Detecting Endometrial Cancer by Blood Spectroscopy: A Diagnostic Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Participant Demographics
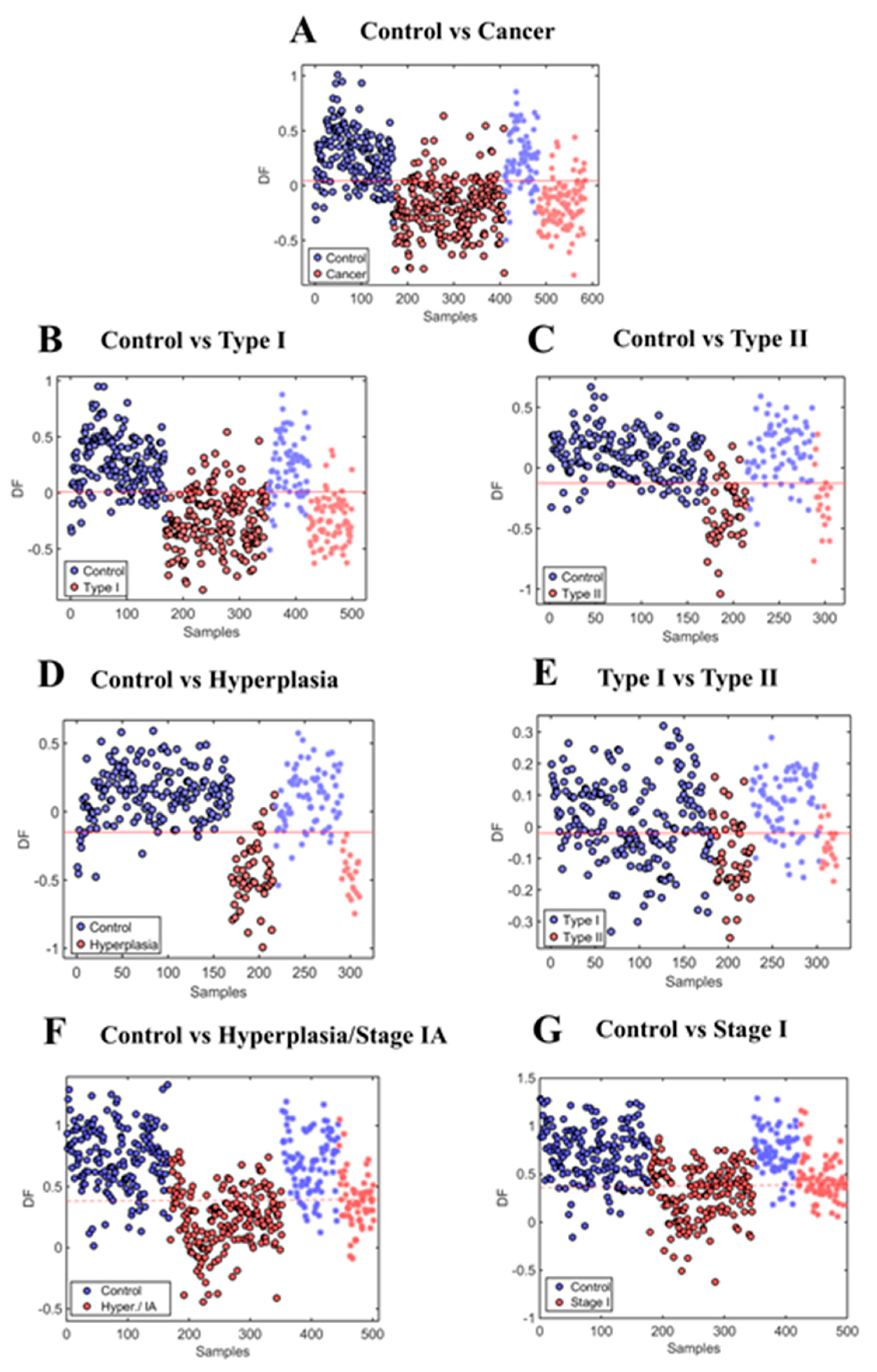
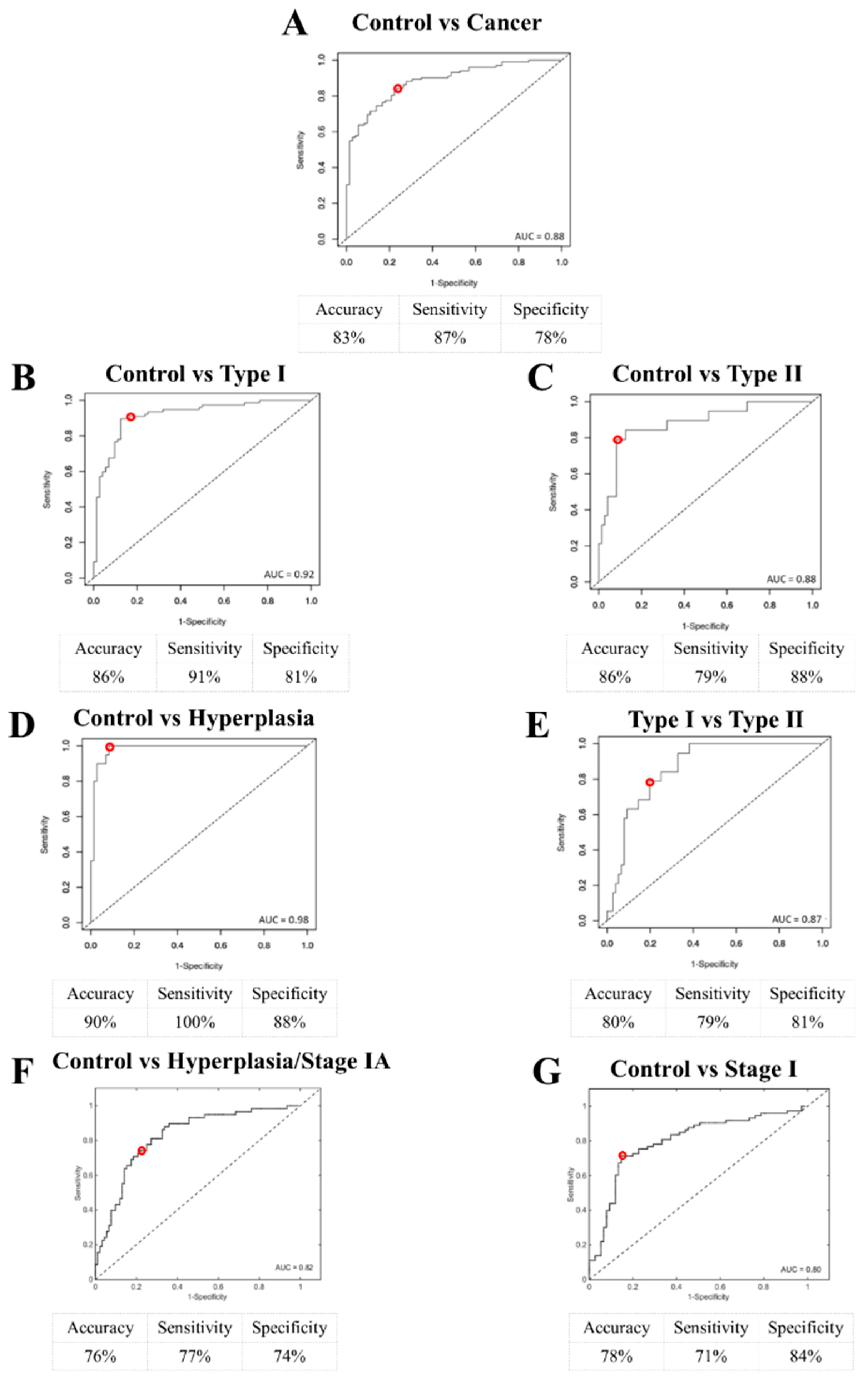
2.2. Endometrial Cancer and Pre-Cancer Diagnosis
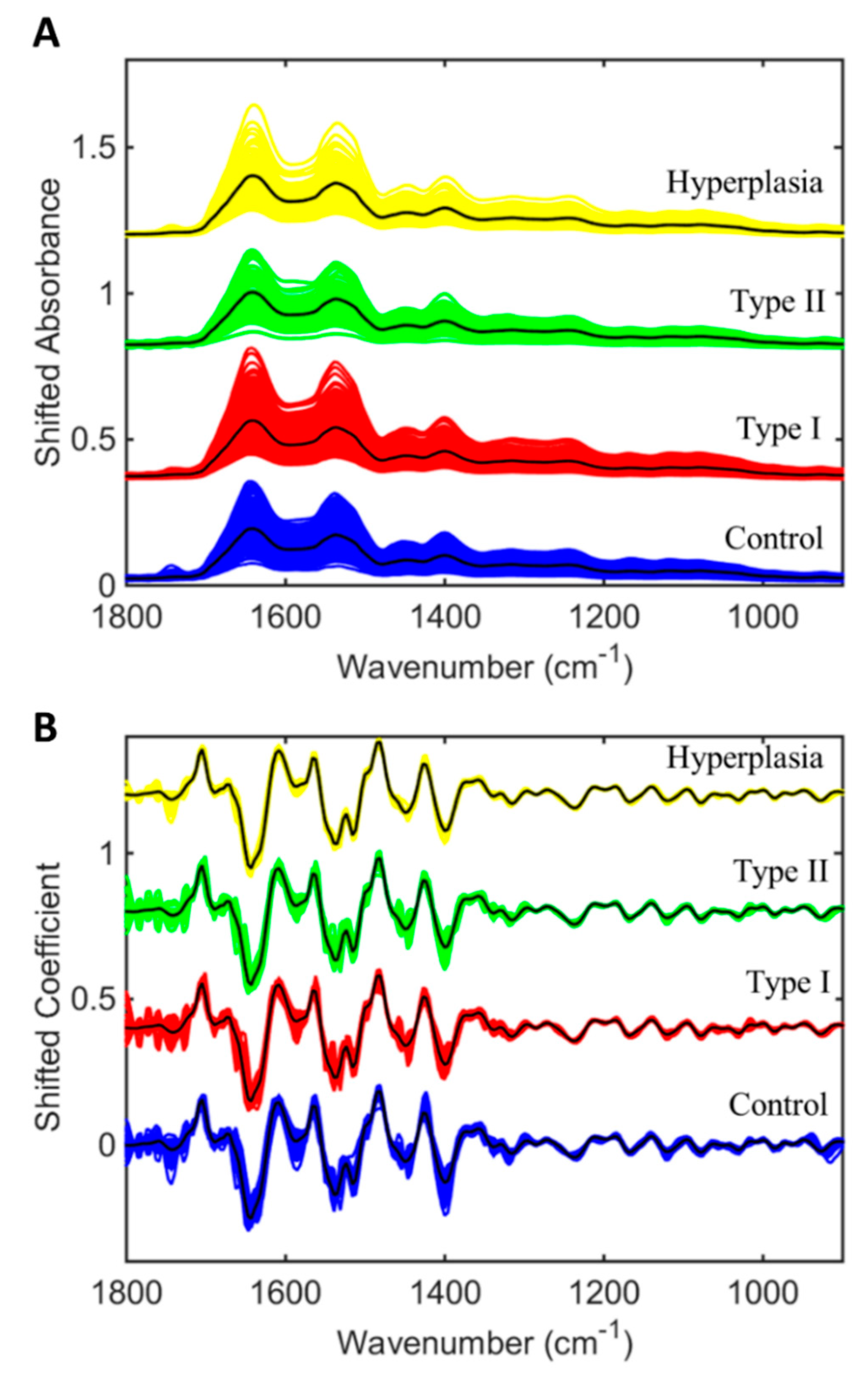
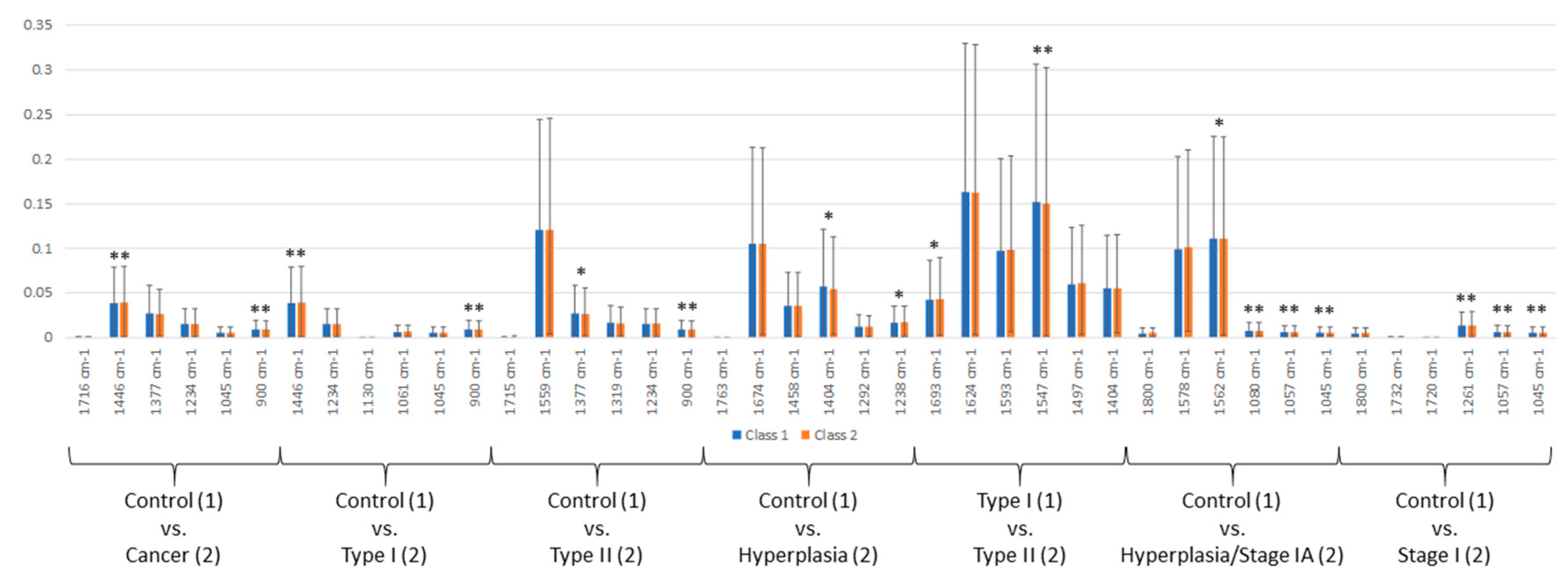
2.3. Panel of Potential Diagnostic Spectral Markers
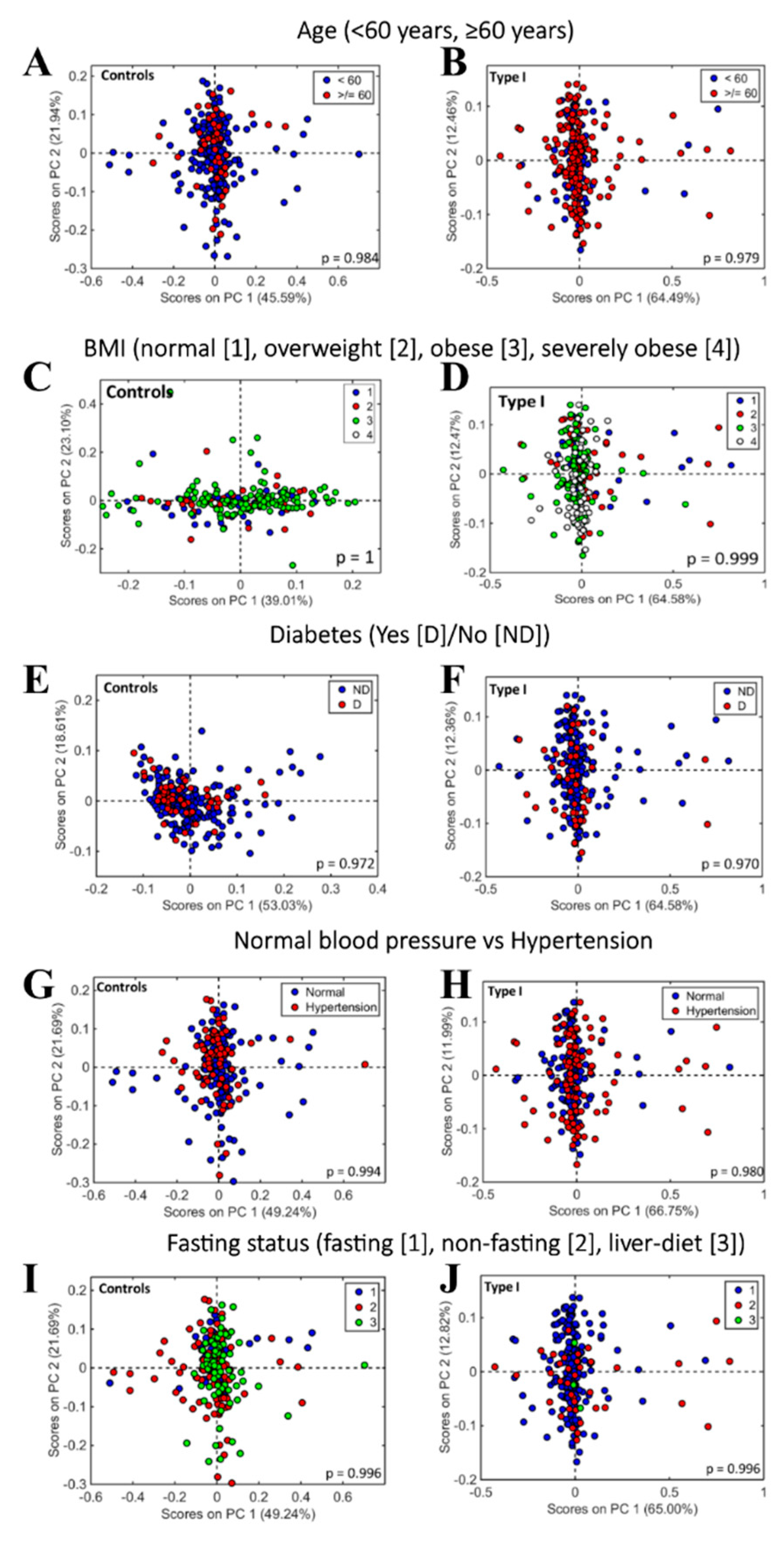
2.4. Consideration of Potential Confounding Factors
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Preparation and Spectroscopic Analysis
4.3. Data Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef]
- Cancer Research, UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer#heading-Zero (accessed on 12 May 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Kitson, S.J.; Lindsay, J.; Sivalingam, V.N.; Rutter, M.K.; Crosbie, E.J. High prevalence of metabolic syndrome in women newly diagnosed with endometrial cancer. Gynecol. Oncol. Rep. 2018, 26, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Saso, S.; Chatterjee, J.; Georgiou, E.; Ditri, A.M.; Smith, J.R.; Ghaem-Maghami, S. Endometrial cancer. BMJ 2011, 343, d3954. [Google Scholar] [CrossRef]
- Cooper, N.A.; Barton, P.M.; Breijer, M.; Caffrey, O.; Opmeer, B.C.; Timmermans, A.; Mol, B.W.; Khan, K.S.; Clark, T.J. Cost-effectiveness of diagnostic strategies for the management of abnormal uterine bleeding (heavy menstrual bleeding and post-menopausal bleeding): A decision analysis. Health Technol. Assess. 2014, 18. [Google Scholar] [CrossRef]
- Jacobs, I.; Gentry-Maharaj, A.; Burnell, M.; Manchanda, R.; Singh, N.; Sharma, A.; Ryan, A.; Seif, M.W.; Amso, N.N.; Turner, G.; et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: A case-control study within the UKCTOCS cohort. Lancet Oncol. 2011, 12, 38–48. [Google Scholar] [CrossRef]
