Evaluation of a Novel Liquid Fiducial Marker, BioXmark®, for Small Animal Image-Guided Radiotherapy Applications
Abstract
1. Introduction
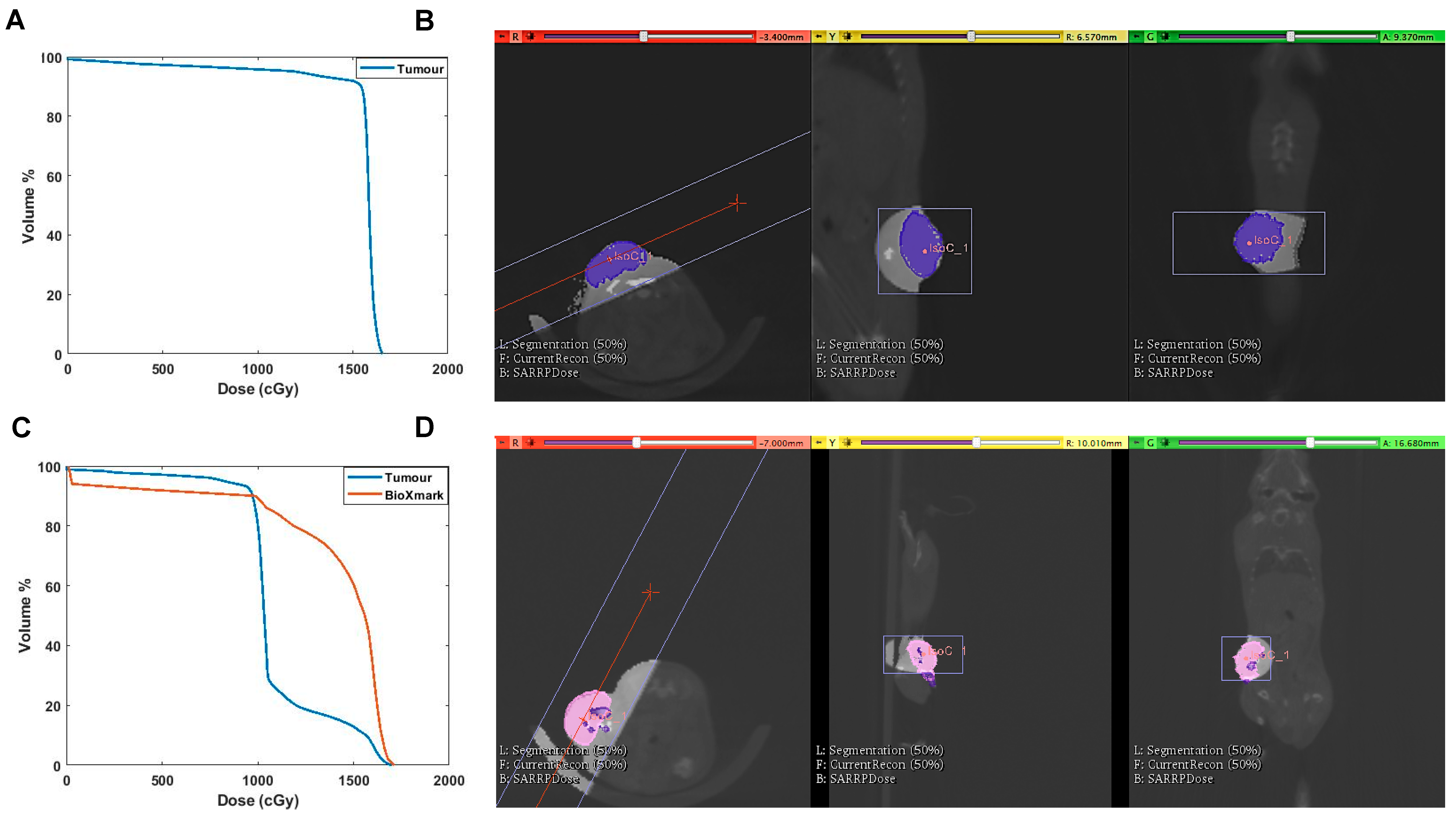
2. Results
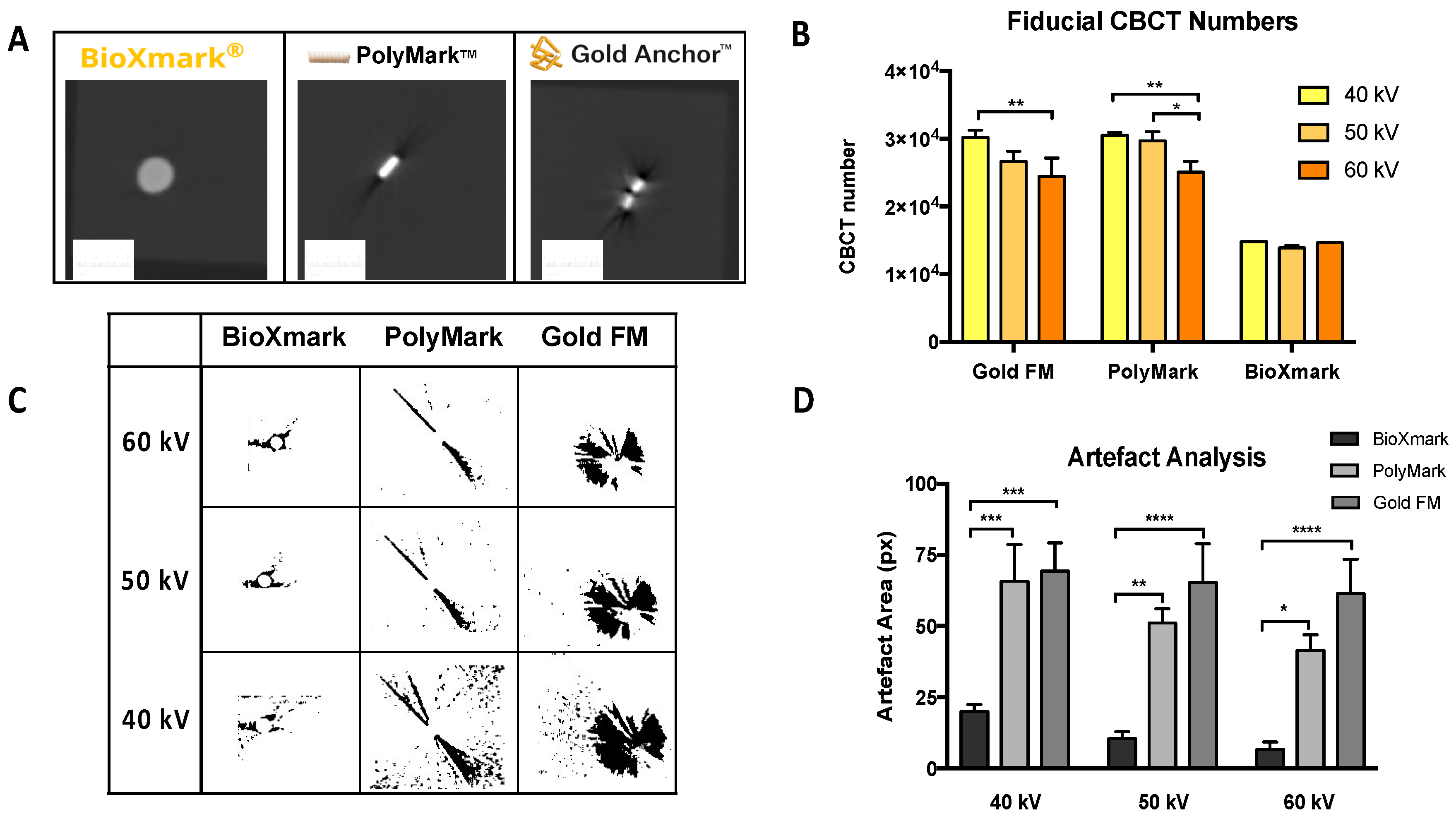
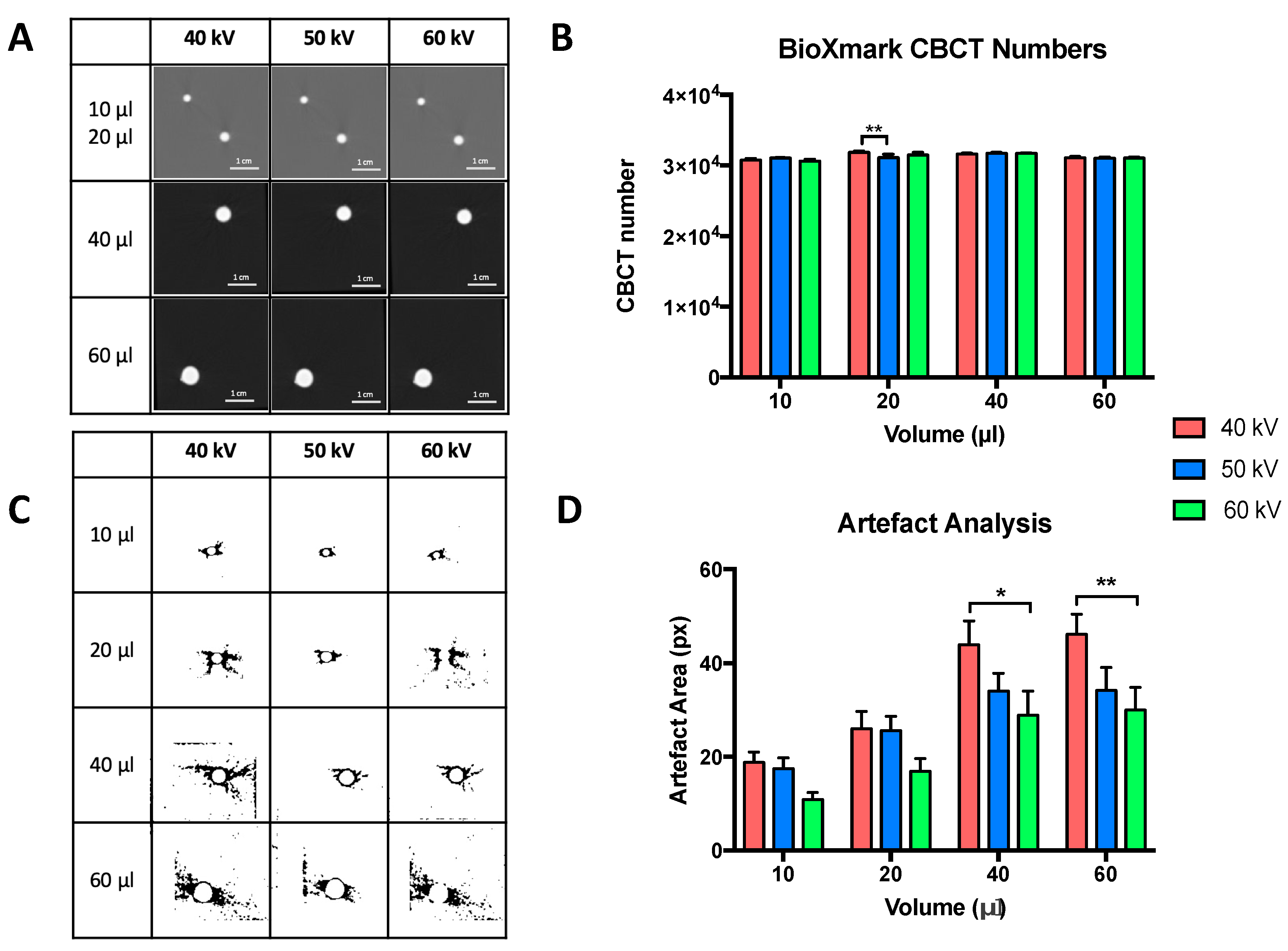
2.1. In Phantom Studies
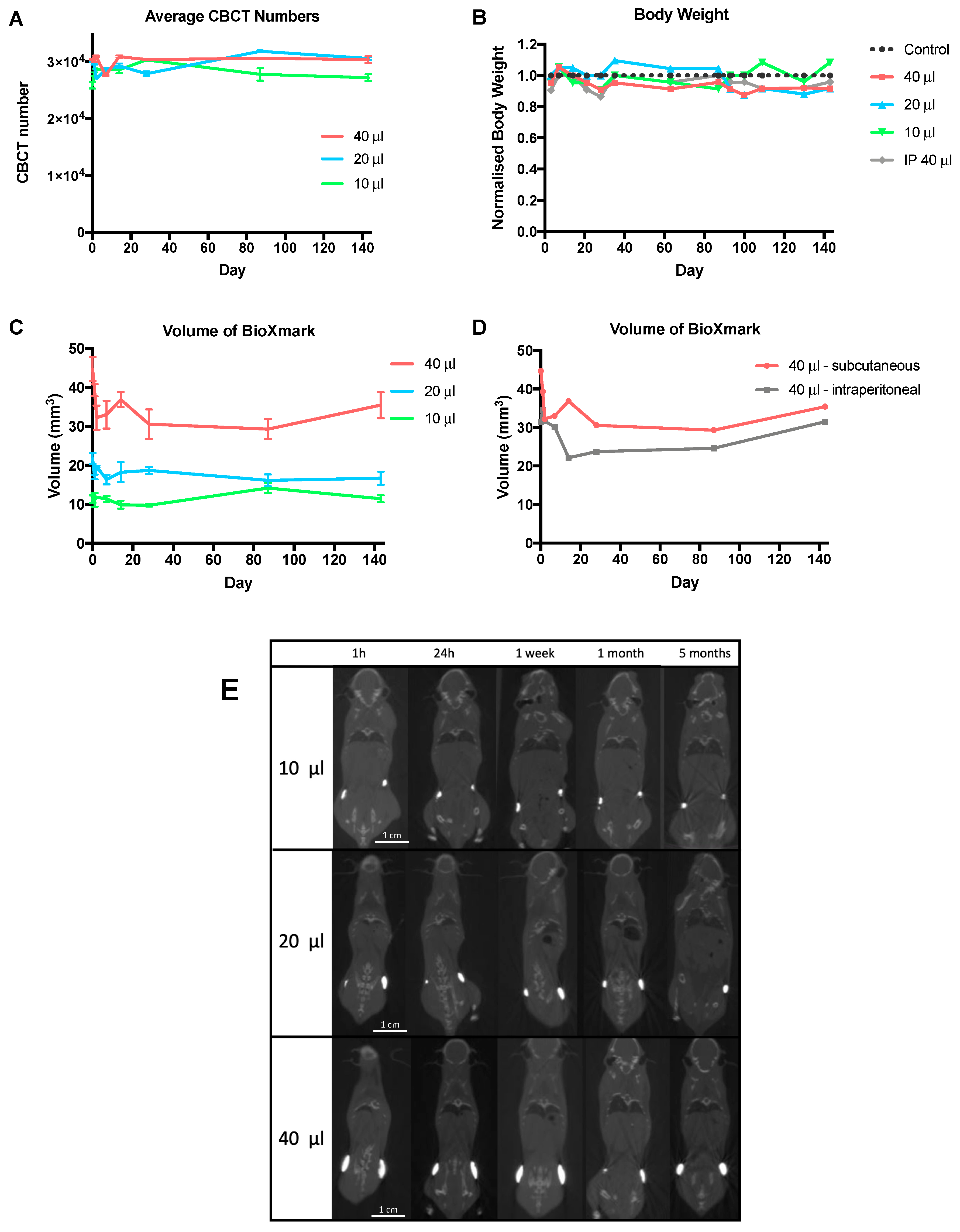
2.2. In Vivo Stability
2.3. In Vivo Tumour Model
3. Discussion
4. Materials and Methods
4.1. Liquid Fiducial Marker
4.2. Solid Fiducial Markers
4.3. Imaging and Irradiation
4.4. In Phantom Studies
Artefact Analysis
4.5. In Vivo Studies
4.5.1. In Vivo Stability
4.5.2. In Vivo Tumour Model
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jolck, R.I.; Rydhog, J.S.; Christensen, A.N.; Hansen, A.E.; Bruun, L.M.; Schaarup-Jensen, H.; von Wenck, A.S.; Borresen, B.; Kristensen, A.T.; Clausen, M.H.; et al. Injectable colloidal gold for use in intrafractional 2D image-guided radiation therapy. Adv. Healthc. Mater. 2015, 4, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Heijmen, B.J.M.; Verellen, D.; Nisbet, A. Automation in intensity modulated radiotherapy treatment planning—A review of recent innovations. Br. J. Radiol. 2018, 91, 20180270. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Clark, C.H.; Urbano, M.T.G.; Bidmead, M.; Dearnaley, D.P.; Harrington, K.J.; A’Hern, R.; Nutting, C.M. The impact of introducing intensity modulated radiotherapy into routine clinical practice. Radiother. Oncol. 2005, 77, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Dahele, M.; Mclaren, D.B. Stereotactic body radiotherapy. Clin. Oncol. 2015, 27, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T. Evolution of the supermodel: Progress in modelling radiotherapy response in mice. Clin. Oncol. R. Coll. Radiol. 