Targeting EpCAM by a Bispecific Trifunctional Antibody Exerts Profound Cytotoxic Efficacy in Germ Cell Tumor Cell Lines
Abstract
1. Introduction
2. Results
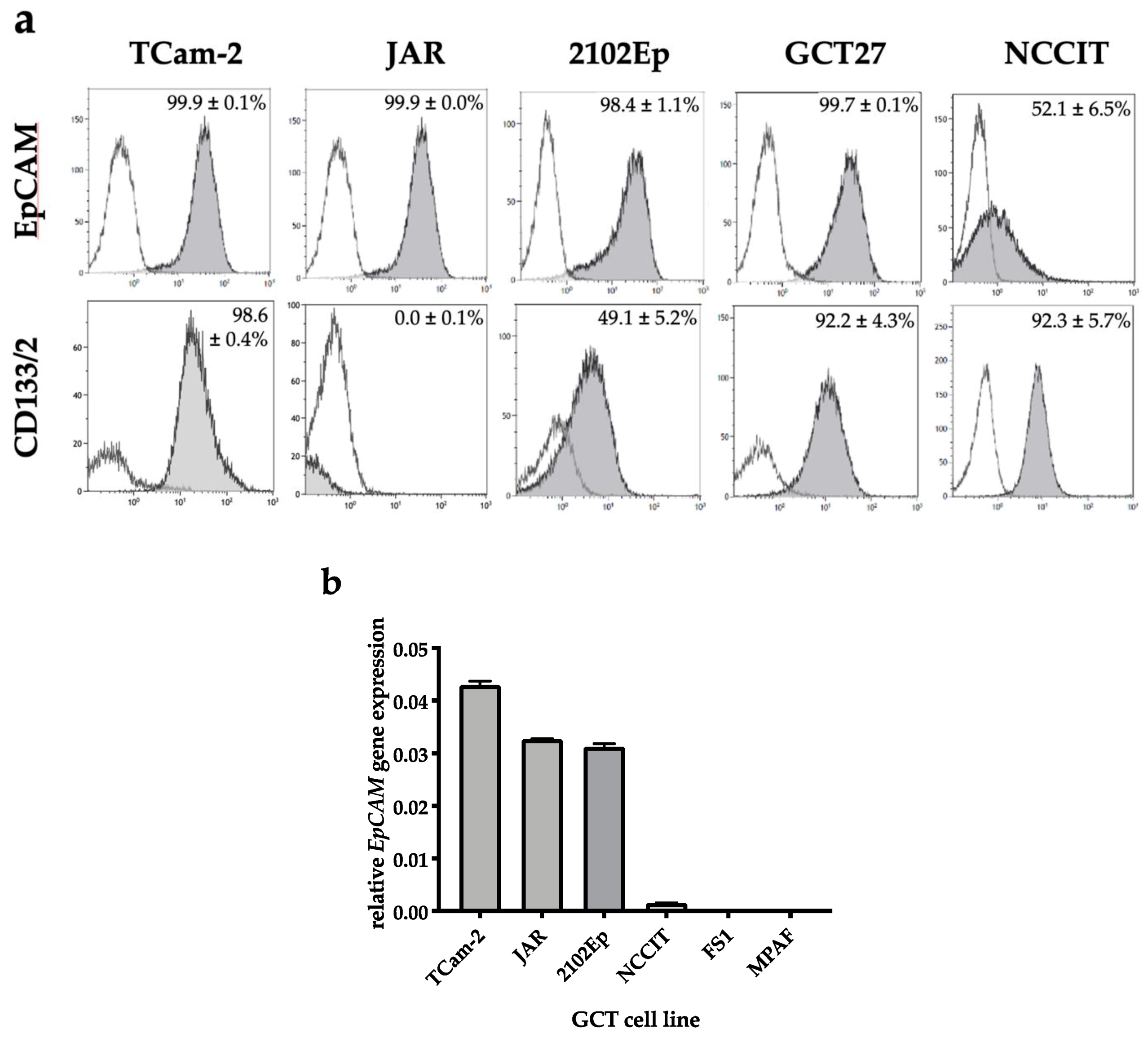
2.1. EpCAM Is Expressed in Seminomatous as Well as Non-Seminomatous GCT Cell Lines
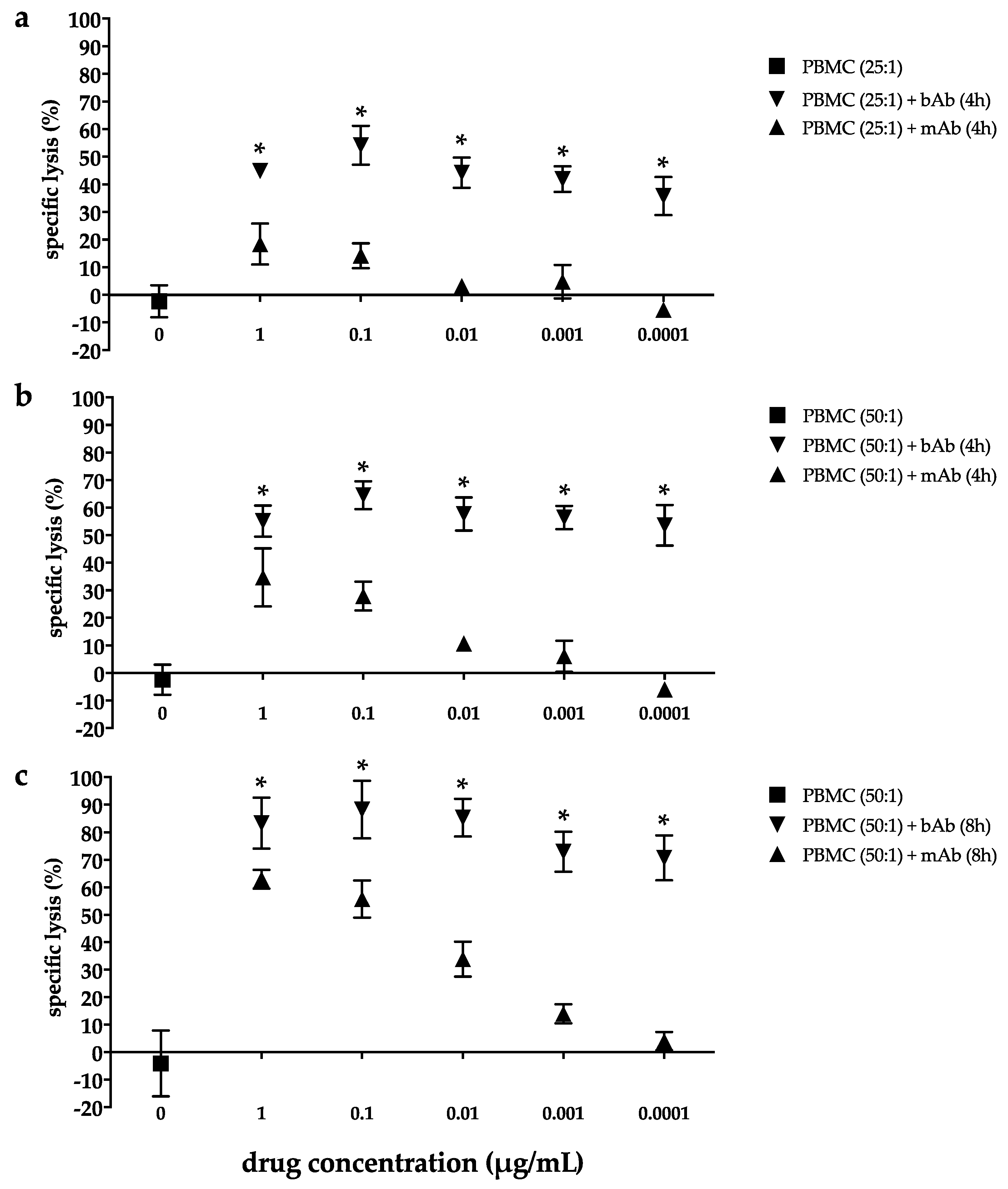
2.2. Marked Cytotoxicity in the EC Line 2102Ep Mediated by the Bispecific EpCAM/CD3 Antibody in the Presence of Peripheral Blood Mononuclear Cells Persists Across a Broad Range of Antibody Dilutions
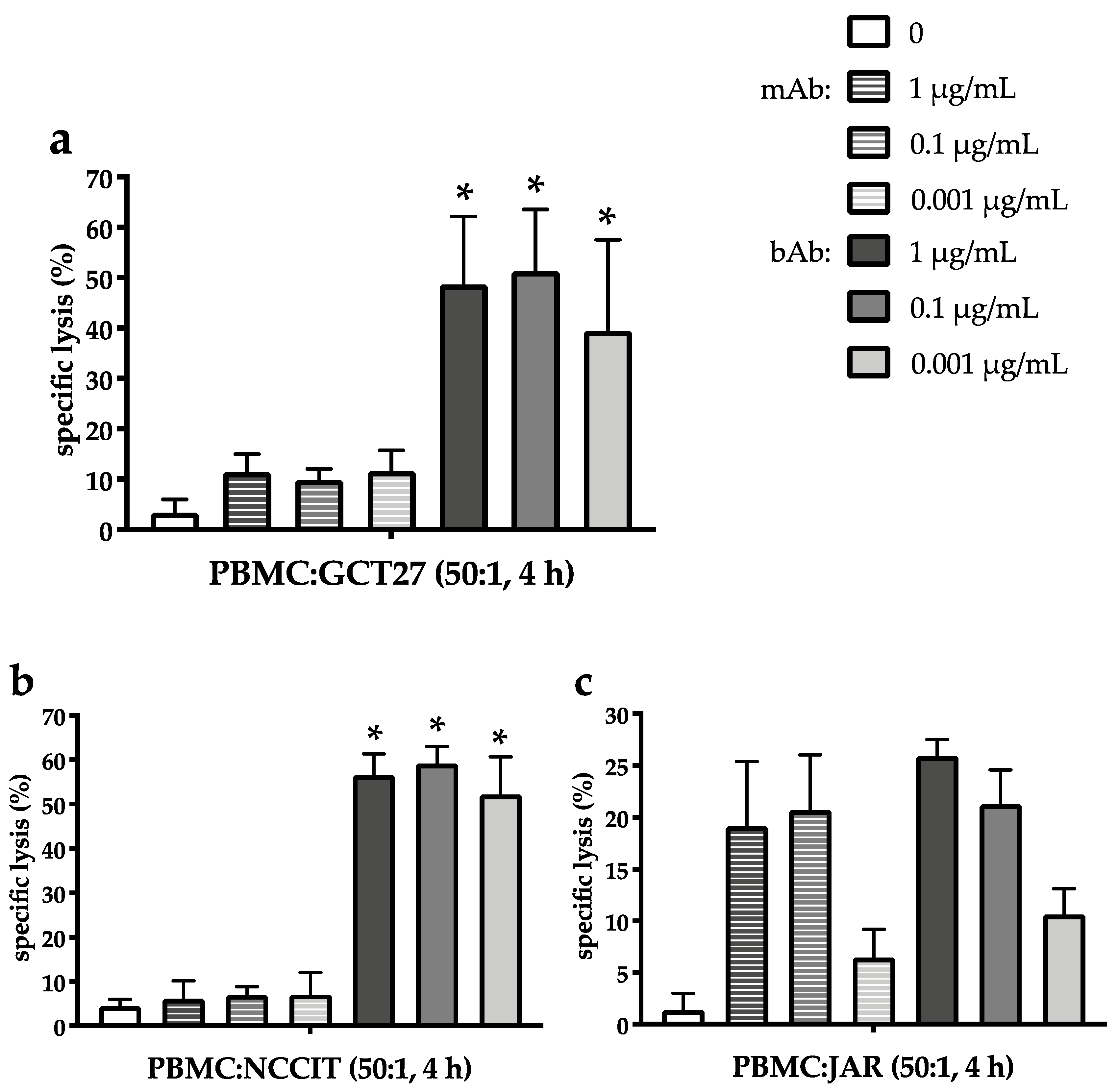
2.3. The EpCAM/CD3-Binding Bispecific Antibody Exerts Potent Cytotoxic Activity in GCT Cell Lines of Different Histologies
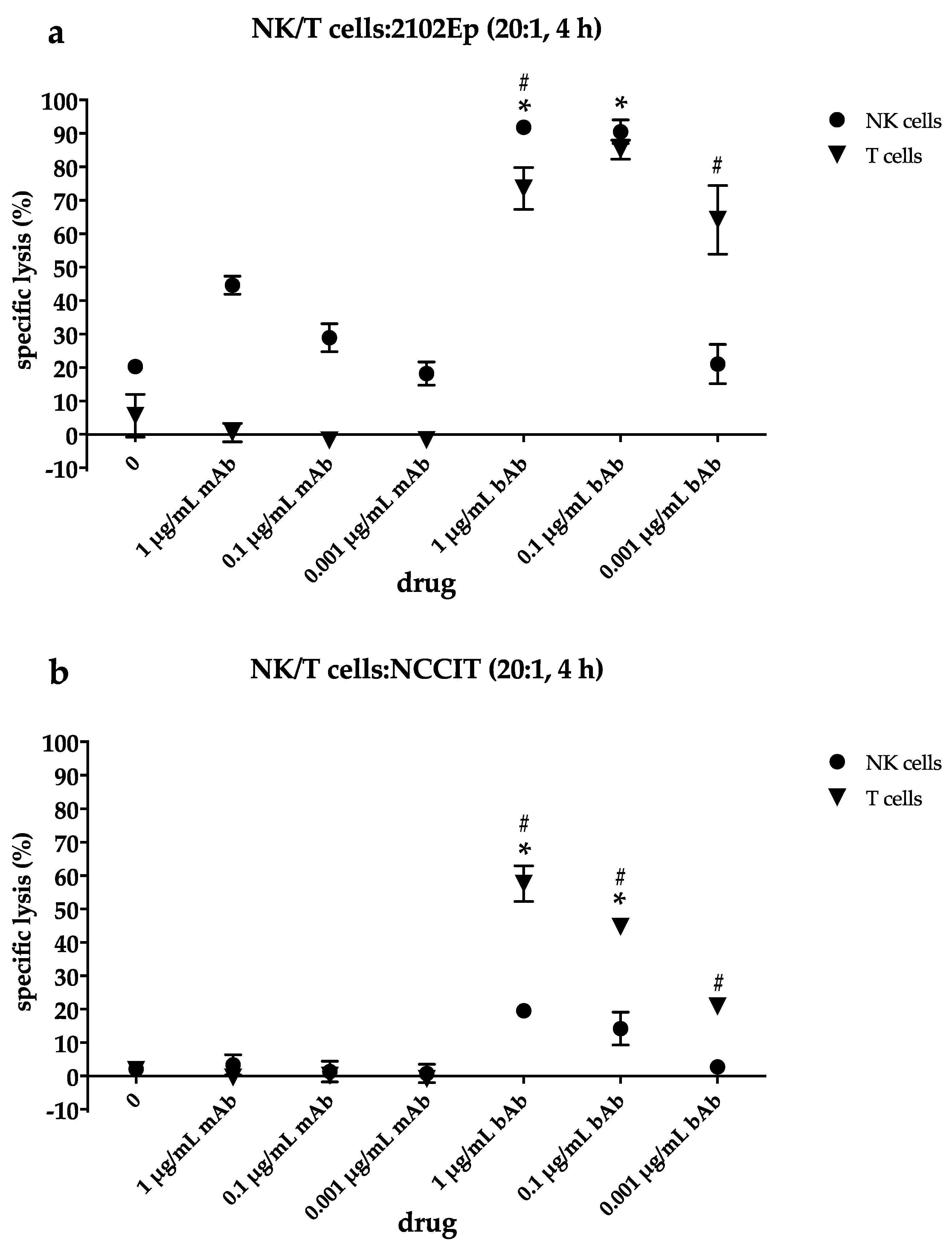
2.4. NK Cells Contribute to Cytotoxic Efficacy of the EpCAM/CD3-bAb at Higher Drug Concentrations While T Cells Still Induce Apoptosis Even at Lower bAb Dosages
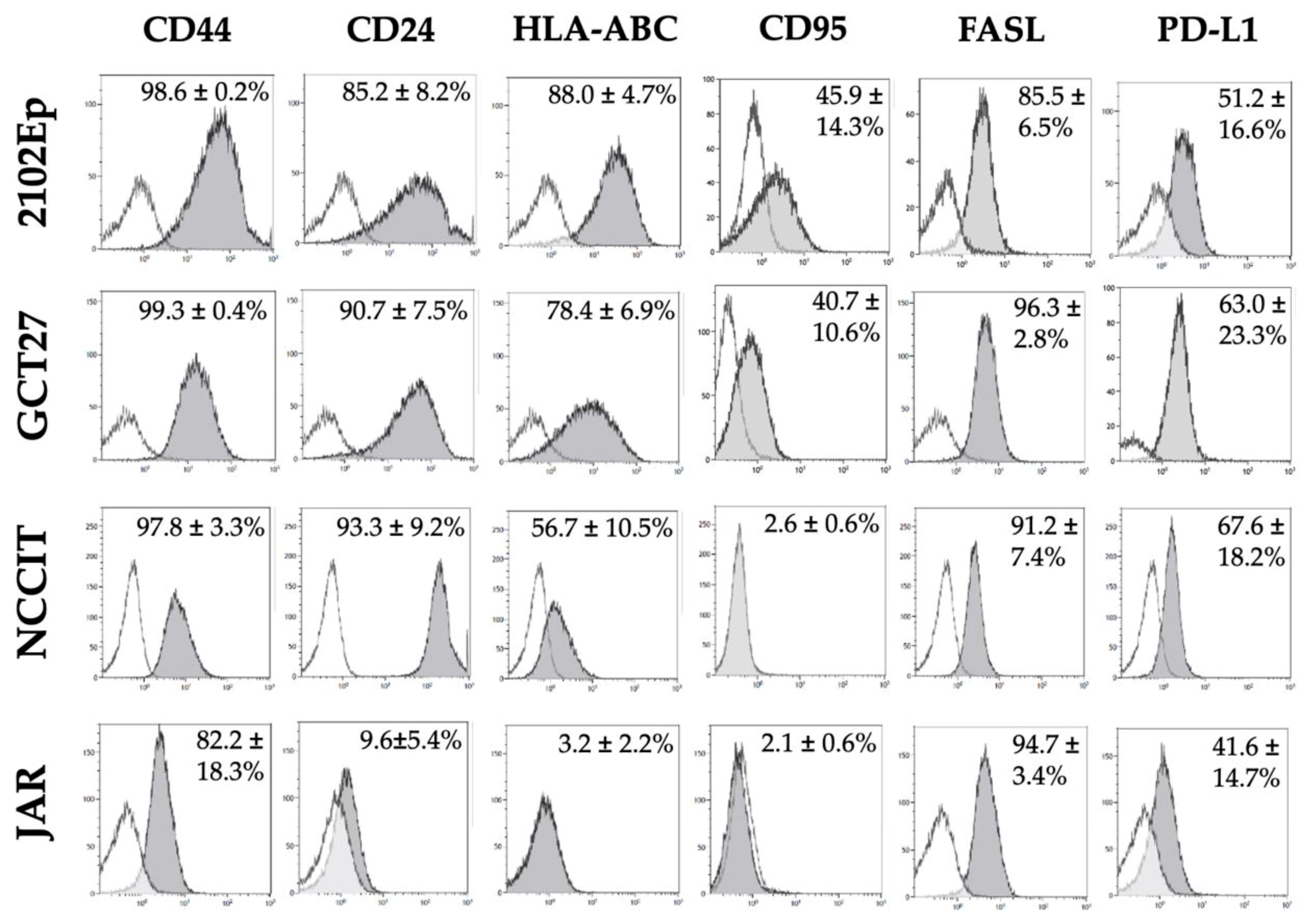
2.5. Phenotypic Characterization of Immunomodulatory Surface Molecules in GCT Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cytofluorimetric Analysis
4.3. Quantitative Real-Time RT-PCR
4.4. Isolation of Natural Killer and T Cells
4.5. Europium Release Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, M.; Schönberger, S. Biology of Germ Cell Tumors. In Pediatric Germ Cell Tumors; Frazier, A., Amatruda, J., Eds.; Springer Berlin Heidelberg: Heidelberg, Germany, 2014; pp. 1–15. [Google Scholar]
- Cheng, L.; Albers, P.; Berney, D.M.; Feldman, D.R.; Daugaard, G.; Gilligan, T.; Looijenga, L.H.J. Testicular cancer. Nat. Rev. Dis. Prim. 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Calaminus, G.; Schneider, D.T.; von Schweinitz, D.; Jurgens, H.; Infed, N.; Schonberger, S.; Olson, T.A.; Albers, P.; Vokuhl, C.; Stein, R.; et al. Age-dependent presentation and clinical course of 1465 patients aged 0 to less than 18 years with ovarian or testicular germ cell tumors; Data of the MAKEI 96 protocol revisited in the light of prenatal germ cell biology. Cancers 2020, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P.; Nicolai, N.; Oldenburg, J.; et al. Guidelines on Testicular Cancer: 2015 Update. Eur. Urol. 2015, 68, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Göbel, U.; Calaminus, G.; Schneider, D.T.; Schmidt, P.; Haas, R.J. Management of germ cell tumors in children: Approaches to cure. Onkologie 2002, 25, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Oing, C.; Seidel, C.; Bokemeyer, C. Therapeutic approaches for refractory germ cell cancer. Expert Rev. Anticancer Ther. 2018, 18, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.T.; Hilgenfeld, E.; Schwabe, D.; Behnisch, W.; Zoubek, A.; Wessalowski, R.; Göbel, U. Acute myelogenous leukemia after treatment for malignant germ cell tumors in children. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 3226–3233. [Google Scholar] [CrossRef]
- Brock, P.R.; Knight, K.R.; Freyer, D.R.; Campbell, K.C.; Steyger, P.S.; Blakley, B.W.; Rassekh, S.R.; Chang, K.W.; Fligor, B.J.; Rajput, K.; et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2408–2417. [Google Scholar] [CrossRef]
- Honecker, F.; Aparicio, J.; Berney, D.; Beyer, J.; Bokemeyer, C.; Cathomas, R.; Clarke, N.; Cohn-Cedermark, G.; Daugaard, G.; Dieckmann, K.P.; et al. ESMO Consensus Conference on testicular germ cell cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, 1658–1686. [Google Scholar] [CrossRef]
- Chovanec, M.; Cierna, Z.; Miskovska, V.; Machalekova, K.; Svetlovska, D.; Kalavska, K.; Rejlekova, K.; Spanik, S.; Kajo, K.; Babal, P.; et al. Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors. Oncotarget 2017, 8, 21794–21805. [Google Scholar] [CrossRef]
- Bols, B.; Jensen, L.; Jensen, A.; Braendstrup, O. Immunopathology of in situ seminoma. Int. J. Exp. Pathol. 2000, 81, 211–217. [Google Scholar] [CrossRef]
