Beyond MicroRNAs: Emerging Role of Other Non-Coding RNAs in HPV-Driven Cancers
Abstract
:1. Introduction
2. HPV-Driven Cancerogenesis
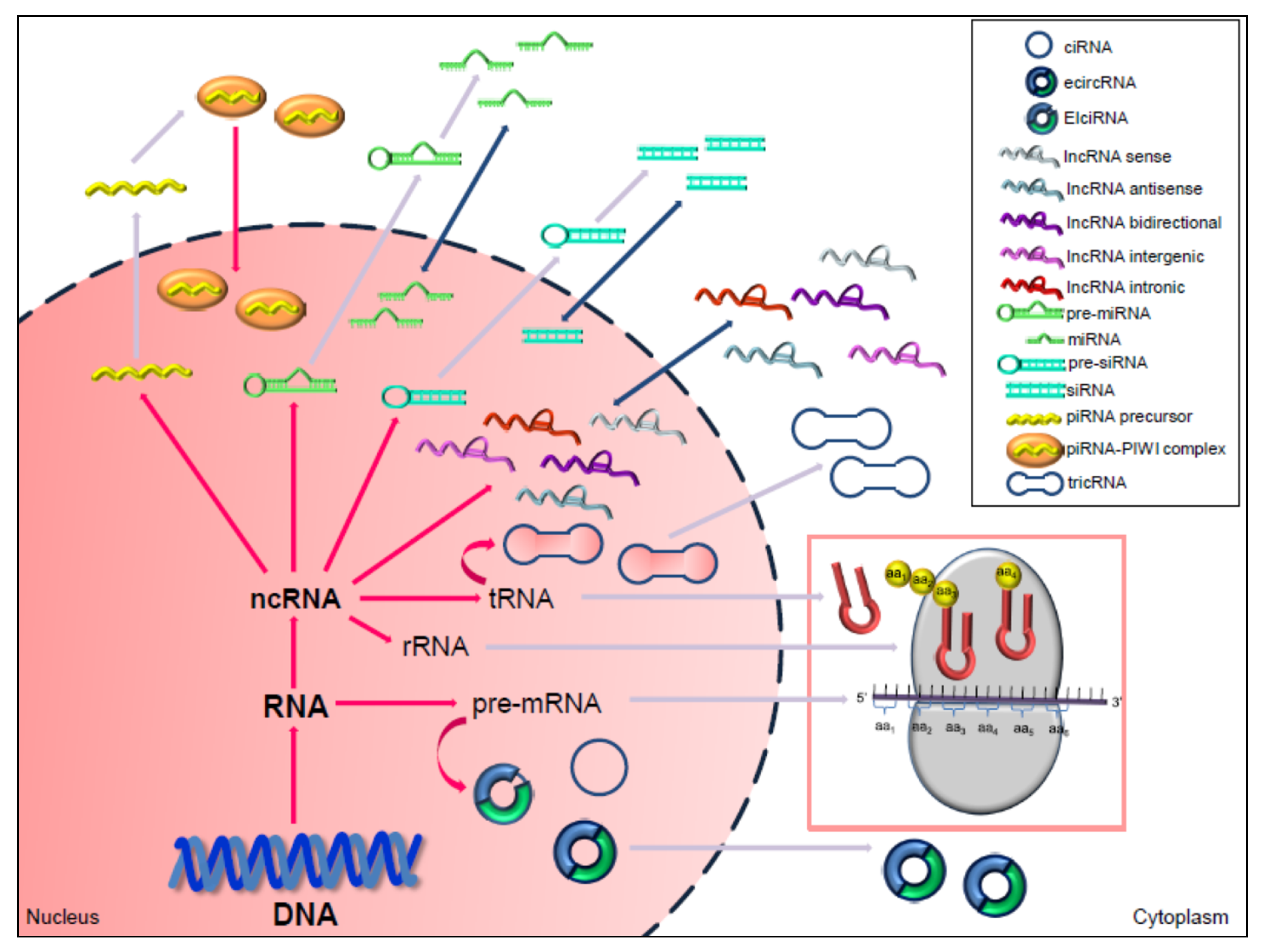
3. Non-Coding RNAs
4. CircRNAs Expression in HPV-Driven Cancers
CSCC
5. PiRNAs and PIWI-Like Proteins Expression in HPV-Driven Cancers
5.1. CSCC
5.2. HNSCC
5.3. SCCA
6. LncRNAs Expression in HPV-Driven Cancers
6.1. CSCC
6.2. HNSCC
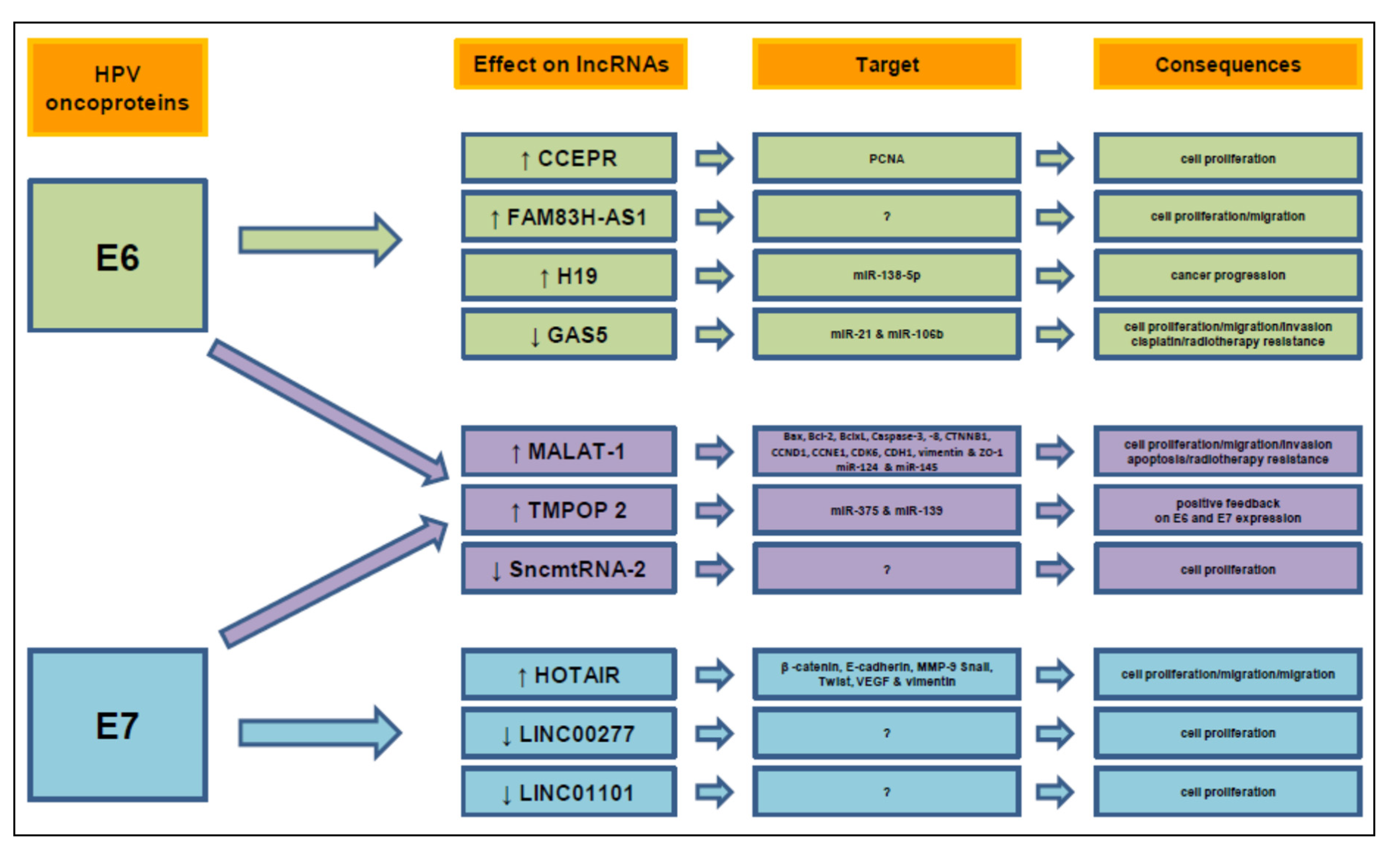
7. NcRNAs Modulated by E6/E7 Oncoproteins
8. HR-HPV-Derived NcRNAs
9. NcRNAs as Potential Diagnostic Biomarkers in HPV-Driven Cancers
10. Therapeutic Targeting of NcRNAs
11. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| LncRNAs ID | HPV-Driven Cancer Type | References |
| CCEPR | CSCC | [133,134,135] |
| GAS5 | CSCC | [139,140,141,142] |
| HOTAIR | CSCC | [144,145,147] |
| MALAT-1 | CSCC | [150,151,152,153,154] |
| MEG3 | CSCC | [94,95,96] |
| PIWI-Like Proteins ID | HPV-Driven Cancer Type | References |
| PIWIL4 | CSCC, HNSCC | [83,86] |
References
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tommasino, M. The human papillomavirus family and its role in carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens--Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Bandolin, L.; Borsetto, D.; Fussey, J.; Da Mosto, M.C.; Nicolai, P.; Menegaldo, A.; Calabrese, L.; Tommasino, M.; Boscolo-Rizzo, P. Beta human papillomaviruses infection and skin carcinogenesis. Rev. Med. Virol. 2020, e2104. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, G.; Anantharaman, D.; Gheit, T.; Abedi-Ardekani, B.; Beachler, D.C.; Conway, D.I.; Olshan, A.F.; Wunsch-Filho, V.; Toporcov, T.N.; Ahrens, W.; et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: A comparison of 1362 cases across three continents. Oral Oncol. 2016, 62, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Castellsagué, X.; Alemany, L.; Quer, M.; Halec, G.; Quirós, B.; Tous, S.; Clavero, O.; Alòs, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjosé, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef]
- Jhaveri, J.; Rayfield, L.; Liu, Y.; Chowdhary, M.; Cassidy, R.J.; Madden, N.A.; Tanenbaum, D.G.; Gillespie, T.W.; Patel, P.R.; Patel, K.R.; et al. Prognostic relevance of human papillomavirus infection in anal squamous cell carcinoma: Analysis of the national cancer data base. J. Gastrointest. Oncol. 2017, 8, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Kidd, L.C.; Chaing, S.; Chipollini, J.; Giuliano, A.R.; Spiess, P.E.; Sharma, P. Relationship between human papillomavirus and penile cancer-implications for prevention and treatment. Transl. Androl. Urol. 2017, 6, 791–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backes, D.M.; Kurman, R.J.; Pimenta, J.M.; Smith, J.S. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 2009, 20, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wilkinson, E.J.; Unger, E.R.; Steinau, M.; Watson, M.; Huang, Y.; Copeland, G.; Cozen, W.; Goodman, M.T.; Hopenhayn, C.; et al. Prevalence of human papillomavirus types in invasive vulvar cancers and vulvar intraepithelial neoplasia 3 in the United States before vaccine introduction. J. Low. Genit. Tract Dis. 2012, 16, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, Y.; Zhang, Z. Prevalence of human papillomavirus and its prognostic value in vulvar cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0204162. [Google Scholar] [CrossRef] [PubMed]
- Insinga, R.P.; Liaw, K.L.; Johnson, L.G.; Madeleine, M.M. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1611–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar] [CrossRef]
- Colombo, N.; Carinelli, S.; Colombo, A.; Marini, C.; Rollo, D.; Sessa, C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, vii27–vii32. [Google Scholar] [CrossRef]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, H.K.; Li, Y.; Hafner, M.; Banerjee, N.S.; Tang, S.; Briskin, D.; Meyers, C.; Chow, L.T.; Xie, X.; et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc. Natl. Acad. Sci. USA 2014, 111, 4262–4267. [Google Scholar] [CrossRef] [Green Version]
- zur Hausen, H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 2000, 92, 690–698. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin-Drubin, M.E.; Munger, K. The human papillomavirus E7 oncoprotein. Virology 2009, 384, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howie, H.L.; Katzenellenbogen, R.A.; Galloway, D.A. Papillomavirus E6 proteins. Virology 2009, 384, 324–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajer, C.B.; Garnæs, E.; Friis-Hansen, L.; Norrild, B.; Therkildsen, M.H.; Glud, M.; Rossing, M.; Lajer, H.; Svane, D.; Skotte, L.; et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: Bridging between HPV-related head and neck cancer and cervical cancer. Br. J. Cancer 2012, 106, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.X.; D’Souza, G.; Westra, W.H.; Wang, S.J.; van Zante, A.; Zhang, Y.; Rettig, E.M.; Ryan, W.R.; Ha, P.K.; Wentz, A.; et al. Prognostic factors for human papillomavirus-positive and negative oropharyngeal carcinomas. Laryngoscope 2018, 128, E287–E295. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Tribius, S. HPV and Oropharyngeal Cancer in the Eighth Edition of the TNM Classification: Pitfalls in Practice. Transl. Oncol. 2019, 12, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Carlander, A.F.; Gronhoj Larsen, C.; Jensen, D.H.; Garnaes, E.; Kiss, K.; Andersen, L.; Olsen, C.H.; Franzmann, M.; Hogdall, E.; Kjaer, S.K.; et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: A Danish population-based study from 2011 to 2014. Eur. J. Cancer 2017, 70, 75–82. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Viscidi, R.; Torres, B.N.; Ingles, D.J.; Sudenga, S.L.; Villa, L.L.; Baggio, M.L.; Abrahamsen, M.; Quiterio, M.; Salmeron, J.; et al. Seroconversion Following Anal and Genital HPV Infection in Men: The HIM Study. Papillomavirus Res. 2015, 1, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliano, A.R.; Nyitray, A.G.; Kreimer, A.R.; Pierce Campbell, C.M.; Goodman, M.T.; Sudenga, S.L.; Monsonego, J.; Franceschi, S. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int. J. Cancer 2015, 136, 2752–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felice, F.; Tombolini, V.; Valentini, V.; de Vincentiis, M.; Mezi, S.; Brugnoletti, O.; Polimeni, A. Advances in the Management of HPV-Related Oropharyngeal Cancer. J. Oncol. 2019, 2019, 9173729. [Google Scholar] [CrossRef]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): An open-label, phase 2, randomised trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Chera, B.S.; Amdur, R.J.; Green, R.; Shen, C.; Gupta, G.; Tan, X.; Knowles, M.; Fried, D.; Hayes, N.; Weiss, J.; et al. Phase II Trial of De-Intensified Chemoradiotherapy for Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2019, 37, 2661–2669. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Marur, S.; Li, S.; Cmelak, A.J.; Gillison, M.L.; Zhao, W.J.; Ferris, R.L.; Westra, W.H.; Gilbert, J.; Bauman, J.E.; Wagner, L.I.; et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2017, 35, 490–497. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Foster, C.C.; Blair, E.A.; Karrison, T.G.; Agrawal, N.; Melotek, J.M.; Portugal, L.; Brisson, R.J.; Dekker, A.; Kochanny, S.; et al. OPTIMA: A phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann. Oncol. 2019, 30, 1673. [Google Scholar] [CrossRef] [Green Version]
- Swisher-McClure, S.; Lukens, J.N.; Aggarwal, C.; Ahn, P.; Basu, D.; Bauml, J.M.; Brody, R.; Chalian, A.; Cohen, R.B.; Fotouhi-Ghiam, A.; et al. A Phase 2 Trial of Alternative Volumes of Oropharyngeal Irradiation for De-intensification (AVOID): Omission of the Resected Primary Tumor Bed After Transoral Robotic Surgery for Human Papilloma Virus-Related Squamous Cell Carcinoma of the Oropharynx. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 725–732. [Google Scholar] [CrossRef]
- Ma, D.J.; Price, K.A.; Moore, E.J.; Patel, S.H.; Hinni, M.L.; Garcia, J.J.; Graner, D.E.; Foster, N.R.; Ginos, B.; Neben-Wittich, M.; et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J. Clin. Oncol. 2019, 37, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Mirghani, H.; Blanchard, P. Treatment de-escalation for HPV-driven oropharyngeal cancer: Where do we stand? Clin. Transl. Radiat. Oncol. 2017, 8, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirian, E.S.; Fickey, P.A., Jr.; Scheurer, M.E.; Chiao, E.Y. Anal cancer incidence and survival: Comparing the greater San-Francisco bay area to other SEER cancer registries. PLoS ONE 2013, 8, e58919. [Google Scholar] [CrossRef] [Green Version]
- Arbyn, M.; de Sanjose, S.; Saraiya, M.; Sideri, M.; Palefsky, J.; Lacey, C.; Gillison, M.; Bruni, L.; Ronco, G.; Wentzensen, N.; et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int. J. Cancer 2012, 131, 1969–1982. [Google Scholar] [CrossRef] [Green Version]
- Glynne-Jones, R.; Nilsson, P.J.; Aschele, C.; Goh, V.; Peiffert, D.; Cervantes, A.; Arnold, D. Anal cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. Ann. Oncol. 2014, 25, iii10–iii20. [Google Scholar] [CrossRef]
- Lampejo, T.; Kavanagh, D.; Clark, J.; Goldin, R.; Osborn, M.; Ziprin, P.; Cleator, S. Prognostic biomarkers in squamous cell carcinoma of the anus: A systematic review. Br. J. Cancer 2010, 103, 1858–1869. [Google Scholar] [CrossRef] [Green Version]
- Horner, S.M.; DeFilippis, R.A.; Manuelidis, L.; DiMaio, D. Repression of the human papillomavirus E6 gene initiates p53-dependent, telomerase-independent senescence and apoptosis in HeLa cervical carcinoma cells. J. Virol. 2004, 78, 4063–4073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todorovic, B.; Hung, K.; Massimi, P.; Avvakumov, N.; Dick, F.A.; Shaw, G.S.; Banks, L.; Mymryk, J.S. Conserved region 3 of human papillomavirus 16 E7 contributes to deregulation of the retinoblastoma tumor suppressor. J. Virol. 2012, 86, 13313–13323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin-Drubin, M.E.; Huh, K.W.; Munger, K. Human papillomavirus type 16 E7 oncoprotein associates with E2F6. J. Virol. 2008, 82, 8695–8705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansma, A.L.; Martinez-Yamout, M.A.; Liao, R.; Sun, P.; Dyson, H.J.; Wright, P.E. The high-risk HPV16 E7 oncoprotein mediates interaction between the transcriptional coactivator CBP and the retinoblastoma protein pRb. J. Mol. Biol. 2014, 426, 4030–4048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Doorslaer, K.; Burk, R.D. Association between hTERT activation by HPV E6 proteins and oncogenic risk. Virology 2012, 433, 216–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiantore, M.V.; Mangino, G.; Iuliano, M.; Capriotti, L.; Di Bonito, P.; Fiorucci, G.; Romeo, G. Human Papillomavirus and carcinogenesis: Novel mechanisms of cell communication involving extracellular vesicles. Cytokine Growth Factor Rev. 2020, 51, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.; Gardiner, A.S.; Board, K.F.; Monzon, F.A.; Edwards, R.P.; Khan, S.A. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene 2008, 27, 2575–2582. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tang, S.; Le, S.Y.; Lu, R.; Rader, J.S.; Meyers, C.; Zheng, Z.M. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS ONE 2008, 3, e2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakenhoff, R.H.; Wagner, S.; Klussmann, J.P. Molecular Patterns and Biology of HPV-Associated HNSCC. Recent Results Cancer Res. 2017, 206, 37–56. [Google Scholar] [CrossRef]