2019, 31, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Kataria, T. Image guidance in radiation therapy: Techniques and applications. Radiol. Res. Pract. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Bedford, J.L.; Chajecka-Szczygielska, H.; Thomas, M.D.R. Quality control of VMAT synchronization using portal imaging. J. Appl. Clin. Med. Phys. 2015, 16, 284–297. [Google Scholar] [CrossRef]
- Bell, K.; Heitfeld, M.; Licht, N.; Rübe, C.; Dzierma, Y. Influence of daily imaging on plan quality and normal tissue toxicity for prostate cancer radiotherapy. Radiat. Oncol. 2017, 12, 1–11. [Google Scholar] [CrossRef]
- Liney, G.P.; Whelan, B.; Oborn, B.; Barton, M.; Keall, P. MRI-linear accelerator radiotherapy systems. Clin. Oncol. 2018, 30, 686–691. [Google Scholar] [CrossRef]
- Habermehl, D.; Henkner, K.; Ecker, S.; Jakel, O.; Debus, J.; Combs, S.E. Evaluation of different fiducial markers for image-guided radiotherapy and particle therapy. J. Radiat. Res. 2013, 54, i61–i68. [Google Scholar] [CrossRef]
- O’Neill, A.G.M.; Jain, S.; Hounsell, A.R.; O’Sullivan, J.M. Fiducial marker guided prostate radiotherapy: A review. Br. J. Radiol. 2016, 89, 20160296. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Hoffe, S.E.; Barthel, J.S.; Vignesh, S.; Klapman, J.B.; Harris, C.; Almhanna, K.; Biagioli, M.C.; Meredith, K.L.; Feygelman, V.; et al. Stability of endoscopic ultrasound-guided fiducial marker placement for esophageal cancer target delineation and image-guided radiation therapy. Pract. Radiat. Oncol. 2013, 3, 32–39. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, C.J.; Nagula, S.; Goodman, K.A.; Ho, A.Y.; Markowitz, A.J.; Schattner, M.A.; Gerdes, H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos). Gastrointest. Endosc. 