- Hvarness, T.; Nielsen, J.E.; Almstrup, K.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Claesson, M.H. Phenotypic characterisation of immune cell infiltrates in testicular germ cell neoplasia. J. Reprod. Immunol. 2013, 100, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zapka, P.; Dorner, E.; Dreschmann, V.; Sakamato, N.; Kristiansen, G.; Calaminus, G.; Vokuhl, C.; Leuschner, I.; Pietsch, T. Type, Frequency, and Spatial Distribution of Immune Cell Infiltrates in CNS Germinomas: Evidence for Inflammatory and Immunosuppressive Mechanisms. J. Neuropathol. Exp. Neurol. 2018, 77, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Mardiak, J.; Mego, M. Immune mechanisms and possible immune therapy in testicular germ cell tumours. Andrology 2019, 7, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Oing, C.; Bokemeyer, C. Biological basis and early clinical results of immunotherapy for cisplatin-resistant germ cell cancer. Curr. Opin. Urol. 2018, 28, 479–484. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Fan, D.; Xiong, D. The development of bispecific antibodies and their applications in tumor immune escape. Exp. Hematol. Oncol. 2017, 6, 12. [Google Scholar] [CrossRef]
- Schönberger, S.; Okpanyi, V.; Calaminus, G.; Heikaus, S.; Leuschner, I.; Nicholson, J.C.; Stoecklein, N.H.; Schneider, D.T.; Borkhardt, A. EPCAM—A novel molecular target for the treatment of pediatric and adult germ cell tumors. Genes Chromosom. Cancer 2013, 52, 24–32. [Google Scholar] [CrossRef]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell. Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef]
- Okpanyi, V.; Schneider, D.T.; Zahn, S.; Sievers, S.; Calaminus, G.; Nicholson, J.C.; Palmer, R.D.; Leuschner, I.; Borkhardt, A.; Schönberger, S. Analysis of the adenomatous polyposis coli (APC) gene in childhood and adolescent germ cell tumors. Pediatr. Blood Cancer 2011, 56, 384–391. [Google Scholar] [CrossRef]