- Virtanen, E.; Pietilä, T.; Nieminen, P.; Qian, K.; Auvinen, E. Low expression levels of putative HPV encoded microRNAs in cervical samples. SpringerPlus 2016, 5, 1856. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Lee, E.E.; Kim, J.; Yang, R.; Chamseddin, B.; Ni, C.; Gusho, E.; Xie, Y.; Chiang, C.M.; Buszczak, M.; et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat Commun 2019, 10, 2300. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.-D. Non-coding RNA: A new frontier in regulatory biology. Natl. Sci. Rev. 2014, 1, 190–204. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamantopoulos, M.A.; Tsiakanikas, P.; Scorilas, A. Non-coding RNAs: The riddle of the transcriptome and their perspectives in cancer. Ann. Transl. Med. 2018, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yang, A. Noncoding RNAs in Therapeutic Resistance of Cancer. Adv. Exp. Med. Biol. 2016, 927, 265–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, X.; Yang, Y.; He, Z.; Qu, X.; Zhang, Y. Long noncoding RNAs in the progression, metastasis, and prognosis of osteosarcoma. Cell Death Amp Dis. 2016, 7, e2389. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, J.; Shi, J.; Guo, Z.; He, C.; Ding, L.; Hai Tang, J.; Hou, Y. Role of Long Non-Coding RNA in Tumor Drug Resistance. Tumour Biol. 2016, 37, 11623–11631. [Google Scholar] [CrossRef]
- Corrà, F.; Agnoletto, C.; Minotti, L.; Baldassari, F.; Volinia, S. The Network of Non-coding RNAs in Cancer Drug Resistance. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Malek, E.; Jagannathan, S.; Driscoll, J.J. Correlation of long non-coding RNA expression with metastasis, drug resistance and clinical outcome in cancer. Oncotarget 2014, 5, 8027–8038. [Google Scholar] [CrossRef] [Green Version]
- Dhamija, S.; Diederichs, S. From junk to master regulators of invasion: LncRNA functions in migration, EMT and metastasis. Int. J. Cancer 2016, 139, 269–280. [Google Scholar] [CrossRef]
- Lin, C.-W.; Lin, P.-Y.; Yang, P.-C. Noncoding RNAs in Tumor Epithelial-to-Mesenchymal Transition. Stem Cells Int. 2016, 2016, 2732705. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Brenn, T.; Brown, E.R.; Doherty, V.; Melton, D.W. Differential expression of microRNAs during melanoma progression: MiR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br. J Cancer 2012, 106, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Snoek, B.C.; Babion, I.; Koppers-Lalic, D.; Pegtel, D.M.; Steenbergen, R.D.M. Altered microRNA processing proteins in HPV-induced cancers. Curr. Opin. Virol. 2019, 39, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.; Li, J. Circular RNAs: Biogenesis, expression and their potential roles in reproduction. J. Ovarian Res. 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Li, S.; Yang, N.; Zou, Y.; Zheng, D.; Xiao, T. Recent progress in circular RNAs in human cancers. Cancer Lett. 2017, 404, 8–18. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Mao, M.; Song, X.; Wu, P.; Zhang, Y.; Jin, Y.; Yang, Y.; Chen, L.-L.; Wang, Y.; et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017, 27, 626–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, D.-H.; Lee, S.K.; Sood, A.K. Circular RNAs in Cancer. Mol. Ther. Nucleic Acids 2019, 16, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Qi, X.; Liu, L.; Hu, X.; Liu, J.; Yang, J.; Yang, J.; Lu, L.; Zhang, Z.; Ma, S.; et al. Emerging Epigenetic Regulation of Circular RNAs in Human Cancer. Mol. Ther. Nucleic Acids 2019, 16, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.; Xiao, Y.; Ma, J.; Tang, Y.; Tian, B.; Zhang, Y.; Li, X.; Wu, Z.; Yang, D.; Zhou, Y.; et al. Circular RNAs in Cancer: Emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol. Cancer 2019, 18, 90. [Google Scholar] [CrossRef]
- Vo, J.N.; Cieslik, M.; Zhang, Y.; Shukla, S.; Xiao, L.; Wu, Y.M.; Dhanasekaran, S.M.; Engelke, C.G.; Cao, X.; Robinson, D.R.; et al. The Landscape of Circular RNA in Cancer. Cell 2019, 176, 869–881. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Yang, J.; Huang, Q.; Hsueh, C.; Zheng, J.; Wu, C.; Chen, H.; Zhou, L. Circular RNAs and their roles in head and neck cancers. Mol. Cancer 2019, 18, 44. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, D.; Long, Z.; Li, W. CircRNA8924 Promotes Cervical Cancer Cell Proliferation, Migration and Invasion by Competitively Binding to MiR-518d-5p /519-5p Family and Modulating the Expression of CBX8. Cell. Physiol. Biochem. 2018, 48, 173–184. [Google Scholar] [CrossRef]
- Ma, H.; Tian, T.; Liu, X.; Xia, M.; Chen, C.; Mai, L.; Xie, S.; Yu, L. Upregulated circ_0005576 facilitates cervical cancer progression via the miR-153/KIF20A axis. Biomed. Pharmacother. 2019, 118, 109311. [Google Scholar] [CrossRef] [PubMed]
- Firmino, N.; Martinez, V.D.; Rowbotham, D.A.; Enfield, K.S.S.; Bennewith, K.L.; Lam, W.L. HPV status is associated with altered PIWI-interacting RNA expression pattern in head and neck cancer. Oral Oncol. 2016, 55, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.R.; Qu, Y.; Li, P.X.; Zou, A.E.; Califano, J.A.; Wang-Rodriguez, J.; Ongkeko, W.M. Computational methods reveal novel functionalities of PIWI-interacting RNAs in human papillomavirus-induced head and neck squamous cell carcinoma. Oncotarget 2017, 9, 4614–4624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Gao, Q.; Chen, K.; Xue, X.; Li, M.; Chen, Q.; Zhu, G.; Gao, Y. Hiwi facilitates chemoresistance as a cancer stem cell marker in cervical cancer. Oncol. Rep. 2014, 32, 1853–1860. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Yan, K.; Zhou, Y.; Liang, H.; Liang, J.; Zhao, W.; Dong, Z.; Ling, B. Piwil2 is reactivated by HPV oncoproteins and initiates cell reprogramming via epigenetic regulation during cervical cancer tumorigenesis. Oncotarget 2016, 7, 64575–64588. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Ren, Z.-J.; Wang, F.; Liu, M.; Li, X.; Tang, H. PIWIL4 regulates cervical cancer cell line growth and is involved in down-regulating the expression of p14ARF and p53. FEBS Lett. 2012, 586, 1356–1362. [Google Scholar] [CrossRef] [Green Version]