- Adambekov, S.; Goughnour, S.L.; Mansuria, S.; Donnellan, N.; Elishaev, E.; Villanueva, H.J.; Edwards, R.P.; Bovbjerg, D.H.; Linkov, F. Patient and provider factors associated with endometrial Pipelle sampling failure. Gynecol. Oncol. 2017, 144, 324–328. [Google Scholar] [CrossRef]
- Pennington, M.; Gentry-Maharaj, A.; Karpinskyj, C.; Miners, A.; Taylor, J.; Manchanda, R.; Iyer, R.; Griffin, M.; Ryan, A.; Jacobs, I.; et al. Long-term secondary care costs of endometrial cancer: A prospective cohort study nested within the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). PLoS ONE 2016, 11, e0165539. [Google Scholar] [CrossRef]
- Srinivas, P.R.; Kramer, B.S.; Srivastava, S. Trends in biomarker research for cancer detection. Lancet Oncol. 2001, 2, 698–704. [Google Scholar] [CrossRef]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Hanash, S.M.; Baik, C.S.; Kallioniemi, O. Emerging molecular biomarkers—Blood-based strategies to detect and monitor cancer. Nat. Rev. Clin. Oncol. 2011, 8, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, K.; Trevisan, J.; Owens, G.; Keating, P.J.; Wood, N.J.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Fourier-transform infrared spectroscopy coupled with a classification machine for the analysis of blood plasma or serum: A novel diagnostic approach for ovarian cancer. Analyst 2013, 138, 3917–3926. [Google Scholar] [CrossRef] [PubMed]
- Sitole, L.; Steffens, F.; Krüger, T.P.; Meyer, D. Mid-ATR-FTIR spectroscopic profiling of HIV/AIDS sera for novel systems diagnostics in global health. OMICS 2014, 18, 513–523. [Google Scholar] [CrossRef]