2010, 71, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; van der Horst, A.; de Jong, R.; van Hooft, J.E.; Kamphuis, M.; van Wieringen, N.; Machiels, M.; Bel, A.; Hulshof, M.C.C.M.; Alderliesten, T. Marker-based quantification of interfractional tumor position variation and the use of markers for setup verification in radiation therapy for esophageal cancer. Radiother. Oncol. 2015, 117, 412–418. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, U.A.; Kotte, A.N.T.J.; Dehnad, H.; Hofman, P.; Lagenijk, J.J.W.; van Vulpen, M. Analysis of fiducial marker-based position verification in the external beam radiotherapy of patients with prostate cancer. Radiother. Oncol. 2007, 82, 38–45. [Google Scholar] [CrossRef]
- Rydhög, J.S.; Jølck, R.I.; Andresen, T.L.; Munck Af Rosenschöld, P. Quantification and comparison of visibility and image artifacts of a new liquid fiducial marker in a lung phantom for image-guided radiation therapy. Med. Phys. 2015, 42, 2818–2826. [Google Scholar] [CrossRef] [PubMed]
- Gurney-Champion, O.J.; Lens, E.; Van Der Horst, A.; Houweling, A.C.; Klaassen, R.; Van Hooft, J.E.; Stoker, J.; Van Tienhoven, G.; Nederveen, A.J.; Bel, A. Visibility and artifacts of gold fiducial markers used for image guided radiation therapy of pancreatic cancer on MRI. Med. Phys. 2015, 42, 2638–2647. [Google Scholar] [CrossRef]
- Schneider, S.; Jolck, R.I.; Troost, E.G.C.; Hoffmann, A.L. Quantification of MRI visibility and artifacts at 3T of liquid fiducial marker in a pancreas tissue-mimicking phantom. Med. Phys. 2018, 45, 37–47. [Google Scholar] [CrossRef]
- Osman, S.O.S.; Russell, E.; King, R.B.; Crowther, K.; Jain, S.; McGrath, C.; Hounsell, A.R.; Prise, K.M.; McGarry, C.K. Fiducial markers visibility and artefacts in prostate cancer radiotherapy multi-modality imaging. Radiat. Oncol. 2019, 14, 237. [Google Scholar] [CrossRef]
