Characteristics of MGUS and Multiple Myeloma According to the Target of Monoclonal Immunoglobulins, Glucosylsphingosine, or Epstein-Barr Virus EBNA-1
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Specificity of Recognition of Purified Monoclonal Igs
2.3. Characteristics of MGUS/SMM Patients with GlcSph-Reactive Igs
2.4. Characteristics of MM Patients with GlcSph-Reactive Igs
2.5. Sialylation Status of GlcSph-Reactive Igs from MGUS, SMM and MM Patients
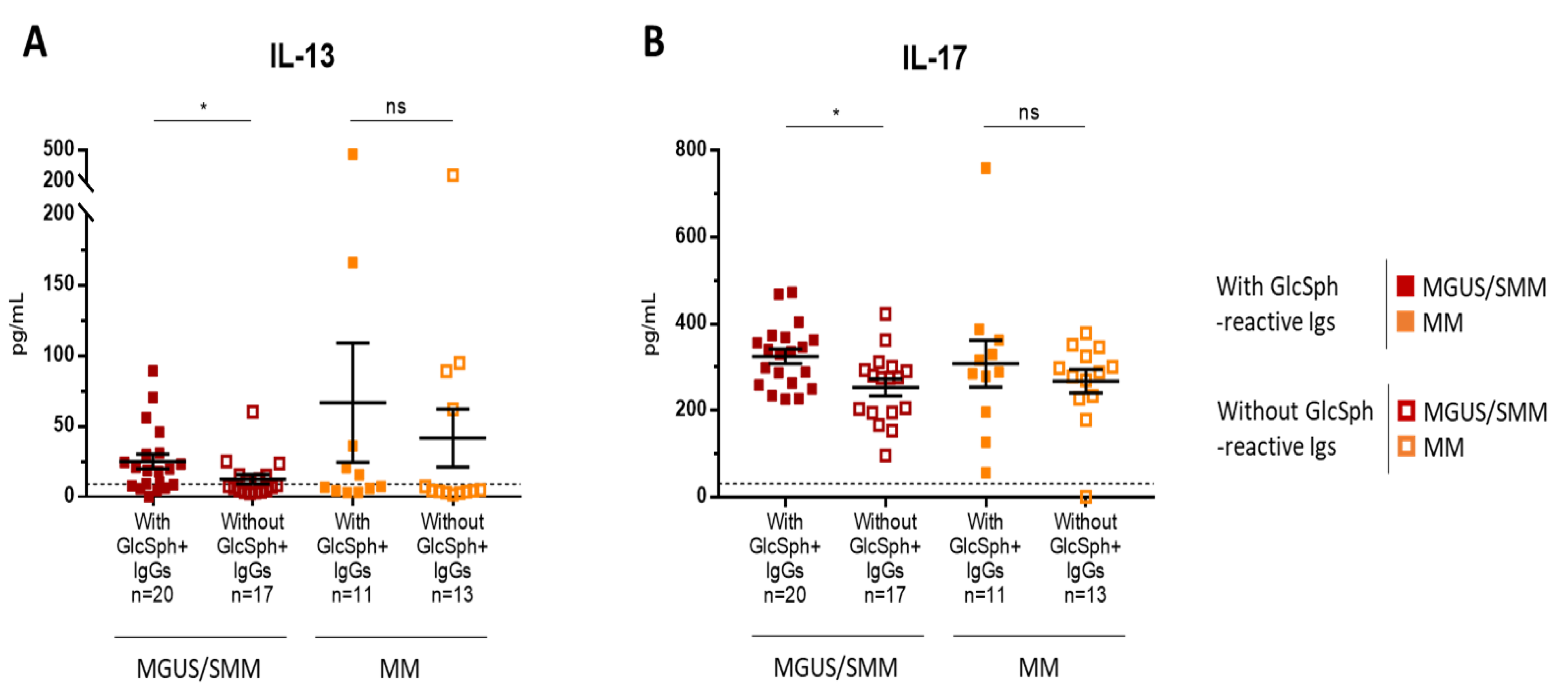
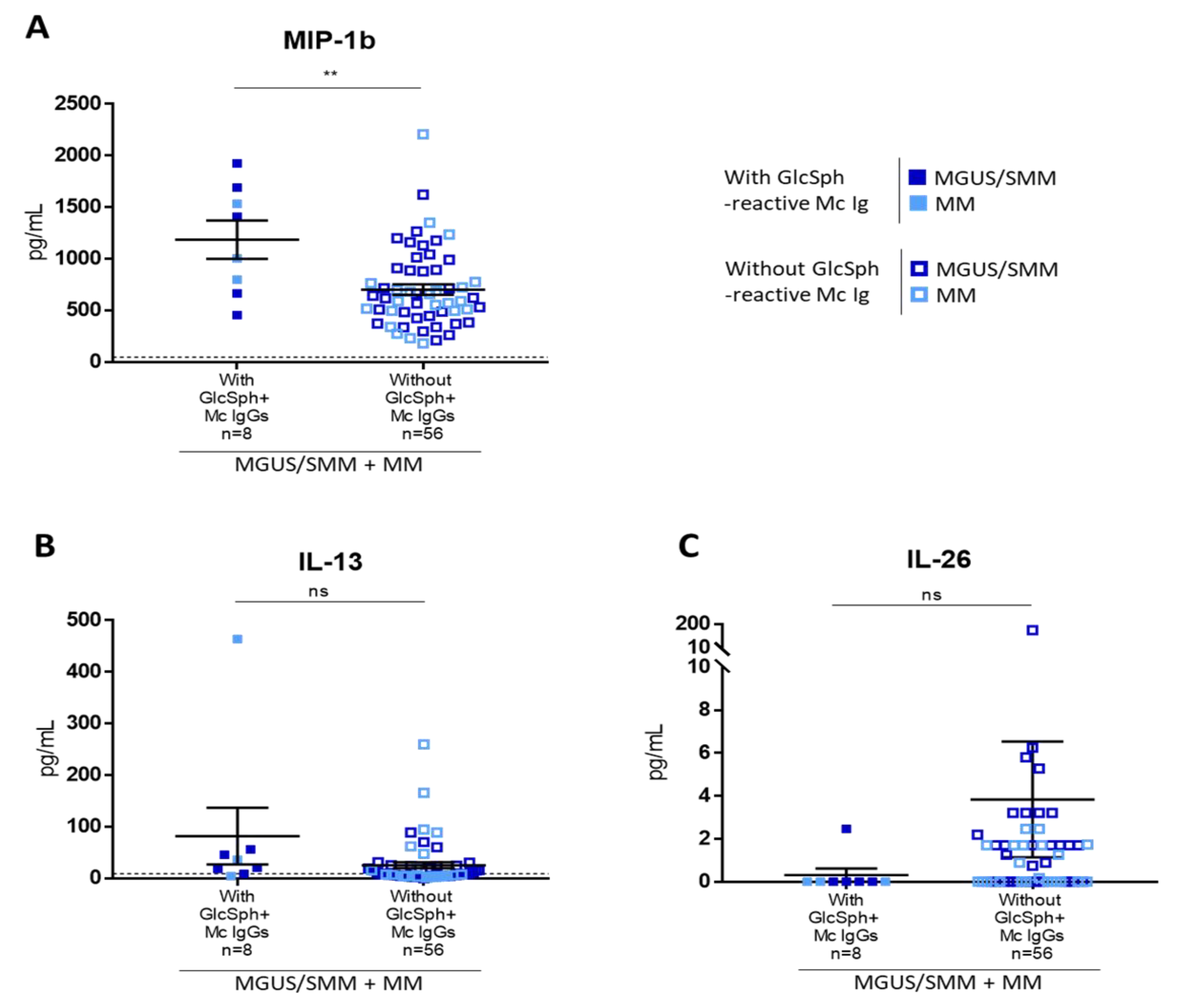
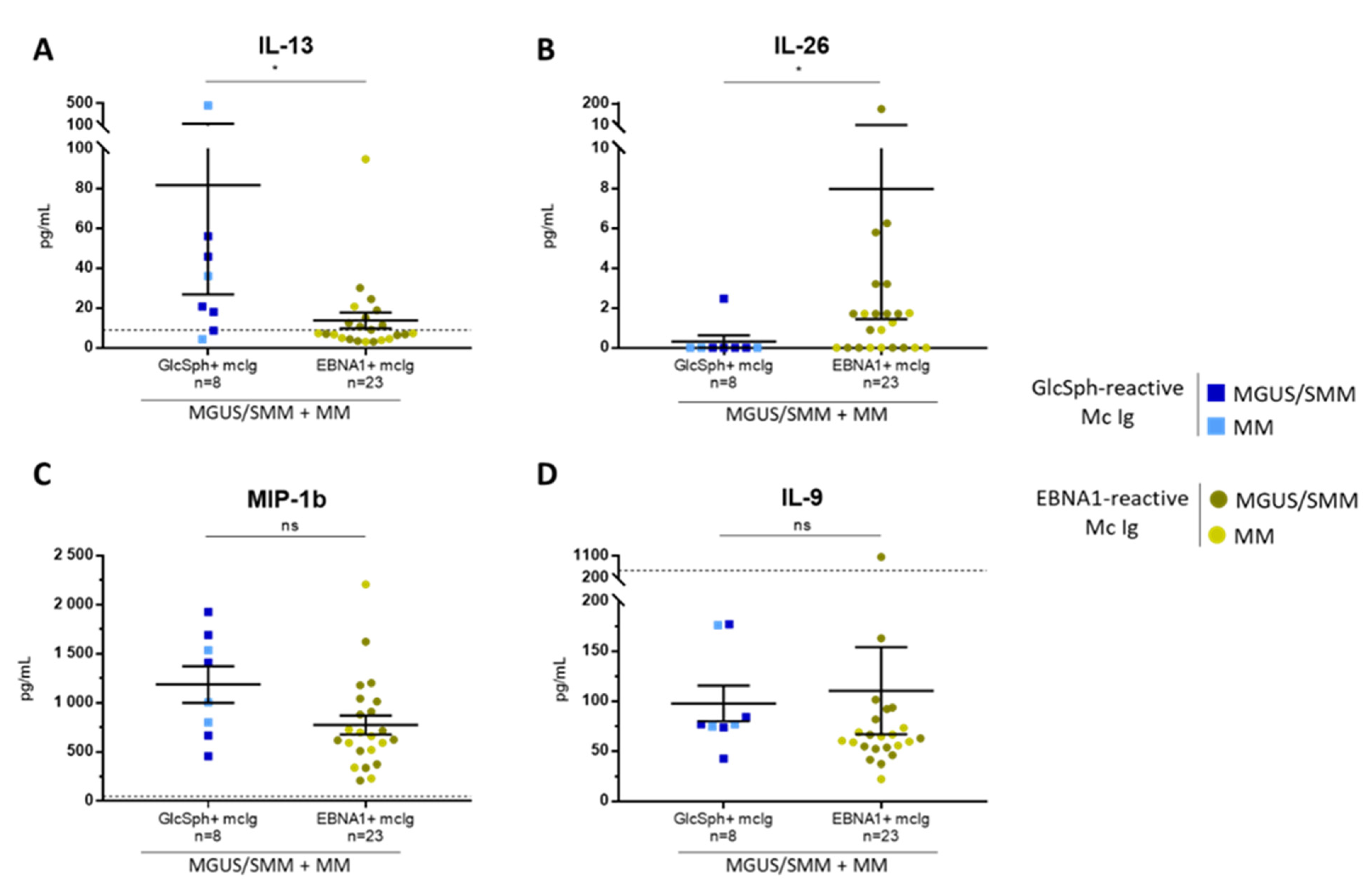
2.6. Inflammation Status of MGUS, SMM, and MM Patients with LGL1-Reactive Igs
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Separation of Monoclonal and Non-Clonal Igs
4.3. Analysis of the Specificity of Recognition of Monoclonal Igs
4.3.1. The GlcSph (LGL1) Assay
4.3.2. The MIAA Assay
4.4. Analysis of IgG sialylation
4.5. Quantification of Inflammation Cytokines
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhodapkar, M.V. MGUS to myeloma: A mysterious gammopathy of underexplored significance. Blood 2016, 128, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.M.; Davies, F.E.; Leleu, X.; Morgan, G.J. Understanding the multiple biological aspects leading to myeloma. Haematologica 2014, 99, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Bosseboeuf, A.; Feron, D.; Tallet, A.; Rossi, C.; Charlier, C.; Garderet, L.; Caillot, D.; Moreau, P.; Cardo-Vila, M.; Pasqualini, R.; et al. Monoclonal IgG in MGUS and multiple myeloma target infectious pathogens. J. Clin. Investig. Insight 2017, 2, 95367. [Google Scholar] [CrossRef]
