1. Introduction
Nanomaterials have unique physical, chemical, electronic, optical, and catalytic properties, which are widely used in chemical industry, biomedicine, energy, environment, food production, aerospace, and other important fields [
1,
2,
3]. The increasing use of nanomaterials will inevitably lead to an increase in the exposure of nanomaterials in the ground, resulting in negative effects in the application process, and its potential impacts have gradually attracted people’s attention in human health and environmental complications [
4,
5,
6,
7]. In humans, drugs may induce toxicity leading to blood vessel division, causing contaminants or harmful microorganisms to enter into the human tissues or organs due to attachment to the nanomaterials, resulting in significant effects [
4,
6]. Over the past few decades, the field of nanotoxicity has significantly evolved through the booming of nanotechnology to address potential prolonged exposure of nanoparticles to the consumers through medical applications [
5,
6].
Titanium dioxide nanoparticles (TiO
2-NPs) are widely used for biomedical applications, drug research and development design, food additives and packaging materials, and so forth [
1,
2,
4,
7,
8]. Additionally, TiO
2-NPs can enter the human body and induce toxicity into different organs including, liver [
7,
8,
9], kidney [
10,
11], lung [
12], brain [
7,
9,
13], heart [
14], ovary and testis [
15,
16,
17]. In relation to this, inflammation is one among of the most important toxic mechanisms following TiO
2-NPs exposure. However, in toxicological researches, oral exposure of TiO
2-NPs to intestinal tract has not been well studied compared with skin contact or inhalation route due to lack of established global standards for toxicity levels [
18].
Most of the TiO2-NPs entering the body are metabolized and absorbed by the intestine and their adverse effects on gastrointestinal tract in humans and animals are rarely concerned. Colon cancer and inflammatory bowel disease (IBD) are the most common occurring diseases worldwide which might be contributed by TiO2-NPs exposure in the intestines. Therefore, there is an urgent need to confirm the safety of TiO2-NPs in human intestines by considering an increasing number of TiO2-NPs exposures.
In recent years, microRNAs have attracted extensive attention in toxicology. MicroRNAs have been shown to play an important role in gene regulation of vertebrate genome [
19,
20,
21]. It is predicted that microRNAs can regulate the expression of one-third of human genes [
21,
22]. Contrary, it has been found that nanoparticles can significantly alter the expression of microRNAs in cells [
23,
24,
25]. Hence, regulation of microRNAs as a key post-transcriptional regulator has attracted much attention in the molecular mechanism of nanoparticles toxicology [
23,
24]. Additionally, microRNAs are considered as a "buffer" for impedance gene expression variation [
26] and they help to maintain the robustness of biological processes [
21,
26]. In the face of stimuli caused by environmental changes, the regulation of microRNAs can be used to maintain biological functions, such as reinforcement of transcriptional programs and attenuation of aberrant transcripts in living organisms.
MicroRNAs may play important regulatory roles in gene expression and protein synthesis in biological systems, while TiO2-NPs are mainly digested and absorbed by the gastrointestinal tract. However, entrance of TiO2-NPs into the cells may lead to changes in miRNAs regulation and possibly body damage, these changes are urgently needed to be confirmed. Additionally, studying the damage and mechanism of TiO2-NPs on colon cells is of great significance in the prevention and treatment of colonic inflammation and colon cancer. Therefore, normal human colon cell lines (NCM460) and human colon cancer cells (HCT116) cell lines were used to investigate the effects of TiO2-NPs on microRNAs expression and correlating the expression of microRNAs with target genes in colon cells.
2. Materials and Methods
2.1. TiO2 Nanoparticles Characterization
TiO2-NPs of 25nm were applied in a concentration range of 3–60 μg/mL (Deke Daojin, Beijing, China). The morphology of nanoparticles was characterized by transmission electron microscopy (TEM) (JEOL Ltd., Tokyo, Japan) in dry form at Xiamen University, and the crystallized form of nanoparticles was characterized by X-ray diffraction (XRD) (BRUKER AXS GMBH, Karlsruhe, Germany).
For the preparation of TiO2-NPs exposure medium, powdered NPs were suspended in a serum-free 1640 basal medium solution, sonicated for 30 min before each use, and then added to the cell culture medium at test concentrations.