- Slagowski, J.M.; Colbert, L.E.; Cazacu, I.M.; Singh, B.S.; Martin, R.; Koay, E.J.; Taniguchi, C.M.; Koong, A.C.; Bhutani, M.S.; Herman, J.M.; et al. Evaluation of the visibility and artifacts of 11 common fiducial markers for image guided stereotactic body radiation therapy in the abdomen. Pract. Radiat. Oncol. 2020. [Google Scholar] [CrossRef]
- Chan, M.F.; Cohen, G.N.; Deasy, J.O. Qualitative evaluation of fiducial markers for radiotherapy imaging. Technol. Cancer Res. Treat. 2015, 14, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Scherman Rydhög, J.; Perrin, R.; Jølck, R.I.; Gagnon-Moisan, F.; Larsen, K.R.; Clementsen, P.; Riisgaard de Blanck, S.; Fredberg Persson, G.; Weber, D.C.; Lomax, T.; et al. Liquid fiducial marker applicability in proton therapy of locally advanced lung cancer. Radiother. Oncol. 2017, 122, 393–399. [Google Scholar] [CrossRef]
- De Roover, R.; Crijns, W.; Poels, K.; Peeters, R.; Draulans, C.; Haustermans, K.; Depuydt, T. Characterization of a novel liquid fiducial marker for multimodal image guidance in stereotactic body radiotherapy of prostate cancer. Med. Phys. 2018, 45, 2205–2217. [Google Scholar] [CrossRef]
- Dobiasch, S.; Kampfer, S.; Burkhardt, R.; Schilling, D.; Schmid, T.E.; Wilkens, J.J.; Combs, S.E. BioXmark for high-precision radiotherapy in an orthotopic pancreatic tumor mouse model: Experiences with a liquid fiducial marker. Strahlenther. Onkol. 2017, 193, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Machiels, M.; Voncken, F.E.M.; Jin, P.; van Dieren, J.M.; Bartels-Rutten, A.; Alderliesten, T.; Aleman, B.M.P.; van Hooft, J.E.; Hulshof, M.C.C.M. A novel liquid fiducial marker in esophageal cancer image guided radiation therapy: Technical feasibility and visibility on imaging. Pract. Radiat. Oncol. 