- Paraskevaidi, M.; Morais, C.L.M.; Lima, K.M.G.; Snowden, J.S.; Saxon, J.A.; Richardson, A.M.T.; Jones, M.; Mann, D.M.A.; Allsop, D.; Martin-Hirsch, P.L.; et al. Differential diagnosis of Alzheimer’s disease using spectrochemical analysis of blood. Proc. Natl. Acad. Sci. USA 2017, 114, E7929–E7938. [Google Scholar] [CrossRef]
- Butler, H.J.; Brennan, P.M.; Cameron, J.M.; Finlayson, D.; Hegarty, M.G.; Jenkinson, M.D.; Palmer, D.S.; Smith, B.R.; Baker, M.J. Development of high-throughput ATR-FTIR technology for rapid triage of brain cancer. Nat. Commun. 2019, 10, 4501. [Google Scholar] [CrossRef]
- Sundar, S.; Balega, J.; Crosbie, E.; Drake, A.; Edmondson, R.; Fotopoulou, C.; Gallos, I.; Ganesan, R.; Gupta, J.; Johnson, N.; et al. BGCS uterine cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 71–97. [Google Scholar] [CrossRef]
- Lalkhen, A.G.; McCluskey, A. Clinical tests: Sensitivity and specificity. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 221–223. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Kumar, K.; Prasad, A.D. Fourier transform infrared spectroscopy an advanced technique for identification of biomolecules. Spectroscopy 2012, 14, 15. [Google Scholar]
- Mistry, D.A.H.; French, P.W. Circulating phospholipids as biomarkers of breast cancer: A review. Breast Cancer Basic Clin. Res. 2016, 10, S40693. [Google Scholar] [CrossRef] [PubMed]
- Hammad, L.A.; Wu, G.; Saleh, M.M.; Klouckova, I.; Dobrolecki, L.E.; Hickey, R.J.; Schnaper, L.; Novotny, M.V.; Mechref, Y. Elevated levels of hydroxylated phosphocholine lipids in the blood serum of breast cancer patients. Rapid Commun. Mass Spectrom. 2009, 23, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Bandu, R.; Mok, H.J.; Kim, K.P. Phospholipids as cancer biomarkers: Mass spectrometry-based analysis. Mass Spectrom. Rev. 2018, 37, 107–138. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef]
