cells: Two junction endonucleases with distinct substrate specificities. EMBO J. 2002, 21, 5577–5585. [Google Scholar] [CrossRef]
- Blais, V.; Gao, H.; Elwell, C.A.; Boddy, M.N.; Gaillard, P.H.; Russell, P.; McGowan, C.H. RNA interference inhibition of Mus81 reduces mitotic recombination in human cells. Mol. Biol. Cell 2004, 15, 552–562. [Google Scholar] [CrossRef]
- Sarbajna, S.; West, S.C. Holliday junction processing enzymes as guardians of genome stability. Trends Biochem. Sci. 2014, 39, 409–419. [Google Scholar] [CrossRef]
- Técher, H.; Koundrioukoff, S.; Carignon, S.; Wilhelm, T.; Millot, G.A.; Lopez, B.S.; Brison, O.; Debatisse, M. Signaling from Mus81-Eme2-Dependent DNA Damage Elicited by Chk1 Deficiency Modulates Replication Fork Speed and Origin Usage. Cell Rep. 2016, 14, 1114–1127. [Google Scholar] [CrossRef]
- Xie, S.; Zheng, H.; Wen, X.; Sun, J.; Wang, Y.; Gao, X.; Guo, L.; Lu, R. MUS81 is associated with cell proliferation and cisplatin sensitivity in serous ovarian cancer. Biochem. Biophys. Res. Commun. 2016, 476, 493–500. [Google Scholar] [CrossRef]
- Ho, S.S.; Zhang, W.Y.; Tan, N.Y.; Khatoo, M.; Suter, M.A.; Tripathi, S.; Cheung, F.S.; Lim, W.K.; Tan, P.H.; Ngeow, J.; et al. The DNA Structure-Specific Endonuclease MUS81 Mediates DNA Sensor STING-Dependent Host Rejection of Prostate Cancer Cells. Immunity 2016, 44, 1177–1189. [Google Scholar] [CrossRef]
- Wu, F.; Chen, W.J.; Yan, L.; Tan, G.Q.; Li, W.T.; Zhu, X.J.; Ge, X.C.; Liu, J.W.; Wang, B.L. Mus81 knockdown improves chemosensitivity of hepatocellular carcinoma cells by inducing S-phase arrest and promoting apoptosis through CHK1 pathway. Cancer Med. 2016, 5, 370–385. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, Y.; Yan, Q.; Lv, J.; Ni, X.; Wu, Y.; Dong, X. Inhibition of Mus81 by siRNA enhances sensitivity to 5-FU in breast carcinoma cell lines. Onco Targets Ther. 2014, 7, 1883–1890. [Google Scholar] [CrossRef][Green Version]
- McPherson, J.P.; Lemmers, B.; Chahwan, R.; Pamidi, A.; Migon, E.; Matysiak-Zablocki, E.; Moynahan, M.E.; Essers, J.; Hanada, K.; Poonepalli, A.; et al. Involvement of Mammalian Mus81 in Genome Integrity and Tumor Suppression. Science 2004, 304, 1822–1826. [Google Scholar] [CrossRef]
- Hiyama, T.; Katsura, M.; Yoshihara, T.; Ishida, M.; Kinomura, A.; Tonda, T.; Asahara, T.; Miyagawa, K. Haploinsufficiency of the Mus81-Eme1 endonuclease activates the intra-S-phase and G2/M checkpoints and promotes rereplication in human cells. Nucleic Acids Res. 2006, 34, 880–892. [Google Scholar] [CrossRef]
- Bisarro Dos Reis, M.; Barros-Filho, M.C.; Marchi, F.A.; Beltrami, C.M.; Kuasne, H.; Pinto, C.A.L.; Ambatipudi, S.; Herceg, Z.; Kowalski, L.P.; Rogatto, S.R. Prognostic Classifier Based on Genome-Wide DNA Methylation Profiling in Well-Differentiated Thyroid Tumors. J. Clin. Endocrinol. Metab. 2017, 102, 4089–4099. [Google Scholar] [CrossRef]
- Goldman, M.; Craft, B.; Hastie, M.; Repečka, K.; Kamath, A.; McDade, F.; Rogers, D.; Brooks, A.N.; Zhu, J.; Haussler, D.; et al. The UCSC Xena platform for public and private cancer genomics data visualization and interpretation. BioRxiv 2018, 326470. [Google Scholar] [CrossRef]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Minikel, E.V.; Vallabh, S.M.; Lek, M.; Estrada, K.; Samocha, K.E.; Sathirapongsasuti, J.F.; McLean, C.Y.; Tung, J.Y.; Yu, L.P.C.; Gambetti, P.; et al. Quantifying prion disease penetrance using large population control cohorts. Sci. Transl. Med. 2016, 8, 322ra9. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, K.C.; Mirabello, L.; Stewart, D.R.; Karlins, E.; Koster, R.; Wang, M.; Gapstur, S.M.; Gaudet, M.M.; Freedman, N.D.; Landi, M.T.; et al. Higher-than-expected population prevalence of potentially pathogenic germline TP53 variants in individuals unselected for cancer history. Hum. Mutat. 2017, 38, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Miranda-Filho, A.; Koifman, S.; Koifman, R.J. Thyroid Cancer Incidences from Selected South America Population-Based Cancer Registries: An Age-Period-Cohort Study. J. Glob. Oncol. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Scheuner, M.T.; McNeel, T.S.; Freedman, A.N. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genet. Med. 2010, 12, 726–735. [Google Scholar] [CrossRef]