- Braciale, T.J.; Hahn, Y.S. Immunity to viruses. Immunol. Rev. 2013, 255, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Takata, M.; Sasaki, M.S.; Sonoda, E.; Fukushima, T.; Morrison, C.; Albala, J.S.; Swagemakers, S.M.A.; Kanaar, R.; Thompson, L.H.; Takeda, S. The Rad51 Paralog Rad51B Promotes Homologous Recombinational Repair. Mol. Cell. Biol. 2000, 20, 6476–6482. [Google Scholar] [CrossRef]
- Yang, L.; Yi, K.; Wang, H.; Zhao, Y.; Xi, M. Comprehensive analysis of lncRNAs microarray profile and mRNA-lncRNA co-expression in oncogenic HPV-positive cervical cancer cell lines. Oncotarget 2016, 7, 49917–49929. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Wu, F.; Cui, Y.; Wei, F.; Meng, Q.; Lv, Q. Long non-coding RNA-OIS1 inhibits HPV-positive, but not HPV-negative cervical squamous cell carcinoma by upregulating MTK-1. Oncol. Lett. 2019, 17, 2923–2930. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Huang, Z.; Gao, R.; Zeng, Z.; Yang, W.; Sun, Y.; Wei, W.; Wu, Z.; Yu, L.; Li, Q.; et al. Expression of Long Noncoding RNA Urothelial Cancer Associated 1 Promotes Cisplatin Resistance in Cervical Cancer. Cancer Biother. Radiopharm. 2017, 32, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, Y.; Wang, L.; Yuan, N.; Ma, M.; Chen, Y. LncRNA SNHG8 accelerates proliferation and inhibits apoptosis in HPV-induced cervical cancer through recruiting EZH2 to epigenetically silence RECK expression. J. Cell. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, L.G.; Wang, P.; Li, J.; Tian, F.; Chu, Z.P.; Kang, S. The expression and significance of lncRNA HOST2 and microRNA let-7b in HPV-positive cervical cancer tissues and cell lines. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, Z.; Gao, Y.; Yao, T. Downregulation of long noncoding RNA MEG3 is associated with poor prognosis and promoter hypermethylation in cervical cancer. J. Exp. Clin. Cancer Res. 2017, 36, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yao, T.; Lin, Z.; Gao, Y. Aberrant Methylation of MEG3 Functions as a Potential Plasma-Based Biomarker for Cervical Cancer. Sci. Rep. 2017, 7, 6271. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, T.; Wang, Y.; Yu, J.; Liu, Y.; Lin, Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol. Ther. 2016, 17, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Nohata, N.; Abba, M.C.; Gutkind, J.S. Unraveling the oral cancer lncRNAome: Identification of novel lncRNAs associated with malignant progression and HPV infection. Oral Oncol. 2016, 59, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Xie, H.; Tong, F.; Yan, B.; Zhang, S.; Fu, E.; Jing, Q.; Wei, L. Association of lnc-IL17RA-11 with increased radiation sensitivity and improved prognosis of HPV-positive HNSCC. J. Cell. Biochem. 2019, 120, 17438–17448. [Google Scholar] [CrossRef]
- Kolenda, T.; Kopczyńska, M.; Guglas, K.; Teresiak, A.; Bliźniak, R.; Łasińska, I.; Mackiewicz, J.; Lamperska, K. EGOT lncRNA in head and neck squamous cell carcinomas. Pol. J. Pathol. 2018, 69, 356–365. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Chen, M.; Cui, J. Identification of Novel Long Non-coding and Circular RNAs in Human Papillomavirus-Mediated Cervical Cancer. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.-L.; Zhang, M.-Y.; Xu, B.; Han, L.-J.; Lan, S.-F.; Chen, J.; Dong, Y.-J.; Cao, L.-L. Circular RNA expression profiles reveal that hsa_circ_0018289 is up-regulated in cervical cancer and promotes the tumorigenesis. Oncotarget 2017, 8, 86625–86633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Zhang, J.; Zheng, X.; Li, F. Circular RNA hsa_circ_0023404 exerts an oncogenic role in cervical cancer through regulating miR-136/TFCP2/YAP pathway. Biochem. Biophys. Res. Commun. 2018, 501, 428–433. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, P.; Xu, M.; Yan, L.; Liu, N.; Wu, X. Circular RNA hsa_circ_0000263 participates in cervical cancer development by regulating target gene of miR-150-5p. J. Cell. Physiol. 2019, 234, 11391–11400. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, Y.; Li, A.; Zhang, J.; Xue, F.; Zhu, L. Overexpressed circ_0067934 acts as an oncogene to facilitate cervical cancer progression via the miR-545/EIF3C axis. J. Cell. Physiol. 2019, 234, 9225–9232. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Du, R.; Chen, T.; Zhang, M.; Zhu, Y.; Luo, X.; Ding, Y. Circular RNA circSLC26A4 Accelerates Cervical Cancer Progression via miR-1287-5p/HOXA7 Axis. Mol. Ther. Nucleic Acids 2020, 19, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Ríos, P.; Simonelig, M. piRNAs and PIWI proteins: Regulators of gene expression in development and stem cells. Development 2018, 145, dev161786. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.W.; Anderson, C.; Marshall, E.A.; Minatel, B.C.; Enfield, K.S.S.; Saprunoff, H.L.; Lam, W.L.; Martinez, V.D. Piwi-interacting RNAs in cancer: Emerging functions and clinical utility. Mol. Cancer 2016, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.J.; Weiner, M.M.; Lin, H. PIWI proteins and PIWI-interacting RNAs in the soma. Nature 2014, 505, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xiao, J.; Hann, S.S. The emerging roles of PIWI-interacting RNA in human cancers. Cancer Manag. Res. 2019, 11, 5895–5909. [Google Scholar] [CrossRef] [Green Version]