2019, 9, e506–e515. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.E.; Mitchell, J.B. Mechanisms of normal tissue injury from irradiation. Semin. Radiat. Oncol. 2017, 27, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.; Tudor, G.; Tudor, J.; Katz, B.P.; MacVittie, T.J. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012, 103, 383–399. [Google Scholar] [CrossRef]
- Ghita, M.; Brown, K.H.; Kelada, O.J.; Graves, E.E.; Butterworth, K.T. Integrating small animal irradiators withfunctional imaging for advanced preclinical radiotherapy research. Cancers 2019, 11, 170. [Google Scholar] [CrossRef]
- Schulze, R.; Heil, U.; Groß, D.; Bruellmann, D.D.; Dranischnikow, E.; Schwanecke, U.; Schoemer, E. Artefacts in CBCT: A review. Dentomaxillofacial Radiol. 2011, 40, 265–273. [Google Scholar] [CrossRef]
- Broder, J. Diagnostic imaging for the emergency physician. In Diagnostic Imaging for the Emergency Physician; Saunders: Centennial, CO, USA, 2011; pp. 445–577. ISBN 978-1-4160-6113-7. [Google Scholar]
- Schneider, S.; Aust, D.E.; Bruckner, S.; Welsch, T.; Hampe, J.; Troost, E.G.C.; Hoffmann, A.L. Detectability and structural stability of a liquid fiducial marker in fresh ex vivo pancreas tumour resection specimens on CT and 3T MRI. Strahlenther. Onkol. 2019, 195, 756–763. [Google Scholar] [CrossRef]
- Verhaegen, F.; Granton, P.; Tryggestad, E. Small animal radiotherapy research platforms. Phys. Med. Biol. 2011, 56, R55–R83. [Google Scholar] [CrossRef] [PubMed]