- Hermouet, S.; Corre, I.; Gassin, M.; Bigot-Corbel, E.; Sutton, C.A.; Casey, J.W. Hepatitis C virus, human herpesvirus 8, and the development of plasma-cell leukemia. N. Engl. J. Med. 2003, 348, 178–179. [Google Scholar] [CrossRef]
- Bigot-Corbel, E.; Gassin, M.; Corre, I.; Le Carrer, D.; Delaroche, O.; Hermouet, S. Hepatitis C virus (HCV) infection, monoclonal immunoglobulin specific for HCV core protein, and plasma-cell malignancy. Blood 2008, 112, 4357–4358. [Google Scholar] [CrossRef][Green Version]
- Nair, S.; Branagan, A.R.; Liu, J.; Boddupalli, C.S.; Mistry, P.K.; Dhodapkar, M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016, 374, 555–561. [Google Scholar] [CrossRef]
- Nair, S.; Sng, J.; Sekhar Boddupalli, C.; Seckinger, A.; Chesi, M.; Fulciniti, M.; Zhang, L.; Rauniyar, N.; Lopez, M.; Neparidze, N.; et al. Antigen-mediated regulation in monoclonal gammopathies and myeloma. J. Clin. Investig. Insight 2018, 3, e98259. [Google Scholar] [CrossRef]
- Cox, T.M.; Rosenbloom, B.E.; Barker, E.A. Gaucher disease and co-morbidities: B-cell malignancies and parkinsonism. Am. J. Hematol. 2015, 90, S25–S28. [Google Scholar] [CrossRef]
- Nair, S.; Bar, N.; Xu, M.L.; Dhodapkar, M.; Mistry, P.K. Glucosylsphingosine but not Saposin C, is the target antigen in Gaucher disease-associated gammopathy. Mol. Genet. Metab. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Panfilio, S.; D’Urso, P.; Annechini, G.; D’Elia, G.M.; De Angelis, F.; Stefanizzi, C.; Pulsoni, A. Regression of a case of Multiple Myeloma with antiviral treatment in a patient with chronic HCV infection. Leuk Res. Rep. 2013, 2, 39–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez García, A.; Linares, M.; Mennesson, N.; Sanchez-Vega, B.; Sanchez, R.; Alonso