- Friberg, E.; Mantzoros, C.S.; Wolk, A. Diabetes and risk of endometrial cancer: A population-based prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 276–280. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Vatten, L.J. Hypertension and the risk of endometrial cancer: A systematic review and meta-analysis of case-control and cohort studies. Sci. Rep. 2017, 7, 44808. [Google Scholar] [CrossRef]
- Rosato, V.; Zucchetto, A.; Bosetti, C.; Dal Maso, L.; Montella, M.; Pelucchi, C.; Negri, E.; Franceschi, S.; La Vecchia, C. Metabolic syndrome and endometrial cancer risk. Ann. Oncol. 2010, 22, 884–889. [Google Scholar] [CrossRef]
- Ali, A.T. Risk factors for endometrial cancer. Ceska Gynekol. 2013, 78, 448–459. [Google Scholar]
- Timmermans, A.; Opmeer, B.C.; Khan, K.S.; Bachmann, L.M.; Epstein, E.; Clark, T.J.; Gupta, J.K.; Bakour, S.H.; van den Bosch, T.; van Doorn, H.C.; et al. Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: A systematic review and meta-analysis. Obstet. Gynecol. 2010, 116, 160–167. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Badrick, E.; Cresswell, K.; Ellis, P.; Crosbie, P.; Hall, P.S.; O’Flynn, H.; Detecting Cancer Early Priority Setting Partnership Steering Group (Appendix); Renehan, A.G.; Crosbie, E.J. Top ten research priorities for detecting cancer early. Lancet Public Health 2019. [Google Scholar] [CrossRef]
- Li, L.M.; Zhu, Y.X.; Zhong, Y.; Su, T.; Fan, X.M.; Xi, Q.; Li, M.Y.; Fu, J.; Tan, H.; Liu, S. Human epididymis protein 4 in endometrial cancer: A meta-analysis. Clin. Chim. Acta 2018, 482, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Huang, L.; Zhang, S. Preoperative