- Biglin, E.R.; Price, G.J.; Chadwick, A.L.; Aitkenhead, A.H.; Williams, K.J.; Kirkby, K.J. Preclinical dosimetry: Exploring the use of small animal phantoms. Radiat. Oncol. 2019, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Koontz, B.F.; Verhaegen, F.; De Ruysscher, D. Tumour and normal tissue radiobiology in mouse models: How close are mice to mini-humans? Br. J. Radiol. 2017, 90, 20160441. [Google Scholar] [CrossRef]
- Butterworth, K.T.; Prise, K.M.; Verhaegen, F. Small animal image-guided radiotherapy: Status, considerations and potential for translational impact. Br. J. Radiol. 2015, 88, 20140634. [Google Scholar] [CrossRef]
- Draeger, E.; Sawant, A.; Johnstone, C.; Koger, B.; Becker, S.; Vujaskovic, Z.; Jackson, I.L.; Poirier, Y. A dose of reality: How 20 years of incomplete physics and dosimetry reporting in radiobiology studies may have contributed to the reproducibility crisis. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Verginadis, I.I.; Kanade, R.; Bell, B.; Koduri, S.; Ben-Josef, E.; Koumenis, C. A novel mouse model to study image-guided, radiation-induced intestinal injury and preclinical screening of radioprotectors. Cancer Res. 2017, 77, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Ciernik, I.F.; Greiss, A.M. Visualization of the tumor cavity after lumpectomy of breast cancer for postoperative radiotherapy. Clin. Transl. Radiat. Oncol. 2019, 14, 47–50. [Google Scholar] [CrossRef] [PubMed]
- de Blanck, S.R.; Scherman-Rydhog, J.; Siemsen, M.; Christensen, M.; Baeksgaard, L.; Irming Jolck, R.; Specht, L.; Andresen, T.L.; Persson, G.F. Feasibility of a novel liquid fiducial marker for use in image guided radiotherapy of oesophageal cancer. Br. J. Radiol. 2018, 91, 20180236. [Google Scholar] [CrossRef]