- Chovanec, M.; Cierna, Z.; Miskovska, V.; Machalekova, K.; Kalavska, K.; Rejlekova, K.; Svetlovska, D.; Macak, D.; Spanik, S.; Kajo, K.; et al. betacatenin is a marker of poor clinical characteristics and suppressed immune infiltration in testicular germ cell tumors. BMC Cancer 2018, 18, 1062. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/beta-catenin signaling pathway regulating T Cell-inflammation in the tumor microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef]
- Strohl, W.R.; Naso, M. Bispecific T-Cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Went, P.T.; Lugli, A.; Meier, S.; Bundi, M.; Mirlacher, M.; Sauter, G.; Dirnhofer, S. Frequent EpCam protein expression in human carcinomas. Hum. Pathol 2004, 35, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Heiss, M.M.; Murawa, P.; Koralewski, P.; Kutarska, E.; Kolesnik, O.O.; Ivanchenko, V.V.; Dudnichenko, A.S.; Aleknaviciene, B.; Razbadauskas, A.; Gore, M.; et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. J. Int. Cancer 2010, 127, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Tayama, S.; Motohara, T.; Narantuya, D.; Li, C.; Fujimoto, K.; Sakaguchi, I.; Tashiro, H.; Saya, H.; Nagano, O.; Katabuchi, H. The impact of EpCAM expression on response to chemotherapy and clinical outcomes in patients with epithelial ovarian cancer. Oncotarget 2017, 8, 44312–44325. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Martin, R.C.G.; Zheng, Q.; Farmer, R.; Pandit, H.; Li, X.; Jacob, K.; Suo, J.; Li, Y. Drug-induced expression of EpCAM contributes to therapy resistance in esophageal adenocarcinoma. Cell. Oncol. 2018, 41, 651–662. [Google Scholar] [CrossRef]
- Mau-Sorensen, M.; Dittrich, C.; Dienstmann, R.; Lassen, U.; Buchler, W.; Martinius, H.; Tabernero, J. A phase I trial of intravenous catumaxomab: A bispecific monoclonal antibody targeting EpCAM and the T cell coreceptor CD3. Cancer Chemother. Pharmacol. 2015, 75, 1065–1073. [Google Scholar] [CrossRef]