- Litwin, M.; Szczepańska-Buda, A.; Piotrowska, A.; Dzięgiel, P.; Witkiewicz, W. The meaning of PIWI proteins in cancer development. Oncol. Lett. 2017, 13, 3354–3362. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-K.; Jiang, X.-Y.; Zhang, Z.-X. Expression of PSCA, PIWIL1 and TBX2 and its correlation with HPV16 infection in formalin-fixed, paraffin-embedded cervical squamous cell carcinoma specimens. Arch. Virol. 2010, 155, 657–663. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Chen, L.; Ye, Y.; Xiao, Y.; Hua, K.; Jarjoura, D.; Nakano, T.; Barsky, S.H.; Shen, R.; Gao, J.-X. Piwil2 expressed in various stages of cervical neoplasia is a potential complementary marker for p16. Am. J. Transl. Res. 2010, 2, 156–169. [Google Scholar] [PubMed]
- Hashim, A.; Rizzo, F.; Marchese, G.; Ravo, M.; Tarallo, R.; Nassa, G.; Giurato, G.; Santamaria, G.; Cordella, A.; Cantarella, C.; et al. RNA sequencing identifies specific PIWI-interacting small non-coding RNA expression patterns in breast cancer. Oncotarget 2014, 5, 9901–9910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, A.; Fernandes, M.; Stirrup, O.; Teixeira, A.L.; Santos, J.; Rodrigues, M.; Rios, E.; Macedo, G.; Medeiros, R. Expression of microRNAs 16, 20a, 150 and 155 in anal squamous intraepithelial lesions from high-risk groups. Sci. Rep. 2019, 9, 1523. [Google Scholar] [CrossRef] [PubMed]
- Myklebust, M.P.; Bruland, O.; Fluge, O.; Skarstein, A.; Balteskard, L.; Dahl, O. MicroRNA-15b is induced with E2F-controlled genes in HPV-related cancer. Br. J. Cancer 2011, 105, 1719–1725. [Google Scholar] [CrossRef] [Green Version]
- Karapetyan, A.R.; Buiting, C.; Kuiper, R.A.; Coolen, M.W. Regulatory Roles for Long ncRNA and mRNA. Cancers 2013, 5, 462–490. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 925–933. [Google Scholar] [CrossRef]
- Khalil, A.M.; Guttman, M.; Huarte, M.; Garber, M.; Raj, A.; Rivea Morales, D.; Thomas, K.; Presser, A.; Bernstein, B.E.; van Oudenaarden, A.; et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 11667–11672. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [Green Version]
- Wang, P. The Opening of Pandora’s Box: An Emerging Role of Long Noncoding RNA in Viral Infections. Front. Immunol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartori, D.A.; Chan, D.W. Biomarkers in prostate cancer: What’s new? Curr. Opin. Oncol. 2014, 26, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Hou, Y.; Li, A.; Li, Z.; Wang, W.; Xie, H.; Rong, Z.; Lou, G.; Li, K. Identification of a six-lncRNA signature associated with recurrence of ovarian cancer. Sci. Rep. 2017, 7, 752. [Google Scholar] [CrossRef] [PubMed]
- Bolha, L.; Ravnik-Glavač, M.; Glavač, D. Long Noncoding RNAs as Biomarkers in Cancer. Dis. Markers 2017, 2017, 7243968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huarte, M. The emerging role of lncRNAs in cancer. Nat. Med. 2015, 21, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Chandra Gupta, S.; Nandan Tripathi, Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer 2017, 140, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B.; et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Song, L.; Yao, D.; Tang, Q.; Zhou, J. Upregulation of lncRNA GATA6-AS suppresses the migration and invasion of cervical squamous cell carcinoma by downregulating MTK-1. Oncol. Lett. 2019, 18, 2605–2611. [Google Scholar] [CrossRef] [Green Version]
- Haque, S.-U.; Niu, L.; Kuhnell, D.; Hendershot, J.; Biesiada, J.; Niu, W.; Hagan, M.C.; Kelsey, K.T.; Casper, K.A.; Wise-Draper, T.M.; et al. Differential expression and prognostic value of long non-coding RNA in HPV-negative head and neck squamous cell carcinoma. Head Neck 2018, 40, 1555–1564. [Google Scholar] [CrossRef]
- Yuan, H.; Qin, Y.; Zeng, B.; Feng, Y.; Li, Y.; Xiang, T.; Ren, G. Long noncoding RNA LINC01089 predicts clinical prognosis and inhibits cell proliferation and invasion through the Wnt/beta-catenin signaling pathway in breast cancer. OncoTargets Ther. 2019, 12, 4883–4895. [Google Scholar] [CrossRef] [Green Version]
- Shin, C.H.; Ryu, S.; Kim, H.H. hnRNPK-regulated PTOV1-AS1 modulates heme oxygenase-1 expression via miR-1207-5p. BMB Rep. 2017, 50, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Wen, Q.; Tan, X.; Piao, J.; Zhang, Q.; Wang, Q.; He, L.; Wang, Y.; Chen, Z.; Liu, G. An integrated nomogram combining lncRNAs classifier and clinicopathologic factors to predict the recurrence of head and neck squamous cell carcinoma. Sci. Rep. 2019, 9, 17460. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Munger, K. Expression of the cervical carcinoma expressed PCNA regulatory (CCEPR) long noncoding RNA is driven by the human papillomavirus E6 protein and modulates cell proliferation independent of PCNA. Virology 2018, 518, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhai, X.; Xia, B.; Wang, Y.; Lou, G. Long noncoding RNA CCHE1 promotes cervical cancer cell proliferation via upregulating PCNA. Tumour Biol. 2015, 36, 7615–7622. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.X.; Sun, X.X.; Wang, C.; Liu, T.F.; Wang, D.J. Long non-coding RNA CCHE1 overexpression predicts a poor prognosis for cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 479–483. [Google Scholar]
- Barr, J.A.; Hayes, K.E.; Brownmiller, T.; Harold, A.D.; Jagannathan, R.; Lockman, P.R.; Khan, S.; Martinez, I. Long non-coding RNA FAM83H-AS1 is regulated by human papillomavirus 16 E6 independently of p53 in cervical cancer cells. Sci. Rep. 2019, 9, 3662. [Google Scholar] [CrossRef] [Green Version]
- White, E.A.; Kramer, R.E.; Tan, M.J.A.; Hayes, S.D.; Harper, J.W.; Howley, P.M. Comprehensive Analysis of Host Cellular Interactions with Human Papillomavirus E6 Proteins Identifies New E6 Binding Partners and Reflects Viral Diversity. J. Virol. 2012, 86, 13174–13186. [Google Scholar] [CrossRef] [Green Version]