- Rydhög, J.S.; Mortensen, S.R.; Larsen, K.R.; Clementsen, P.; Jølck, R.I.; Josipovic, M.; Aznar, M.C.; Specht, L.; Andresen, T.L.; Rosenschöld, P.M.; et al. Liquid fiducial marker performance during radiotherapy of locally advanced non small cell lung cancer. Radiother. Oncol. 2016, 121, 64–69. [Google Scholar] [CrossRef]
- de Blanck, S.R.; Rydhog, J.S.; Larsen, K.R.; Clementsen, P.F.; Josipovic, M.; Aznar, M.C.; Rosenschold, P.M.; Jolck, R.I.; Specht, L.; Andresen, T.L.; et al. Long term safety and visibility of a novel liquid fiducial marker for use in image guided radiotherapy of non-small cell lung cancer. Clin. Transl. Radiat. Oncol. 2018, 13, 24–28. [Google Scholar] [CrossRef]
- Nanovi(A/S). BioXmark. Available online: http://nanovi.com/bioxmark/ (accessed on 5 December 2018).
- Bertholet, J.; Knopf, A.; Mcclelland, J.; Grimwood, A. Real-time intrafraction motion monitoring in external beam radiotherapy. Phys. Med. Biol. 2019, 64, 15TR01. [Google Scholar] [CrossRef] [PubMed]
- Vaniqui, A.; Schyns, L.E.J.R.; Almeida, I.P.; Van Der Heyden, B.; Podesta, M.; Verhaegen, F. The effect of different image reconstruction techniques on pre-clinical quantitative imaging and dual-energy cT. Br. J. Radiol. 2019, 92, 20180447. [Google Scholar] [CrossRef] [PubMed]
- Bazalova, M.; Zhou, H.; Keall, P.J.; Graves, E.E. Kilovoltage beam Monte Carlo dose calculations in submillimeter voxels for small animal radiotherapy. Med. Phys. 2009, 36, 4991–4999. [Google Scholar] [CrossRef] [PubMed]
- Van Hoof, S.J.; Granton, P.V.; Verhaegen, F. Development and validation of a treatment planning system for small animal radiotherapy: SmART-Plan. Radiother. Oncol. 2013, 109, 361–366. [Google Scholar] [CrossRef]
- Rosser, K.E. The IPEMB code of practice for the determination of absorbed dose for x-rays below 300 kV generating potential (0.035 mm Al-4 mm Cu HVL; 10-300 kV generating potential). Phys. Med. Biol. 1996, 41, 2605–2625. [Google Scholar]