- Zeidler, R.; Reisbach, G.; Wollenberg, B.; Lang, S.; Chaubal, S.; Schmitt, B.; Lindhofer, H. Simultaneous activation of T cells and accessory cells by a new class of intact bispecific antibody results in efficient tumor cell killing. J. Immunol. 1999, 163, 1246–1252. [Google Scholar]
- Goere, D.; Flament, C.; Rusakiewicz, S.; Poirier-Colame, V.; Kepp, O.; Martins, I.; Pesquet, J.; Eggermont, A.; Elias, D.; Chaput, N.; et al. Potent immunomodulatory effects of the trifunctional antibody catumaxomab. Cancer Res. 2013, 73, 4663–4673. [Google Scholar] [CrossRef]
- Li, O.; Zheng, P.; Liu, Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J. Exp. Med. 2004, 200, 1083–1089. [Google Scholar] [CrossRef]
- Jarvis, J.N.; Zhao, L.; Moore, H.T.; Long, P.M.; Vani Gutta, P. Regulation of cytokine mRNA expression in activated lymphocytes by human choriocarcinoma JAR cells. Cell. Immunol. 1996, 168, 251–257. [Google Scholar] [CrossRef]
- Rajashekhar, G.; Loganath, A.; Roy, A.C.; Mongelli, J.M. Co-expression of Fas (APO-1, CD95)/Fas ligand by BeWo and NJG choriocarcinoma cell lines. Gynecol. Oncol. 2003, 91, 101–111. [Google Scholar] [CrossRef]
- Ka, H.; Hunt, J.S. Temporal and spatial patterns of expression of inhibitors of apoptosis in human placentas. Am. J. Pathol. 2003, 163, 413–422. [Google Scholar] [CrossRef]
- Cierna, Z.; Mego, M.; Miskovska, V.; Machalekova, K.; Chovanec, M.; Svetlovska, D.; Hainova, K.; Rejlekova, K.; Macak, D.; Spanik, S.; et al. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann. Oncol. 2016, 27, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Rodrigues, A.; Guimaraes, R.; Cantante, M.; Lopes, P.; Mauricio, J.; Oliveira, J.; Jeronimo, C.; Henrique, R. Detailed characterization of immune cell infiltrate and expression of immune checkpoint molecules PD-L1/CTLA-4 and MMR proteins in testicular germ cell tumors disclose novel disease biomarkers. Cancers 2019, 11, 1535. [Google Scholar] [CrossRef]