Fernandez, R.; Bigot-Corbel, E.; Hermouet, S.; Martinez Lopez, J. The role of Hepatitis C virus in the development of multiple myeloma: A case study. In Proceedings of the 60th Annual Meeting of the American Society of Hematology (ASH), San Diego, CA, USA, 1–4 December 2018. Abstract Nbr: 5592, Submission ID: 112842. [Google Scholar]
- Feron, D.; Charlier, C.; Gourain, V.; Garderet, L.; Coste-Burel, M.; Le Pape, P.; Weigel, P.; Jacques, Y.; Hermouet, S.; Bigot-Corbel, E. Multiplexed infectious protein microarray immunoassay suitable for the study of the specificity of monoclonal immunoglobulins. Anal. Biochem. 2013, 433, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Bosseboeuf, A.; Allain, S.; Mennesson, N.; Tallet, A.; Rossi, C.; Garderet, L.; Caillot, D.; Moreau, P.; Piver, E.; Girodon, F.; et al. Pro-inflammatory state in MGUS and Myeloma is characterized by low sialylation of pathogen-specific and other monoclonal and polyclonal immunoglobulin G. Front. Immunol. 2017, 8, 1347, eCollection 2017. [Google Scholar] [CrossRef]
- Preuss, K.D.; Hollak, C.E.M.; Fadle, N.; van Oers, M.; Regitz, E.; Pfreundschuh, M. Saposin C is a frequent target of paraproteins in Gaucher disease-associated MGUS/multiple myeloma. Br. J. Haematol. 2019, 184, 384–391. [Google Scholar] [CrossRef]
- Zheng, M.M.; Zhang, Z.; Bemis, K.; Belch, A.R.; Pilarski, L.M.; Shively, J.E.; Kirshner, J. The Systemic Cytokine Environment Is Permanently Altered in Multiple Myeloma. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Jurczyszyn, A.; Czepiel, J.; Biesiada, G.; Gdula-Argasińska, J.; Cibor, D.; Owczarek, D.; Perucki, W.; Skotnicki, A.B. HGF, sIL-6R and TGF-β1 Play a Significant Role in the Progression of Multiple Myeloma. J. Cancer 2014, 5, 518–524. [Google Scholar] [CrossRef]