- Hubbell, J.H.; Seltzer, S.M. X-ray mass attenuation coefficients. Radiat. Phys. Div. PML NIST 2004. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef]
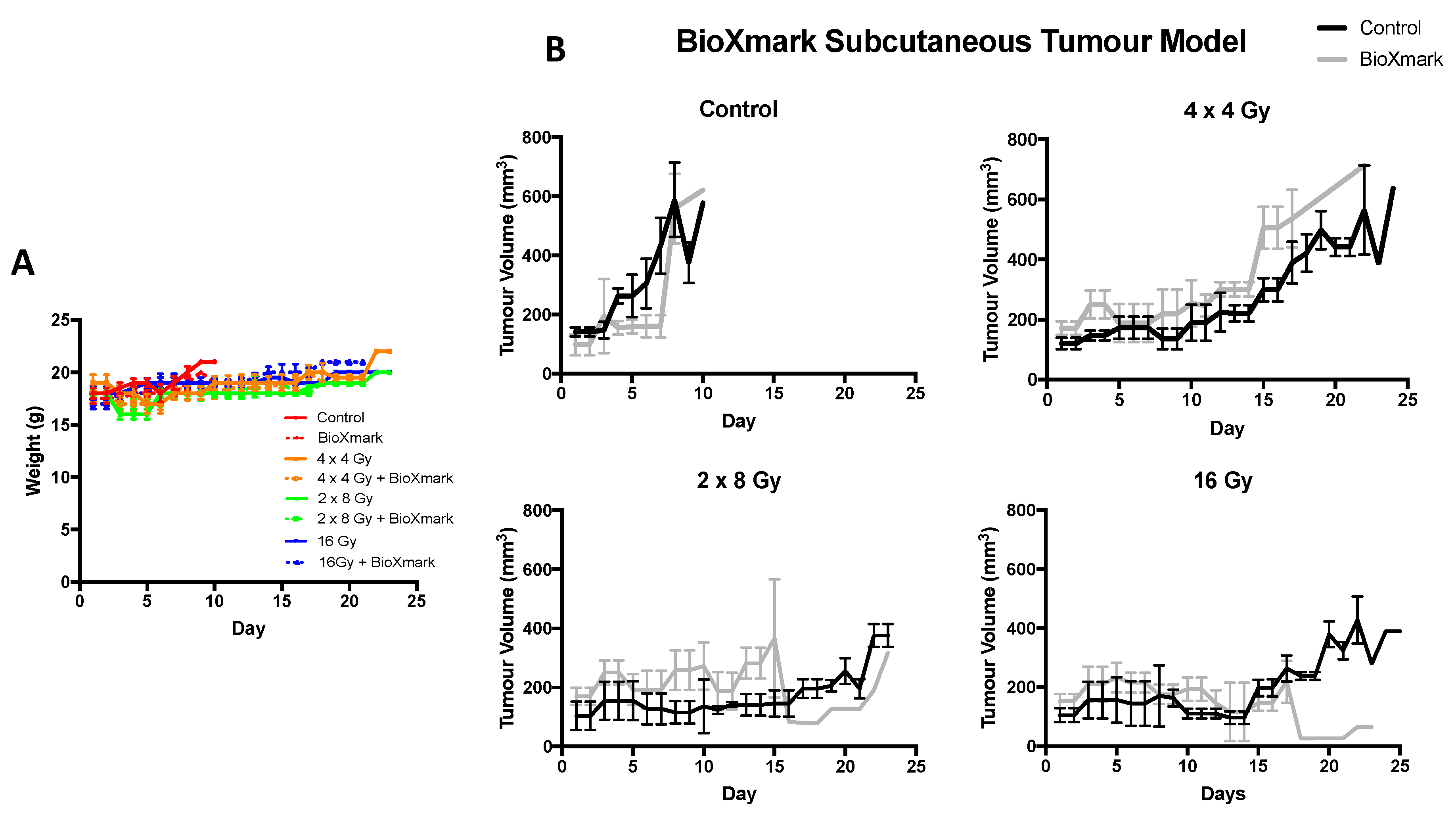
| Dose Schedule | BED (Gy) | EQD2 (Gy) | Average Total Dose Delivered (cGy ± SEM) | Difference in Dose Compared to Non-BioXmark® Control (%) |
|---|---|---|---|---|
| 4 × 4 Gy | 36.6 | 22.3 | 1609 ± 7 | |
| 4 × 4 Gy + BioXmark® | 1442 ± 113 | −10.4 | ||
| 2 × 8 Gy | 57.3 | 34.8 | 1606 ± 8 | |
| 2 × 8 Gy + BioXmark® | 1254 ± 153 | −21.9 | ||
| 16 Gy | 98.6 | 59.9 | 1611 ± 14 | |
| 16 Gy + BioXmark® | 1307 ± 160 | −18.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, K.H.; Ghita, M.; Schettino, G.; Prise, K.M.; Butterworth, K.T. Evaluation of a Novel Liquid Fiducial Marker, BioXmark®, for Small Animal Image-Guided Radiotherapy Applications. Cancers 2020, 12, 1276. https://doi.org/10.3390/cancers12051276
Brown KH, Ghita M, Schettino G, Prise KM, Butterworth KT. Evaluation of a Novel Liquid Fiducial Marker, BioXmark®, for Small Animal Image-Guided Radiotherapy Applications. Cancers. 2020; 12(5):1276. https://doi.org/10.3390/cancers12051276
Chicago/Turabian StyleBrown, Kathryn H., Mihaela Ghita, Giuseppe Schettino, Kevin M. Prise, and Karl T. Butterworth. 2020. "Evaluation of a Novel Liquid Fiducial Marker, BioXmark®, for Small Animal Image-Guided Radiotherapy Applications" Cancers 12, no. 5: 1276. https://doi.org/10.3390/cancers12051276
APA StyleBrown, K. H., Ghita, M., Schettino, G., Prise, K. M., & Butterworth, K. T. (2020). Evaluation of a Novel Liquid Fiducial Marker, BioXmark®, for Small Animal Image-Guided Radiotherapy Applications. Cancers, 12(5), 1276. https://doi.org/10.3390/cancers12051276