- Deisting, W.; Raum, T.; Kufer, P.; Baeuerle, P.A.; Munz, M. Impact of diverse immune evasion mechanisms of cancer cells on T cells engaged by EpCAM/CD3-bispecific antibody construct AMG 110. PLoS ONE 2015, 10, e0141669. [Google Scholar] [CrossRef]
- Deppisch, N.; Ruf, P.; Eissler, N.; Lindhofer, H.; Mocikat, R. Potent CD4+ T cell-associated antitumor memory responses induced by trifunctional bispecific antibodies in combination with immune checkpoint inhibition. Oncotarget 2017, 8, 4520–4529. [Google Scholar] [CrossRef]
- Tamai, T.; Mizukoshi, E.; Kumagai, M.; Terashima, T.; Iida, N.; Kitahara, M.; Shimakami, T.; Kitamura, K.; Arai, K.; Yamashita, T.; et al. A novel alpha-fetoprotein-derived helper T-lymphocyte epitope with strong immunogenicity in patients with hepatocellular carcinoma. Sci. Rep. 2020, 10, 4021. [Google Scholar] [CrossRef]
- Li, Z.; Gong, H.; Liu, Q.; Wu, W.; Cheng, J.; Mei, Y.; Chen, Y.; Zheng, H.; Yu, X.; Zhong, S.; et al. Identification of an HLA-A*24:02-restricted alpha-fetoprotein signal peptide-derived antigen and its specific T-cell receptor for T-cell immunotherapy. Immunology 2020, 159, 384–392. [Google Scholar] [CrossRef]
- Ruf, P.; Lindhofer, H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood 2001, 98, 2526–2534. [Google Scholar] [CrossRef]
- Borlak, J.; Langer, F.; Spanel, R.; Schondorfer, G.; Dittrich, C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcgamma receptors. Oncotarget 2016, 7, 28059–28074. [Google Scholar] [CrossRef]