- Wang, X.S.; Shi, Q.; Shah, N.D.; Heijnen, C.J.; Cohen, E.N.; Reuben, J.M.; Orlowski, R.Z.; Qazilbash, M.H.; Johnson, V.E.; Mendoza, T.R.; et al. Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin. Cancer Res. 2014, 20, 1366–1374. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ghobrial, I.M.; Roodman, G.D. Chemokines in multiple myeloma. Exp. Hematol. 2006, 34, 1289–1295. [Google Scholar] [CrossRef]
- Hashimoto, T.; Abe, M.; Oshima, T.; Shibata, H.; Ozaki, S.; Inoue, D.; Matsumoto, T. Ability of myeloma cells to secrete macrophage inflammatory protein (MIP)-1α and MIP-1β correlates with lytic bone lesions in patients with multiple myeloma. Br. J. Haematol. 2004, 125, 38–41. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Haugg, B.; et al. Regulation of autoantibody activity by the IL-23–TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 2016, 18, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Corvaisier, M.; Delneste, Y.; Jeanvoine, H.; Preisser, L.; Blanchard, S.; Garo, E.; Hoppe, E.; Barré, B.; Audran, M.; Bouvard, B.; et al. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 2012, 10, e1001395. [Google Scholar] [CrossRef]
- Ciccia, F.; Guggino, G.; Ferrante, A.; Cipriani, P.; Giacomelli, R.; Triolo, G. Interleukin-9 and T helper type 9 cells in rheumatic diseases. Clin. Exp. Immunol. 2016, 185, 125–132. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Chang, C.; Lu, L.; Lau, C.S.; Lu, Q. Th9 Cells and IL-9 in Autoimmune Disorders: Pathogenesis and Therapeutic Potential. Hum. Immunol. 2017, 78, 120–128. [Google Scholar] [CrossRef]