- Ou, L.; Wang, D.; Zhang, H.; Yu, Q.; Hua, F. Decreased Expression of miR-138-5p by lncRNA H19 in Cervical Cancer Promotes Tumor Proliferation. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 401–410. [Google Scholar] [CrossRef]
- Cao, S.; Liu, W.; Li, F.; Zhao, W.; Qin, C. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 6776–6783. [Google Scholar]
- Li, Y.; Wan, Y.P.; Bai, Y. Correlation between long strand non-coding RNA GASS expression and prognosis of cervical cancer patients. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 943–949. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, Y.; Lyu, H.; Xu, X.; Wu, Q.; Liu, N.; Yin, Q.; Li, J.; Sheng, X. Long Noncoding RNA GAS5, Which Acts as a Tumor Suppressor via microRNA 21, Regulates Cisplatin Resistance Expression in Cervical Cancer. Int. J. Gynecol. Cancer 2017, 27, 1096–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Liu, L.; Li, G.; Cai, M.; Tan, C.; Han, X.; Han, L. LncRNA GAS5 confers the radio sensitivity of cervical cancer cells via regulating miR-106b/IER3 axis. Int. J. Biol. Macromol. 2019, 126, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y. Long noncoding RNA GAS5-AS1 suppresses growth and metastasis of cervical cancer by increasing GAS5 stability. Am. J. Transl. Res. 2019, 11, 4909–4921. [Google Scholar] [PubMed]
- Sharma, S.; Mandal, P.; Sadhukhan, T.; Roy Chowdhury, R.; Ranjan Mondal, N.; Chakravarty, B.; Chatterjee, T.; Roy, S.; Sengupta, S. Bridging Links between Long Noncoding RNA HOTAIR and HPV Oncoprotein E7 in Cervical Cancer Pathogenesis. Sci. Rep. 2015, 5, 11724. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Lee, D.W.; Yim, G.W.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Long non-coding RNA HOTAIR is associated with human cervical cancer progression. Int. J. Oncol. 2015, 46, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Bhan, A.; Mandal, S.S. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta 2015, 1856, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Sharma Saha, S.; Roy Chowdhury, R.; Mondal, N.R.; Chakravarty, B.; Chatterjee, T.; Roy, S.; Sengupta, S. Identification of genetic variation in the lncRNA HOTAIR associated with HPV16-related cervical cancer pathogenesis. Cell. Oncol. 2016, 39, 559–572. [Google Scholar] [CrossRef]
- Ma, X.; Sheng, S.; Wu, J.; Jiang, Y.; Gao, X.; Cen, X.; Wu, J.; Wang, S.; Tang, Y.; Tang, Y.; et al. LncRNAs as an intermediate in HPV16 promoting myeloid-derived suppressor cell recruitment of head and neck squamous cell carcinoma. Oncotarget 2017, 8, 42061–42075. [Google Scholar] [CrossRef] [Green Version]
- Iancu, I.V.; Anton, G.; Botezatu, A.; Huica, I.; Nastase, A.; Socolov, D.G.; Stanescu, A.D.; Dima, S.O.; Bacalbasa, N.; Plesa, A. LINC01101 and LINC00277 expression levels as novel factors in HPV-induced cervical neoplasia. J. Cell. Mol. Med. 2017, 21, 3787–3794. [Google Scholar] [CrossRef]
- Guo, F.; Li, Y.; Liu, Y.; Wang, J.; Li, G. Inhibition of metastasis-associated lung adenocarcinoma transcript 1 in CaSki human cervical cancer cells suppresses cell proliferation and invasion. Acta Biochim. Biophys. Sin. (Shanghai) 2010, 42, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Li, Y.; Fang, S.; Jiang, B.; Qin, C.; Xie, P.; Zhou, G.; Li, G. The role of MALAT1 correlates with HPV in cervical cancer. Oncol. Lett. 2014, 7, 2135–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Qin, C.; Jiang, B.; Fang, S.; Pan, X.; Peng, L.; Liu, Z.; Li, W.; Li, Y.; Li, G. Down-regulation of MALAT1 inhibits cervical cancer cell invasion and metastasis by inhibition of epithelial-mesenchymal transition. Mol. Biosyst. 2016, 12, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Song, L.; Zeng, S.; Zhang, L. MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells. Tumor Biol. 2016, 37, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; He, Y.; Lin, L.; Qi, Z.; Ma, L.; Li, L.; Su, Y. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour. Biol. 2016, 37, 1683–1691. [Google Scholar] [CrossRef]
- He, H.; Liu, X.; Liu, Y.; Zhang, M.; Lai, Y.; Hao, Y.; Wang, Q.; Shi, D.; Wang, N.; Luo, X.-G.; et al. Human Papillomavirus E6/E7 and Long Noncoding RNA TMPOP2 Mutually Upregulated Gene Expression in Cervical Cancer Cells. J. Virol. 2019, 93, e01808-01818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villegas, J.; Burzio, V.; Villota, C.; Landerer, E.; Martinez, R.; Santander, M.; Pinto, R.; Vera, M.I.; Boccardo, E.; Villa, L.L.; et al. Expression of a novel non-coding mitochondrial RNA in human proliferating cells. Nucleic Acids Res. 2007, 35, 7336–7347. [Google Scholar] [CrossRef] [Green Version]
- Burzio, V.A.; Villota, C.; Villegas, J.; Landerer, E.; Boccardo, E.; Villa, L.L.; Martínez, R.; Lopez, C.; Gaete, F.; Toro, V.; et al. Expression of a family of noncoding mitochondrial RNAs distinguishes normal from cancer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 9430–9434. [Google Scholar] [CrossRef] [Green Version]
- Landerer, E.; Villegas, J.; Burzio, V.A.; Oliveira, L.; Villota, C.; Lopez, C.; Restovic, F.; Martinez, R.; Castillo, O.; Burzio, L.O. Nuclear localization of the mitochondrial ncRNAs in normal and cancer cells. Cell. Oncol. 2011, 34, 297–305. [Google Scholar] [CrossRef]
- Villota, C.; Campos, A.; Vidaurre, S.; Oliveira-Cruz, L.; Boccardo, E.; Burzio, V.A.; Varas, M.; Villegas, J.; Villa, L.L.; Valenzuela, P.D.; et al. Expression of mitochondrial non-coding RNAs (ncRNAs) is modulated by high risk human papillomavirus (HPV) oncogenes. J. Biol. Chem. 2012, 287, 21303–21315. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.-R.; Zhang, H.-R.; Zhang, Z.-F.; Lai, S.-Y.; Huang, L.-J.; Liu, J.; Bai, X.; Ding, K.; Zhou, J.-Y. Human papillomavirus 16 E7 oncoprotein alters the expression profiles of circular RNAs in Caski cells. J. Cancer 2018, 9, 3755–3764. [Google Scholar] [CrossRef] [Green Version]