- English, D.P.; Bellone, S.; Schwab, C.L.; Roque, D.M.; Lopez, S.; Bortolomai, I.; Cocco, E.; Bonazzoli, E.; Chatterjee, S.; Ratner, E.; et al. Solitomab, an epithelial cell adhesion molecule/CD3 bispecific antibody (BiTE), is highly active against primary chemotherapy-resistant ovarian cancer cell lines in vitro and fresh tumor cells ex vivo. Cancer 2015, 121, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kebenko, M.; Goebeler, M.E.; Wolf, M.; Hasenburg, A.; Seggewiss-Bernhardt, R.; Ritter, B.; Rautenberg, B.; Atanackovic, D.; Kratzer, A.; Rottman, J.B.; et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE(R)) antibody construct, in patients with refractory solid tumors. Oncoimmunology 2018, 7, e1450710. [Google Scholar] [CrossRef] [PubMed]
- Karches, C.H.; Benmebarek, M.R.; Schmidbauer, M.L.; Kurzay, M.; Klaus, R.; Geiger, M.; Rataj, F.; Cadilha, B.L.; Lesch, S.; Heise, C.; et al. Bispecific antibodies enable synthetic agonistic receptor-transduced t cells for tumor immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5890–5900. [Google Scholar] [CrossRef] [PubMed]
- Quintarelli, C.; Orlando, D.; Boffa, I.; Guercio, M.; Polito, V.A.; Petretto, A.; Lavarello, C.; Sinibaldi, M.; Weber, G.; Del Bufalo, F.; et al. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology 2018, 7, e1433518. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.X.; Li, Z.; Chi, Z.; Du, S.H.; Chen, C.; Tay, J.C.; Toh, H.C.; Connolly, J.E.; Xu, X.H.; Wang, S. Intraperitoneal immunotherapy with T cells stably and transiently expressing anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis. Oncotarget 2017, 8, 13545–13559. [Google Scholar] [CrossRef]
- Hong, L.K.; Chen, Y.; Smith, C.C.; Montgomery, S.A.; Vincent, B.G.; Dotti, G.; Savoldo, B. CD30-Redirected Chimeric Antigen Receptor T Cells Target CD30(+) and CD30(-) Embryonal Carcinoma via Antigen-Dependent and Fas/FasL Interactions. Cancer Immunol. Res. 2018. [Google Scholar] [CrossRef]