- Nagata, M.; Izumi, Y.; Ishikawa, E.; Kiyotake, R.; Doi, R.; Iwai, S.; Omahdi, Z.; Yamaji, T.; Miyamoto, T.; Bamba, T.; et al. Intracellular metabolite beta-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc. Natl. Acad. Sci. USA 2017, 114, E3285–E3294. [Google Scholar] [CrossRef]
- Nair, S.; Boddupalli, C.S.; Verma, R.; Liu, J.; Yang, R.; Pastores, G.M.; Mistry, P.K.; Dhodapkar, M.V. Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation. Blood 2015, 125, 1256–1271. [Google Scholar] [CrossRef]
- Pandey, M.K.; Burrow, T.A.; Rani, R.; Martin, L.J.; Witte, D.; Setchell, K.D.; McKay, M.A.; Magnusen, A.F.; Zhang, W.; Liou, B.; et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature 2017, 543, 108–112. [Google Scholar] [CrossRef]
- Kumar, S.; Larson, D.R.; Dispenzieri, A.; Therneau, T.M.; Murray, D.L.; Bergsagel, P.L.; Kyle, R.A.; Rajkumar, S.V. Polyclonal serum free light chain elevation is associated with increased risk of monoclonal gammopathies. Blood Cancer J. 2019, 9, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.J.; Rasche, L. Maintaining therapeutic progress in multiple myeloma by integrating genetic and biological advances into the clinic. Expert Rev. Hematol. 2018, 11, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, E.V.; Archer, J.; Wang, S.; Dekker, N.; Aerts, J.M.; Karlsson, S.; Cox, T.M. Inhibition of UDP-glucosylceramide synthase in mice prevents Gaucher disease-associated malignancy. J. Pathol. 2015, 235, 113–124. [Google Scholar] [CrossRef]