serum CA125: A useful marker for surgical management of endometrial cancer. BMC Cancer 2015, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- Farias-Eisner, G.; Su, F.; Robbins, T.; Kotlerman, J.; Reddy, S.; Farias-Eisner, R. Validation of serum biomarkers for detection of early- and late-stage endometrial cancer. Am. J. Obstet. Gynecol. 2010, 202, 73.e1–73.e5. [Google Scholar] [CrossRef] [PubMed]
- Elshimali, Y.I.; Khaddour, H.; Sarkissyan, M.; Wu, Y.; Vadgama, J.V. The clinical utilization of circulating cell free DNA (CCFDNA) in blood of cancer patients. Int. J. Mol. Sci. 2013, 14, 18925–18958. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.-J.; Xu, K.; Xu, H.; Shen, X.-Z.; Tu, R.-Q. Circulating microRNAs as a fingerprint for endometrial endometrioid adenocarcinoma. PLoS ONE 2014, 9, e110767. [Google Scholar] [CrossRef]
- Jia, W.; Wu, Y.; Zhang, Q.; Gao, G.; Zhang, C.; Xiang, Y. Identification of four serum microRNAs from a genome-wide serum microRNA expression profile as potential non-invasive biomarkers for endometrioid endometrial cancer. Oncol. Lett. 2013, 6, 261–267. [Google Scholar] [CrossRef]
- Torres, A.; Torres, K.; Pesci, A.; Ceccaroni, M.; Paszkowski, T.; Cassandrini, P.; Zamboni, G.; Maciejewski, R. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients. Int. J. Cancer 2013, 132, 1633–1645. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Zwahlen, M.; Kitchener, H.C.; Egger, M.; Renehan, A.G. Body mass index, hormone replacement therapy, and endometrial cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 3119–3130. [Google Scholar] [CrossRef]