- Schönberger, S.; van Beekum, C.; Götz, B.; Nettersheim, D.; Schorle, H.; Schneider, D.T.; Casati, A.; Craveiro, R.B.; Calaminus, G.; Dilloo, D. Brentuximab vedotin exerts profound antiproliferative and pro-apoptotic efficacy in CD30-positive as well as cocultured CD30-negative germ cell tumour cell lines. J. Cell. Mol. Med. 2018, 22, 568–575. [Google Scholar] [CrossRef]
- Nettersheim, D.; Jostes, S.; Sharma, R.; Schneider, S.; Hofmann, A.; Ferreira, H.J.; Hoffmann, P.; Kristiansen, G.; Esteller, M.B.; Schorle, H. BMP inhibition in seminomas initiates acquisition of pluripotency via nodal signaling resulting in reprogramming to an embryonal carcinoma. PLoS Genet. 2015, 11, e1005415. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönberger, S.; Kraft, D.; Nettersheim, D.; Schorle, H.; Casati, A.; Craveiro, R.B.; Mohseni, M.M.; Calaminus, G.; Dilloo, D. Targeting EpCAM by a Bispecific Trifunctional Antibody Exerts Profound Cytotoxic Efficacy in Germ Cell Tumor Cell Lines. Cancers 2020, 12, 1279. https://doi.org/10.3390/cancers12051279
Schönberger S, Kraft D, Nettersheim D, Schorle H, Casati A, Craveiro RB, Mohseni MM, Calaminus G, Dilloo D. Targeting EpCAM by a Bispecific Trifunctional Antibody Exerts Profound Cytotoxic Efficacy in Germ Cell Tumor Cell Lines. Cancers. 2020; 12(5):1279. https://doi.org/10.3390/cancers12051279
Chicago/Turabian StyleSchönberger, Stefan, Daniela Kraft, Daniel Nettersheim, Hubert Schorle, Anna Casati, Rogerio B. Craveiro, Mahsa Mir Mohseni, Gabriele Calaminus, and Dagmar Dilloo. 2020. "Targeting EpCAM by a Bispecific Trifunctional Antibody Exerts Profound Cytotoxic Efficacy in Germ Cell Tumor Cell Lines" Cancers 12, no. 5: 1279. https://doi.org/10.3390/cancers12051279
APA StyleSchönberger, S., Kraft, D., Nettersheim, D., Schorle, H., Casati, A., Craveiro, R. B., Mohseni, M. M., Calaminus, G., & Dilloo, D. (2020). Targeting EpCAM by a Bispecific Trifunctional Antibody Exerts Profound Cytotoxic Efficacy in Germ Cell Tumor Cell Lines. Cancers, 12(5), 1279. https://doi.org/10.3390/cancers12051279