- Nooij, F.J.; Van der Sluijs-Gelling, A.J.; Jol-Van der Zijde, C.M.; Van Tol, M.J.; Haas, H.; Radl, J. Immunoblotting techniques for the detection of low level homogeneous immunoglobulin components in serum. J. Immunol. Methods 1990, 134, 273–281. [Google Scholar] [CrossRef]
- Braun, W.; Abraham, R. Modified diffusion blotting for rapid and efficient protein transfer with PhastSystem. Electrophoresis 1989, 10, 249–253. [Google Scholar] [CrossRef]
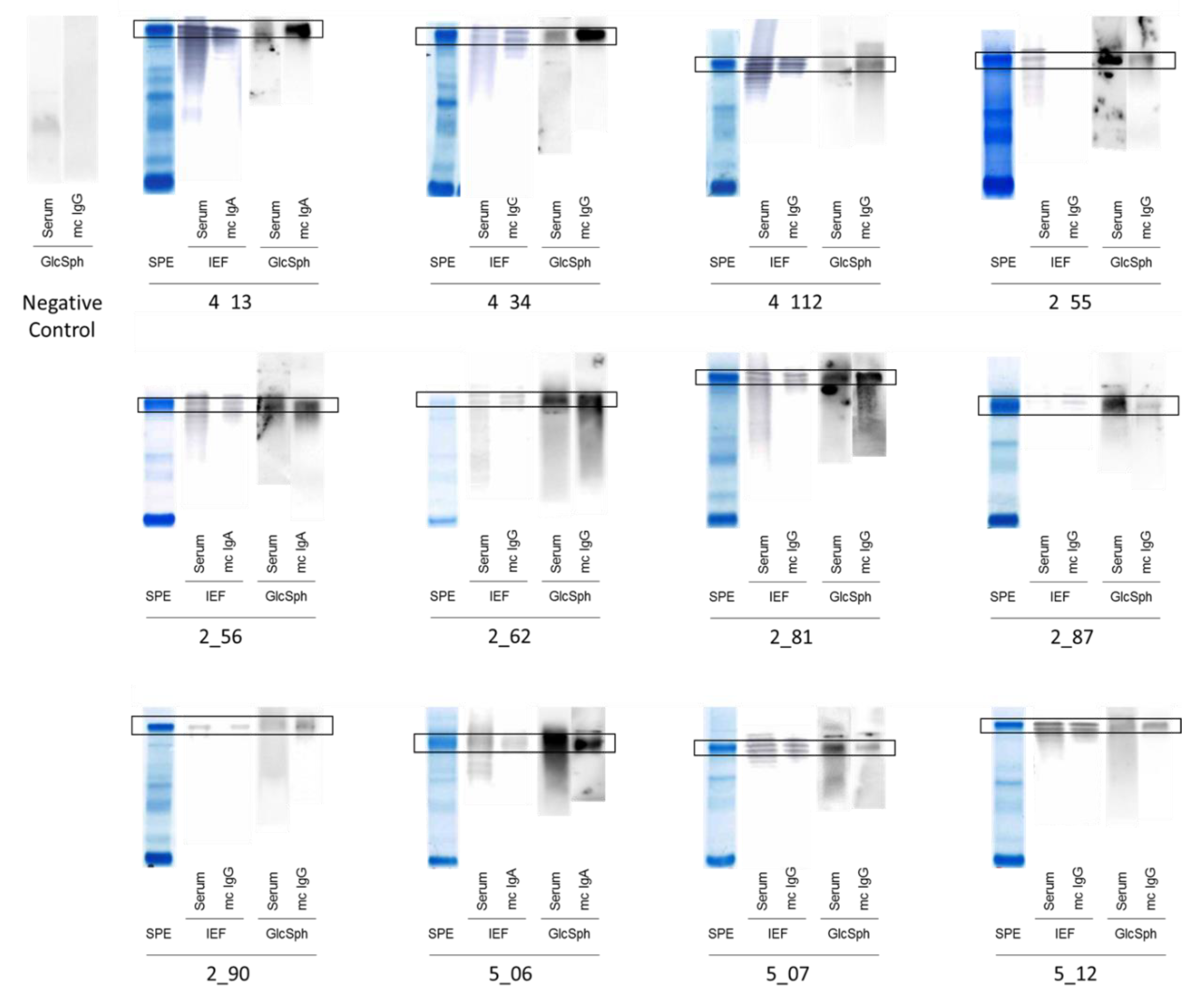
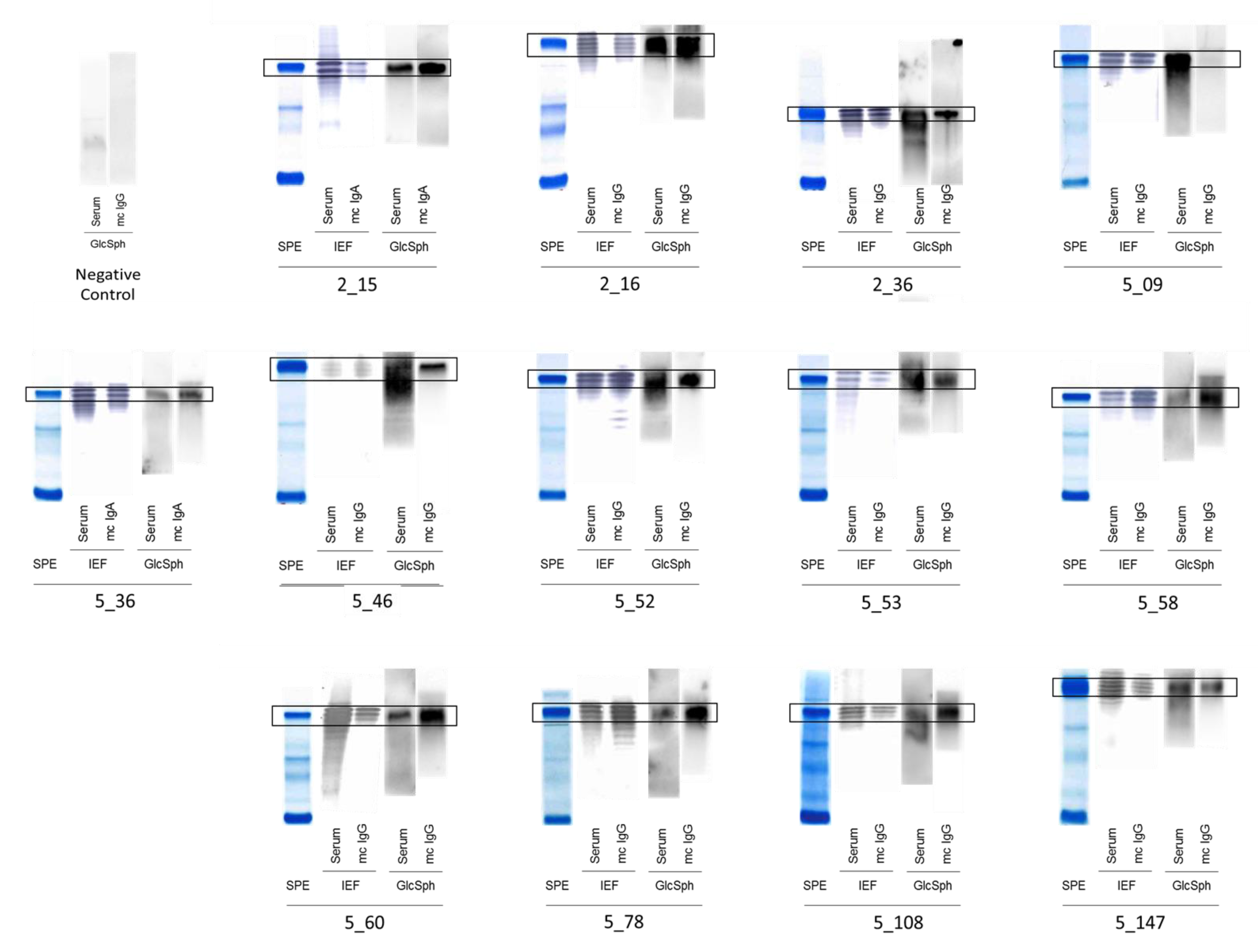
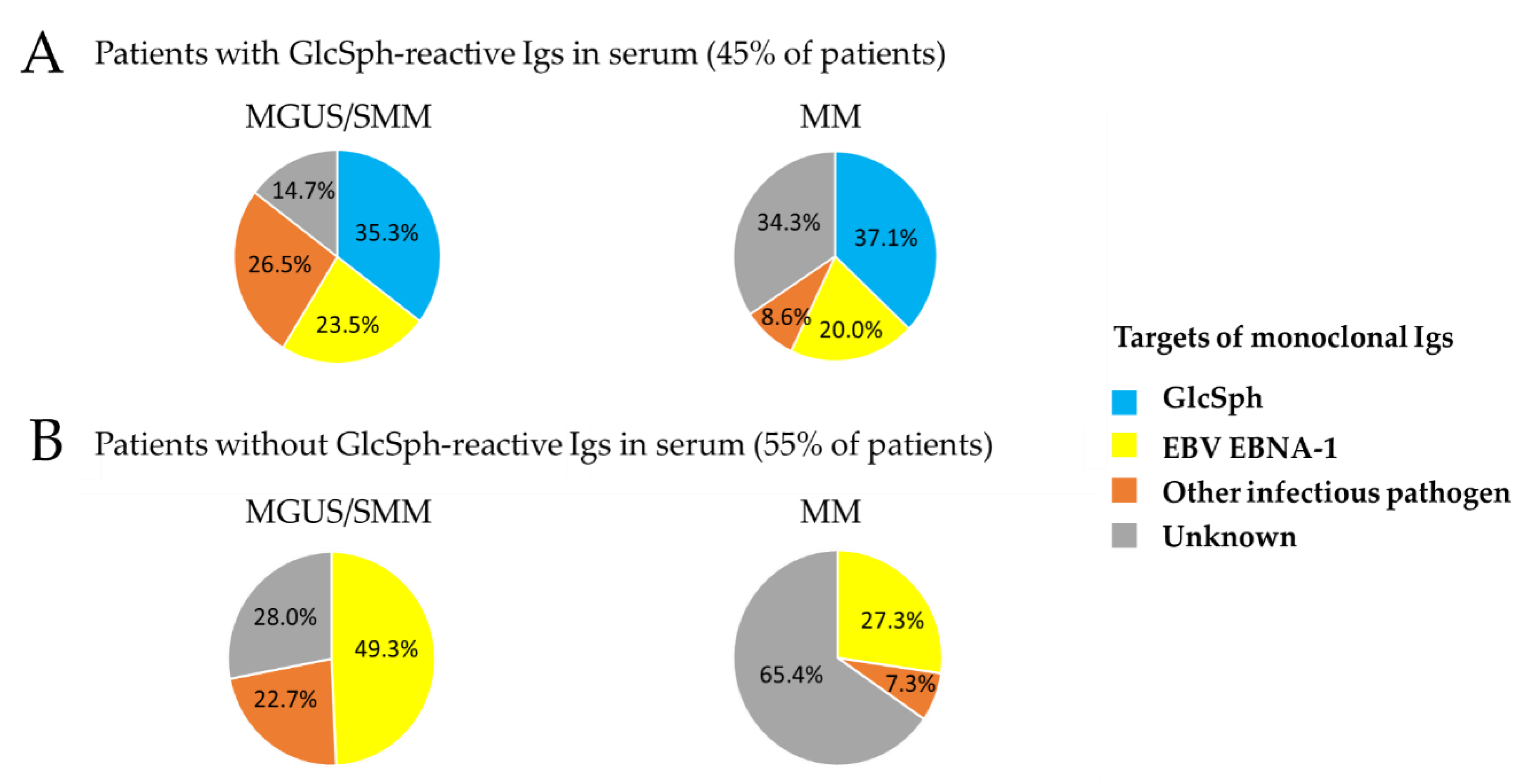
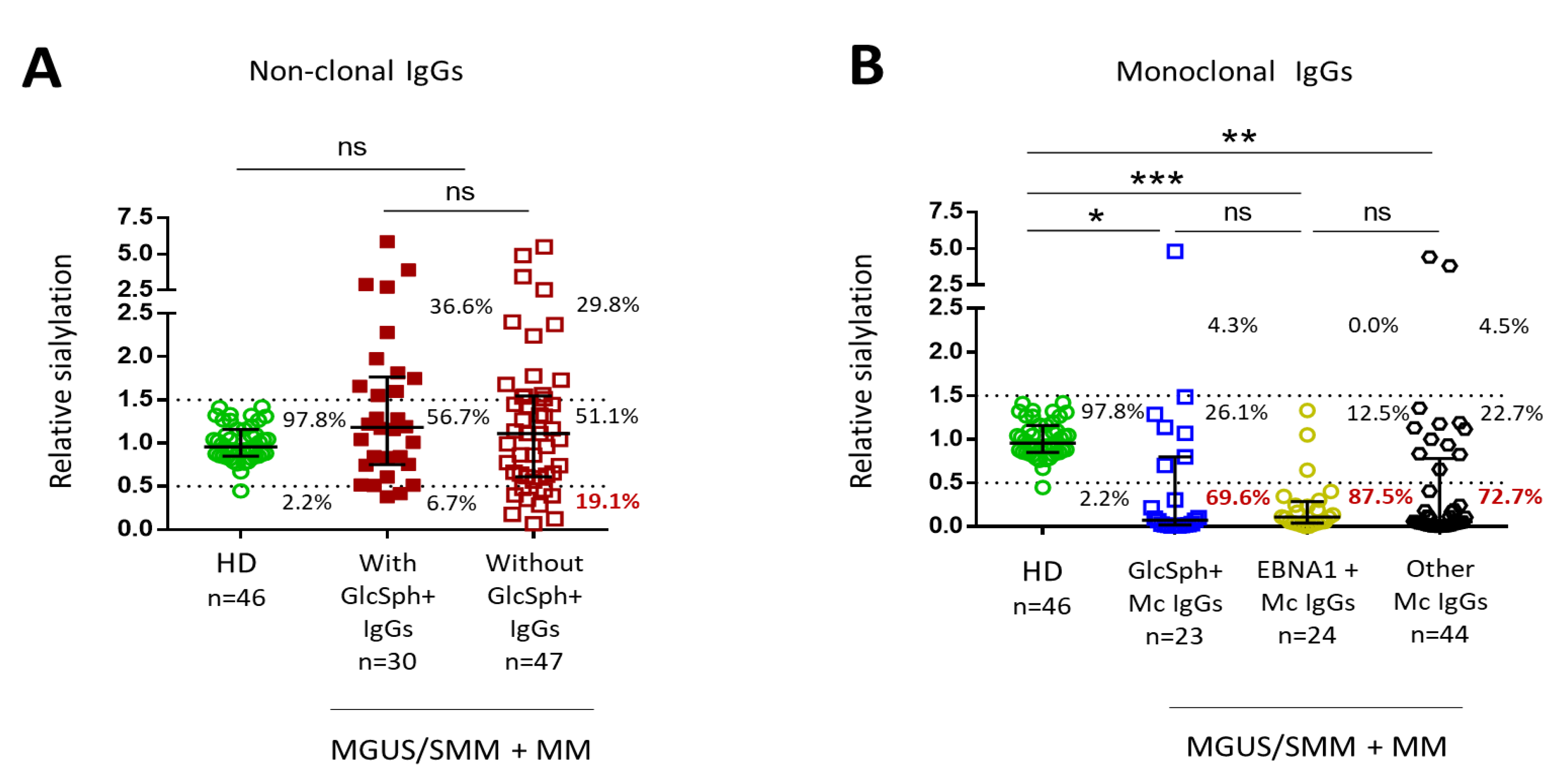
| Serum Ig Reactivity | Healthy Donors (n = 41) * | MGUS/SMM (n = 143) * | MM (n = 90) | p Value, MM vs. MGUS/SMM ** |
|---|---|---|---|---|
| Serum with GlcSph-reactive immunoglobulins (Igs) | 2 (4.9%) | 68/138 (49.3%) 1 | 35/90 (38.9%) 1 | p = 0.223 |
| Reactivity of purified Mc Ig | ||||
| GlcSph | - | 24 (16.8%) | 13 (14.4%) | NS |
| Epstein-Barr virus (EBV) nuclear antigen-1 (EBNA-1) | - | 53 (37.1%) | 22 (24.4%) | p = 0.0608 |
| Other pathogen of the multiplexed infectious antigen microarray (MIAA) | - | 35 (24.5%) | 7 (7.8%) | p = 0.0014 |
| Unknown | 31 (21.7%) | 48 (53.3%) | p < 0.00001 |
| Patients * | Without GlcSph-Reactive Igs | With Non-Clonal GlcSph-Reactive Igs * | p Value |
|---|---|---|---|
| MGUS/SMM | n = 75 | n = 44 | – |
| EBV (EBNA-1) | 37 (49.3%) 1 | 16 (36.4%) | NS |
| Other pathogen of the MIAA | 17 (22.7%) 2 | 18 (40.9%) 4 | p = 0.0398 6 |
| Unknown | 21 (28.0%) 3 | 10 (22.7%) 5 | NS |
| MM | n = 55 | n = 22 | – |
| EBV (EBNA-1) | 15 (27.3%) | 7 (31.8%) | NS |
| Other pathogen of the MIAA | 4 (7.3%) | 3 (13.6%) | NS |
| Unknown | 36 (65.4%) | 12 (54.5%) | NS |
| Characteristics of MM Patients | No GlcSph-Reactive Ig (n = 55) | With GlcSph-Reactive Igs (n = 35) | Analysis According to Mc Ig Specificity | ||