- Chamseddin, B.H.; Lee, E.E.; Kim, J.; Zhan, X.; Yang, R.; Murphy, K.M.; Lewis, C.; Hosler, G.A.; Hammer, S.T.; Wang, R.C. Assessment of circularized E7 RNA, GLUT1, and PD-L1 in anal squamous cell carcinoma. Oncotarget 2019, 10, 5958–5969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajan, S.; Hutvagner, G. RNA-Based Therapeutics: From Antisense Oligonucleotides to miRNAs. Cells 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutschner, T.; Hammerle, M.; Eissmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Gross, M.; et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013, 73, 1180–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arun, G.; Diermeier, S.; Akerman, M.; Chang, K.C.; Wilkinson, J.E.; Hearn, S.; Kim, Y.; MacLeod, A.R.; Krainer, A.R.; Norton, L.; et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016, 30, 34–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-S.; Harford, J.B.; Moghe, M.; Rait, A.; Pirollo, K.F.; Chang, E.H. Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide. Nucleic Acids Res. 2018, 46, 1424–1440. [Google Scholar] [CrossRef] [Green Version]
- Jost, I.; Shalamova, L.A.; Gerresheim, G.K.; Niepmann, M.; Bindereif, A.; Rossbach, O. Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges. RNA Biol. 2018, 15, 1032–1039. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Meng, X.; Pan, J.; Jiang, N.; Zhou, C.; Wu, Z.; Gong, Z. CRISPR/Cas9-mediated noncoding RNA editing in human cancers. RNA Biol. 2018, 15, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Connelly Colleen, M.; Moon Michelle, H.; Schneekloth John, S., Jr. The Emerging Role of RNA as a Therapeutic Target for Small Molecules. Cell Chem. Biol. 2016, 23, 1077–1090. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Duca, M. Synthetic small-molecule RNA ligands: Future prospects as therapeutic agents. MedChemComm 2019, 10, 1242–1255. [Google Scholar] [CrossRef]
- Xia, C.; Liang, S.; He, Z.; Zhu, X.; Chen, R.; Chen, J. Metformin, a first-line drug for type 2 diabetes mellitus, disrupts the MALAT1/miR-142-3p sponge to decrease invasion and migration in cervical cancer cells. Eur. J. Pharmacol. 2018, 830, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Q.; Lin, F.; Zhu, L.; Huang, F.; Zhao, L.; Ou, R. Casiopeina II-gly acts on lncRNA MALAT1 by miR-17-5p to inhibit FZD2 expression via the Wnt signaling pathway during the treatment of cervical carcinoma. Oncol. Rep. 2019, 42, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Su, C.; Jiang, M.; Shen, Y.; Shi, A.; Zhao, F.; Chen, R.; Shen, Z.; Bao, J.; Tang, W. Fenofibrate inhibited pancreatic cancer cells proliferation via activation of p53 mediated by upregulation of LncRNA MEG3. Biochem. Biophys. Res. Commun. 2016, 471, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-H.; Chan, C.-W.; Fang, J.-Y.; Shih, Y.-M.; Liu, Y.-W.; Wang, T.-C.V.; Chen, C.-Y. 2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells. Cell Death Dis. 2017, 8, e2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özeş, A.R.; Wang, Y.; Zong, X.; Fang, F.; Pilrose, J.; Nephew, K.P. Therapeutic targeting using tumor specific peptides inhibits long non-coding RNA HOTAIR activity in ovarian and breast cancer. Sci. Rep. 2017, 7, 894. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Wang, R.-P.; Zou, X. Dioscin inhibits gastric tumor growth through regulating the expression level of lncRNA HOTAIR. BMC Complement. Altern. Med. 2016, 16, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Tumor Site | Predominant HPV Types * | HPV Attributable Fraction (%) | New Cases Attributable to HPV | Prognostic Significance of HPV-Positivity | References |
|---|---|---|---|---|---|
| Head and neck cancer | |||||
| Oropharynx | HPV16; HPV33; HPV35 | 30.1 | 42,000 | Better survival | [1,6,7,8] |
| Oral cavity | HPV16; HPV52; HPV35 | 2.2 | 5900 | Inconclusive | [1,6,7,8] |
| Larynx | HPV16; HPV31; HPV33 | 2.4 | 4100 | Inconclusive | [1,6,7,8] |
| Cervical cancer | HPV16; HPV18; HPV45 | 100 | 570,000 | - | [1,6,9] |
| Anal cancer | HPV16; HPV18 | 88.0 | 29,000 | Better prognosis in men | [1,6,10] |
| Penile cancer | HPV16; HPV6; HPV18 | 50.0 | 18,000 | Inconclusive | [1,6,11,12] |
| Vulval cancer | HPV16; HPV33 | 24.9 | 11,000 | Better survival | [1,6,13,14] |
| Vaginal cancer | HPV16; HPV18; HPV73 | 78.0 | 14,000 | - | [1,6,15] |
| HPV-Driven Cancer Type | CircRNAs ID | Sample Source | Expression Change | Function/Effect | Targets | Prognostic Value | Notes | References |
| CSCC | CircRNA8924 | Tissues, Cell lines | Up | Promote proliferation, cell cycle progression, migration, and invasion | MiR-518-d-5p/miR-519-5p | [80] | ||
| Circ_0005576 | Tissues, Cell lines | Up | Promote tumor progression | MiR-153 | Correlated with advanced FIGO stage, lymph node metastasis and poor prognosis | [81] | ||
| HPV-Driven Cancer Type | PiRNAs ID | Samples Source | Expression Change | Function/Effect | Targets | Prognostic Value | Notes | References |
| HNSCC | FR018916, FR140858, FR197104, FR237180, FR298757 | TCGA | Down | PiRNA expression signature can predict OS in HPV positive patients | Downregulated in HPV16/18 HNSCC samples compared to cases harboring other HPV types | [82] | ||
| PiR-36742 | TCGA | Up | Deregulated in smoking HPV-positive patients | [83] | ||||
| PiR-33519 | Up | |||||||
| PiR-36743 | Up | |||||||
| PiR-34291 | Up | |||||||
| PiR-36340 | Down | |||||||
| PiR-62011 | Down | |||||||
| PiR-30652 | TCGA | Up | Significantly predictive of patient outcome | Significantly associated with higher histologic grade | ||||