- Ryan, N.A.; Morris, J.; Green, K.; Lalloo, F.; Woodward, E.R.; Hill, J.; Crosbie, E.J.; Evans, D.G. Association of mismatch repair mutation with age at cancer onset in Lynch syndrome: Implications for stratified surveillance strategies. JAMA Oncol. 2017, 3, 1702–1706. [Google Scholar] [CrossRef]
- MacKintosh, M.L.; Derbyshire, A.E.; McVey, R.J.; Bolton, J.; Nickkho-Amiry, M.; Higgins, C.L.; Kamieniorz, M.; Pemberton, P.W.; Kirmani, B.H.; Ahmed, B.; et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int. J. Cancer 2019, 144, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Perez-Guaita, D.; Andrew, D.W.; Richards, J.S.; McNaughton, D.; Heraud, P.; Wood, B.R. Simultaneous ATR-FTIR Based Determination of Malaria Parasitemia, Glucose and Urea in Whole Blood Dried onto a Glass Slide. Anal. Chem. 2017, 89, 5238–5245. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Beckmann, M.W.; Schmidt, D.; Mallmann, P.; Uterus commission of the Gynecological Oncology Working Group. New WHO Classification of Endometrial Hyperplasias. Geburtshilfe Frauenheilkd 2015, 75, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.L.; Kelly, J.G.; Llabjani, V.; Martin-Hirsch, P.L.; Patel, I.I.; Trevisan, J.; Fullwood, N.J.; Walsh, M.J. Distinguishing cell types or populations based on the computational analysis of their infrared spectra. Nat. Protoc. 2010, 5, 1748–1760. [Google Scholar] [CrossRef]
| Patient Characteristics | Controls (n = 242) | Cancer (n = 342) | Atypical Hyperplasia (n = 68) | All (n = 652) | ||
|---|---|---|---|---|---|---|
| Type I (n = 258) | Type II (n = 64) | Mixed * (n = 20) | ||||
| Age, years | p-value: 0.268 | |||||
| Mean (SD) | 52 (11) | 63 (13) | 69 (10) | 69 (10) | 52 (15) | - |
| <60, n/N (%) | 194/242 (80) | 92/258 (36) | 10/64 (16) | 4/20 (20) | 45/68 (66) | 345/652 (53) |
| ≥60, n/N (%) | 48/242 (20) | 166/258 (64) | 54/64 (84) | 16/20 (80) | 23/68 (34) | 307/652 (47) |