|---|---|---|---|---|---|
| GlcSph (n = 13) | EBV EBNA-1 (n = 22) | Unknown (n = 45) | |||
| Sex | |||||
| Nbr | 55 | 34 | 13 | 22 | 45 |
| M/F (male %) | 27/28 (49.1%) | 23/12 (65.7%) | 7/6 (53.8%) | 12/10 (54.5%) | 24/21 (53.3%) |
| Age at diagnosis (yrs) | |||||
| Nbr | 53 | 35 | 13 | 22 | 41 |
| Median | 64.0 | 69.7 | 61.0 | 61.1 | 64.5 |
| Range (Min-Max) | 41–90 | 43–90 | 54–80 | 46–87 | 41–90 |
| Amount of Mc Ig (g/L) | |||||
| Nbr | 55 | 33 | 13 | 22 | 42 |
| Median | 20.0 | 27.0 | 24.0 | 27.0 | 22.9 |
| Range (Min-Max) | 8.5–68 | 4.0–68 | 11–68 | 11–59 | 4–55 |
| BM plasma cells (%) | |||||
| Nbr | 41 | 27 | 10 | 18 | 33 |
| Median | 16.0 | 26.5 | 35.5 | 32.5 | 16.0 |
| Range (Min-Max) | 1–93 | 1–98 | 11–65 | 2–98 | 1–89 |
| β2-microglobulin (mg/L) | |||||
| Nbr | 29 | 19 | 9 | 22 | 21 |
| Median | 3.1 | 3.9 | 2.5 | 4.1 | 3.1 |
| Range (Min-Max) | 1.3–14.0 | 1.9–12.1 | 1.9–12.1 | 1.3–16.0 | 1.5–14.0 |
| Nbr > 3.5 mg/L | 11 (37.9%) | 10 (55%) | 4 (44.4%) | 13 (59.1%) | 8 (38.1%) |
| Bone lesions | |||||
| Nbr | 52 | 34 | 13 | 22 | 43 |
| Nbr with lesions (%) | 38 (73.1%) | 21 (61.8%) | 7 (53.8%) 1 | 16 (72.7%) | 30 (69.7%) |
| ISS Stage | |||||
| Nbr | 30 | 20 | 8 | 16 | 19 |
| Stage I | 16 (53.3%) | 10 (50%) | 5 (62.5%) | 6 (37.4%) | 11 (57.9%) |
| Stage II | 6 (20.0%) | 4 (20%) | 2 (25.0%) | 3 (18.8%) | 5 (26.3%) |
| Stage III (%) | 8 (26.7%) | 6 (30%) | 1 (12.5%) | 7 (43.8%) | 3 (15.8%) |
| DSS Stage | |||||
| Nbr | 45 | 28 | 13 | 22 | 34 |
| Stage I | 11 (24.4%) | 12 (42.9%) | 7 (53.8%) | 6 (27.3%) | 10 (29.4%) |
| Stage II | 12 (26.7%) | 6 (21.4%) | 4 (30.8%) | 3 (13.6) | 12 (35.3) |