- Achatz, M.I.; Zambetti, G.P. The Inherited p53 Mutation in the Brazilian Population. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Naslavsky, M.S.; Yamamoto, G.L.; De Almeida, T.F.; Ezquina, S.A.M.; Sunaga, D.Y.; Pho, N.; Bozoklian, D.; Sandberg, T.O.M.; Brito, L.A.; Lazar, M.; et al. Exomic variants of an elderly cohort of Brazilians in the ABraOM database. Hum. Mutat. 2017, 38, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Witten, D.M.; Jain, P.; O’Roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef]
- Jian, X.; Boerwinkle, E.; Liu, X. In silico prediction of splice-altering single nucleotide variants in the human genome. Nucleic Acids Res. 2014, 42, 13534–13544. [Google Scholar] [CrossRef]
- Djebbari, A.; Ali, M.; Otasek, D.; Kotlyar, M.; Fortney, K.; Wong, S.; Hrvojic, A.; Jurisica, I. NAViGaTOR: Large scalable and interactive navigation and analysis of large graphs. Internet Math. 2011, 7, 314–347. [Google Scholar] [CrossRef]
- Nozima, B.H.; Mendes, T.B.; Pereira, G.; Araldi, R.P.; Iwamura, E.S.M.; Smaili, S.S.; Carvalheira, G.M.G.; Cerutti, J.M. FAM129A regulates autophagy in thyroid carcinomas in an oncogene-dependent manner. Endocr. Relat. Cancer 2019, 26, 227–238. [Google Scholar] [CrossRef]
| Sample Set | Allele | |||||
|---|---|---|---|---|---|---|
| G (REF) | Frequency | A (ALT) | Frequency | Total (100%) | p * | |
| Families | 35 | 0.875 | 5 | 0.125 | 40 | Reference |
| Healthy | 713 | 0.985 | 11 | 0.015 | 724 | 8.7 × 10−4 |
| Sporadic | 173 | 0.983 | 3 | 0.017 | 176 | 6.3 × 10−3 |
| Genotype | ||||||
| GG (WT) | Frequency | GA (HET) | Frequency | Total (100%) | p * | |
| Families | 15 | 0.750 | 5 | 0.250 | 20 | Reference |
| Healthy | 351 | 0.970 | 11 | 0.030 | 362 | 7.1 × 10−4 |
| Sporadic | 85 | 0.966 | 3 | 0.034 | 88 | 5.3 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, M.; Lupinacci, F.C.S.; Santiago, K.M.; Drigo, S.A.; Marchi, F.A.; Fonseca-Alves, C.E.; Andrade, S.C.d.S.; Aagaard, M.M.; Basso, T.R.; dos Reis, M.B.; et al. Germline Mutation in MUS81 Resulting in Impaired Protein Stability is Associated with Familial Breast and Thyroid Cancer. Cancers 2020, 12, 1289. https://doi.org/10.3390/cancers12051289
Pinheiro M, Lupinacci FCS, Santiago KM, Drigo SA, Marchi FA, Fonseca-Alves CE, Andrade SCdS, Aagaard MM, Basso TR, dos Reis MB, et al. Germline Mutation in MUS81 Resulting in Impaired Protein Stability is Associated with Familial Breast and Thyroid Cancer. Cancers. 2020; 12(5):1289. https://doi.org/10.3390/cancers12051289
Chicago/Turabian StylePinheiro, Maisa, Fernanda Cristina Sulla Lupinacci, Karina Miranda Santiago, Sandra Aparecida Drigo, Fabio Albuquerque Marchi, Carlos Eduardo Fonseca-Alves, Sonia Cristina da Silva Andrade, Mads Malik Aagaard, Tatiane Ramos Basso, Mariana Bisarro dos Reis, and et al. 2020. "Germline Mutation in MUS81 Resulting in Impaired Protein Stability is Associated with Familial Breast and Thyroid Cancer" Cancers 12, no. 5: 1289. https://doi.org/10.3390/cancers12051289
APA StylePinheiro, M., Lupinacci, F. C. S., Santiago, K. M., Drigo, S. A., Marchi, F. A., Fonseca-Alves, C. E., Andrade, S. C. d. S., Aagaard, M. M., Basso, T. R., dos Reis, M. B., Villacis, R. A. R., Roffé, M., Hajj, G. N. M., Jurisica, I., Kowalski, L. P., Achatz, M. I., & Rogatto, S. R. (2020). Germline Mutation in MUS81 Resulting in Impaired Protein Stability is Associated with Familial Breast and Thyroid Cancer. Cancers, 12(5), 1289. https://doi.org/10.3390/cancers12051289