| p-value | - | <0.0001 | <0.0001 | <0.0001 | 1.00 | - |
| BMI, n/N (%) | p-value: 0.412 | |||||
| Underweight (<18) | 0/242 (0) | 1/258 (0) | 2/64 (3) | 0/20 (0) | 0/68 (0) | 3/652 (<1) |
| Normal weight (18.5–24.9) | 33/242 (14) | 33/258 (13) | 17/64 (26.5) | 2/20 (10) | 1/68 (1) | 86/652 (13) |
| Overweight (25–29.9) | 42/242 (17) | 58/258 (22) | 17/64 (26.5 | 6/20 (30) | 5/68 (7) | 128/652 (20) |
| Obese (30–39.9) | 41/242 (17) | 95/258 (37) | 20/64 (31) | 8/20 (40) | 14/68 (21) | 178/652 (27) |
| Severely obese (>40) | 124/242 (51) | 71/258 (28) | 7/64 (11) | 4/20 (20) | 48/68 (71) | 254/652 (39) |
| Unknown | 2/242 (1) | 0/258 (0) | 1/64 (2) | 0/20 (0) | 0/68 (0) | 3/652 (<1) |
| p-value | - | 0.220 | 0.241 | 0.220 | 0.220 | - |
| Diabetes, n/N (%) | p-value: 0.268 | |||||
| Yes | 58/242 (24) | 47/258 (18) | 9/64 (14) | 4/20 (20) | 21/68 (31) | 139/652 (21) |
| No | 184/242 (76) | 210/258 (81) | 54/64 (84) | 15/20 (75) | 47/68 (69) | 510/652 (78) |
| Unknown | 0/242 (0) | 1/258 (<1) | 1/64 (2) | 1/20 (5) | 0/68 (0) | 3/652 (<1) |
| p-value | - | 0.157 | 0.157 | 0.157 | 0.157 | - |
| Blood pressure, n/N (%) | p-value: 0.268 | |||||
| Normotension | 128/242 (53) | 129/258 (50) | 38/64 (59) | 9/20 (45) | 30/68 (44) | 334/652 (51) |
| Hypertension | 74/242 (31) | 108/258 (42) | 14/64 (22) | 3/20 (15) | 36/68 (53) | 235/652 (36) |
| Unknown | 40/242 (16) | 21/258 (8) | 12/64 (19) | 8/20 (40) | 2/68 (3) | 83/652 (13) |
| P-value | - | 0.157 | 0.157 | 0.157 | 0.157 | - |
| Fasting status, n/N (%) | p-value: 0.345 | |||||
| Fasting | 36/242 (15) | 188/258 (73) | 36/64 (56) | 7/20 (35) | 43/68 (63) | 310/652 (47) |
| Non-fasting | 93/242 (38) | 38/258 (15) | 12/64 (19) | 3/20 (15) | 16/68 (24) | 162/652 (25) |