| Stage III (%) | 22 (48.9%) | 10 (35.7%) | 2 (15.4%) 2 | 13 (59.1%) | 12 (35.3%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosseboeuf, A.; Mennesson, N.; Allain-Maillet, S.; Tallet, A.; Piver, E.; Decaux, O.; Moreau, C.; Moreau, P.; Lehours, P.; Mégraud, F.; et al. Characteristics of MGUS and Multiple Myeloma According to the Target of Monoclonal Immunoglobulins, Glucosylsphingosine, or Epstein-Barr Virus EBNA-1. Cancers 2020, 12, 1254. https://doi.org/10.3390/cancers12051254
Bosseboeuf A, Mennesson N, Allain-Maillet S, Tallet A, Piver E, Decaux O, Moreau C, Moreau P, Lehours P, Mégraud F, et al. Characteristics of MGUS and Multiple Myeloma According to the Target of Monoclonal Immunoglobulins, Glucosylsphingosine, or Epstein-Barr Virus EBNA-1. Cancers. 2020; 12(5):1254. https://doi.org/10.3390/cancers12051254
Chicago/Turabian StyleBosseboeuf, Adrien, Nicolas Mennesson, Sophie Allain-Maillet, Anne Tallet, Eric Piver, Olivier Decaux, Caroline Moreau, Philippe Moreau, Philippe Lehours, Francis Mégraud, and et al. 2020. "Characteristics of MGUS and Multiple Myeloma According to the Target of Monoclonal Immunoglobulins, Glucosylsphingosine, or Epstein-Barr Virus EBNA-1" Cancers 12, no. 5: 1254. https://doi.org/10.3390/cancers12051254
APA StyleBosseboeuf, A., Mennesson, N., Allain-Maillet, S., Tallet, A., Piver, E., Decaux, O., Moreau, C., Moreau, P., Lehours, P., Mégraud, F., Salle, V., Bigot-Corbel, E., Harb, J., & Hermouet, S. (2020). Characteristics of MGUS and Multiple Myeloma According to the Target of Monoclonal Immunoglobulins, Glucosylsphingosine, or Epstein-Barr Virus EBNA-1. Cancers, 12(5), 1254. https://doi.org/10.3390/cancers12051254