| PiR-33686 | Up | |||||||
| PiR-36340 | Down | |||||||
| PiR-45029 | Down | |||||||
| HPV-Driven Cancer Type | PIWI-Like Proteins ID | Samples Source | Expression Change | Function/Effect | Targets | Prognostic Value | Notes | References |
| CSCC | PIWIL1 | Tissues, Cell lines | Up | Promote tumorigenesis and tumor progression, suppress chemotherapy sensitivity | [84] | |||
| PIWIL2 | Tissues, Cell lines | Up | Promote tumorigenesis, induce H3K9 acetylation and reduce H3K9 trimethylation | PIWIL2 activation in CSCC appears to depend on the integration of HR-HPV DNA | [85] | |||
| PIWIL4 | Tissues, Cell lines | Up | Promote proliferation, inhibit apoptosis | P14ARF/p53 pathway | [86] | |||
| HNSCC | PIWIL4 | TCGA consortium | Up | Upregulated in smoking HPV-positive patients | [83] | |||
| HPV-Driven Cancer Type | LncRNAs ID | Samples Source | Expression Change | Function/Effect | Targets | Prognostic Value | Notes | References |
| CSCC | ENST00000503812 | Cell lines | Up | Impair DNA repair, induce immune response | Negatively correlated with RAD51B and IL-28A expression | [87,88] | ||
| ENST00000420168, ENST00000564977 | Cell lines | Up | Promote proliferation, inhibit apoptosis | Correlation with the increased expression of FOX Q1 and the reduced expression of caspase-3 | [89] | |||
| TCONS_00010232 | Cell lines | Down | ||||||
| OIS1 | Tissues, Serum Cell lines | Down | Suppress cell proliferation | MTK-1 | Significant association between OIS1 serum levels and tumor size | [90] | ||
| UCA1 | Cell lines | Up | Induce cisplatin resistance and inhibit apoptosis | [91] | ||||
| SNHG8 | Cell lines | Up | Promote proliferation and migration, inhibit apoptosis | EZH2 | [92] | |||
| HOST2 | Tissues, Cell lines | Up | Promote proliferation, migration and invasion, inhibit apoptosis | MiRNA let-7b | [93] | |||
| MEG3 | Tissues, Serum, Cell lines | Down | Suppress proliferation, promote apoptosis | MiR-21-5p | Correlated with increased tumor size, advanced FIGO stage, lymph node metastasis, HPV infection, recurrence-free survival and OS | [94,95,96] | ||
| HNSCC | LINC01089 | TCGA | Up | [97] | ||||
| PTOV1-AS1 | TCGA | Up | [97] | |||||
| IL17RA-11 | TCGA Cell lines | Up | Induce radiotherapy sensitivity | Correlated with better prognosis | HPV infection stimulates ERα to increase lnc-IL17RA-11 expression; this finding suggests why HPV-positive HNSCC are more sensitive to radiotherapy | [98] | ||
| EGOT | TCGA data | Up | Promote tumor progression | EGOT expression levels vary according to age, N-stage, grade, location, lymph node dissection, and HPV16 status | [99] |
| LncRNAs ID | Sample Description | Expression Change | Prognostic Value | References |
|---|---|---|---|---|
| CCEPR | Cell lines, Tissues | Up | Positively correlated with advanced FIGO stage, lymph node metastasis, HPV infection, and poor prognosis | [133,134,135] |
| FAM83H-AS1 | Cell lines, Tissues | Up | Poor prognosis | [136] |
| GAS5 | Cell lines, Tissues | Down | Poor prognosis | [139,140] |
| HOTAIR | Cell lines, Tissues | Up | Disease recurrence and poor prognosis | [145] |
| Cell lines, Tissues | Rs2366152C polymorphism associates to reduced HOTAIR expression and CSCC metastatic molecular signatures | [147] | ||
| LINC00277, LINC01101 | Cell lines, Tissues | Down | Poor prognosis | [149] |
| LncRNAs ID | Cohort Size | Source of LncRNAs | Sensitivity | Specificity | AUC | Diagnostic Value | References | |
|---|---|---|---|---|---|---|---|---|
| MEG3 | 72 cases and 72 normal tissues | Tissues | 56.1% | 80.6% | 0.745 | Tumor-size <4 cm or ≥ 4 cm | [94] | |
| 70.5% | 67.9% | 0.716 | Lymph node metastasis | |||||
| 108 cases | 54.8% | 84.8% | 0.753 | Tumor-size <4 cm or ≥ 4 cm | ||||
| 76.1% | 85.4% | 0.862 | Lymph node metastasis | |||||
| MEG3 methylation | 160 CIN I-III, 168 cases, and 328 healthy patients divided into training set and test set randomly and averagely | Training set | Plasma | 73.7% | 94.7% | 0.831 | CIN III | [95] |
| 75.8% | 88.9% | 0.815 | HR-HPV infection | |||||
| 93.3% | 51.9% | 0.741 | Lymph node metastasis | |||||
| Test set | 84.2% | 52.6% | 0.788 | CIN III | ||||
| 78.1% | 70.0% | 0.730 | HR-HPV infection | |||||
| 82.4% | 72.0% | 0.804 | Lymph node metastasis | |||||
| OIS1 | 22 HPV-negative patients, 70 HPV-positive patients, and 40 healthy patients | Plasma | n.a. * | n.a. * | 0.9207 | Serum OIS1 may be used to diagnose HPV-positive, but not HPV-negative CSCC | [90] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarotto, M.; Fanetti, G.; Guerrieri, R.; Palazzari, E.; Lupato, V.; Steffan, A.; Polesel, J.; Boscolo-Rizzo, P.; Fratta, E. Beyond MicroRNAs: Emerging Role of Other Non-Coding RNAs in HPV-Driven Cancers. Cancers 2020, 12, 1246. https://doi.org/10.3390/cancers12051246
Casarotto M, Fanetti G, Guerrieri R, Palazzari E, Lupato V, Steffan A, Polesel J, Boscolo-Rizzo P, Fratta E. Beyond MicroRNAs: Emerging Role of Other Non-Coding RNAs in HPV-Driven Cancers. Cancers. 2020; 12(5):1246. https://doi.org/10.3390/cancers12051246
Chicago/Turabian StyleCasarotto, Mariateresa, Giuseppe Fanetti, Roberto Guerrieri, Elisa Palazzari, Valentina Lupato, Agostino Steffan, Jerry Polesel, Paolo Boscolo-Rizzo, and Elisabetta Fratta. 2020. "Beyond MicroRNAs: Emerging Role of Other Non-Coding RNAs in HPV-Driven Cancers" Cancers 12, no. 5: 1246. https://doi.org/10.3390/cancers12051246
APA StyleCasarotto, M., Fanetti, G., Guerrieri, R., Palazzari, E., Lupato, V., Steffan, A., Polesel, J., Boscolo-Rizzo, P., & Fratta, E. (2020). Beyond MicroRNAs: Emerging Role of Other Non-Coding RNAs in HPV-Driven Cancers. Cancers, 12(5), 1246. https://doi.org/10.3390/cancers12051246