| Liver diet | 73/242 (30) | 3/258 (1) | 0/64 (0) | 0/20 (0) | 7/68 (10) | 83/652 (13) |
| Unknown | 40/242 (17) | 29/258 (11) | 16/64 (25) | 10/20 (50) | 2/68 (3) | 97/652 (15) |
| p-value | - | 0.199 | 0.199 | 0.199 | 0.199 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevaidi, M.; Morais, C.L.M.; Ashton, K.M.; Stringfellow, H.F.; McVey, R.J.; Ryan, N.A.J.; O’Flynn, H.; Sivalingam, V.N.; Kitson, S.J.; MacKintosh, M.L.; et al. Detecting Endometrial Cancer by Blood Spectroscopy: A Diagnostic Cross-Sectional Study. Cancers 2020, 12, 1256. https://doi.org/10.3390/cancers12051256
Paraskevaidi M, Morais CLM, Ashton KM, Stringfellow HF, McVey RJ, Ryan NAJ, O’Flynn H, Sivalingam VN, Kitson SJ, MacKintosh ML, et al. Detecting Endometrial Cancer by Blood Spectroscopy: A Diagnostic Cross-Sectional Study. Cancers. 2020; 12(5):1256. https://doi.org/10.3390/cancers12051256
Chicago/Turabian StyleParaskevaidi, Maria, Camilo L. M. Morais, Katherine M. Ashton, Helen F. Stringfellow, Rhona J. McVey, Neil A. J. Ryan, Helena O’Flynn, Vanitha N. Sivalingam, Sarah J. Kitson, Michelle L. MacKintosh, and et al. 2020. "Detecting Endometrial Cancer by Blood Spectroscopy: A Diagnostic Cross-Sectional Study" Cancers 12, no. 5: 1256. https://doi.org/10.3390/cancers12051256
APA StyleParaskevaidi, M., Morais, C. L. M., Ashton, K. M., Stringfellow, H. F., McVey, R. J., Ryan, N. A. J., O’Flynn, H., Sivalingam, V. N., Kitson, S. J., MacKintosh, M. L., Derbyshire, A. E., Pow, C., Raglan, O., Lima, K. M. G., Kyrgiou, M., Martin-Hirsch, P. L., Martin, F. L., & Crosbie, E. J. (2020). Detecting Endometrial Cancer by Blood Spectroscopy: A Diagnostic Cross-Sectional Study. Cancers, 12(5), 1256. https://doi.org/10.3390/cancers12051256