2.2. Cell Culture and Treatment
Human colon cancer cell lines (HCT116) and normal colon cell lines (NCM460) were provided by the College of Food Science and Engineering of the Central South University of Forestry and Technology, Changsha, China. The content of nano-TiO
2 in food is supposed to be 5–10g/kg according to the National Standard of the People’s Republic of China Food Safety National Standard GB2760-2011 [
27]. Fu et al. reported that Sprague Dawley rats were treated by intratracheal instillation with TiO
2-NPs at doses of 0.5, 4, and 32 mg/kg body weight, micro-TiO
2 with 32 mg/kg body weight and 0.9% NaCl, respectively [
28]. In other related studies, it was found that TiO
2-NPs (5, 15, or 30 μg/mL) significantly inhibited the viability of rat Sertoli cells in a concentration-dependent manner [
29]. Moreover, we found that 3 and 6 ug/mL TiO
2-NPs concentration can promote cell proliferation in our pre-experimental research. Based on the above considerations, we selected the concentration gradients as follows: 3, 6, 30, and 60 μg/mL. The cells were cultured in RPMI-1640 medium (Invitrogen Gibco, New York, USA) which contains fetal bovine serum (FBS), 1% Penicillin-Streptomycin. The cells were maintained in petri dish 10 cm in diameter at 37 °C and 5% CO
2. The RPMI-1640 medium was replaced every second day, and cells were passaged when adherence rate was approximately 85 % by washing with D-Hanks Buffered Saline Solution for three times, then with 0.25% Trypsin-EDTA Solution [
30]. Microscopically, when cells became round and dispersed, new medium was added to terminate the activity of trypsin, and trypsin was removed by centrifugation. The cells were suspended in fresh medium and subcultured in HCT116 and NCM460 cell lines, TiO
2-NPs exposures were performed for 24 h. The two types of cell lines were exposed to 0, 3, 6, 15, 30, and 60 μg/mL TiO
2-NPs to screen for cell viability.
2.3. Cell Viability Assays
MTT cell proliferation and cytotoxicity assay kits (Sangon, Shanghai, China) were used to detect colon cell viability after exposure to different concentrations of TiO
2-NPs. Cells were seeded in 96-well plates at 1 × 10
5 cells/well. After 12 h, the adherent growth of cells was observed under a microscope about 60%. Then the cells were exposed to 100 mL of different concentrations of TiO
2-NPs for 24 h. The cells were washed with D-Hanks and cultured for 4 h at 37 ℃ with 10 μL MTT (5 mg/mL). The formazan crystals were solubilised in 100 μL formazan solubilization solution for 10 min and absorbance read at 570 nm (Molecular Devices, San Jose, CA, USA) [
31]. The percentage of cell viability changes was calculated and compared with untreated cells.
2.4. Determination of Intracellular ROS Production
Intracellular ROS levels were measured using a fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) according to the manufacturer’s instructions (Beyotime, Shanghai, China) [
32]. The cells were inoculated in a 24-well plate with 1 × 10
5 cells/holes. After 12 h, the adherent growth of cells was observed under a microscope about 60%. The cells were exposed to 0, 3, 6, 15, 30, and 60 μg/mL TiO
2-NPs for 24 h, respectively. The cells were then washed three times with D-Hanks and placed in a fresh 1640 medium (without FBS) with 5 μM DCFH-DA for 20–30 min. Then, cells were washed with D-Hank. Immediately, the fluorescence of samples was measured at 488 and 525 nm excitation and emission wavelengths in Spectra Max i3x (Molecular Devices, California, USA). Compared to the cells that were not incubated with TiO
2-NPs, the inducing rate of ROS formation was calculated to increase the fluorescence intensity after exposure to TiO
2-NPs.
2.5. Small RNA Sequencing
HCT116 colon cancer cell lines were treated in triplicate with 0, 3 and 30 μg/mL TiO
2-NPs for 24 h for microRNA changes. Then, cells were washed 2× with D-Hanks, and the total RNA was extracted by SanPrep Column RNA Extraction Kit (Sangon Biotech, Shanghai, China) by following the manufacturer’s protocol. The extracted RNA samples were then gel purified and 18–30 nt fragment construction library was selected [
33]. Two kinds of adapters were respectively ligated to the ends of the small RNA fragments, and the prepared RNA was amplified by reverse transcription-polymerase chain reaction (RT-PCR). The 100–120 bp range PCR product was separated by PAGE electrophoresis, and by-products such as primer dimer were effectively removed. The isolated PCR product was loaded onto the BGISEQ-500 platform for sequencing. Experimental testing and data processing were performed by the Beijing Genomics Institute (BGI).
2.6. Prediction of microRNAs Target Genes
The data for microRNA profiling of TiO2-NPs-3 μg/mL and 30 μg/mL treated HCT116 cells are reported in this paper. MicroRNAs target mRNA were predicted using RNAhybrid, miRanda, and TargetScan. The final results of the target genes were predicted in combination of the three databases.
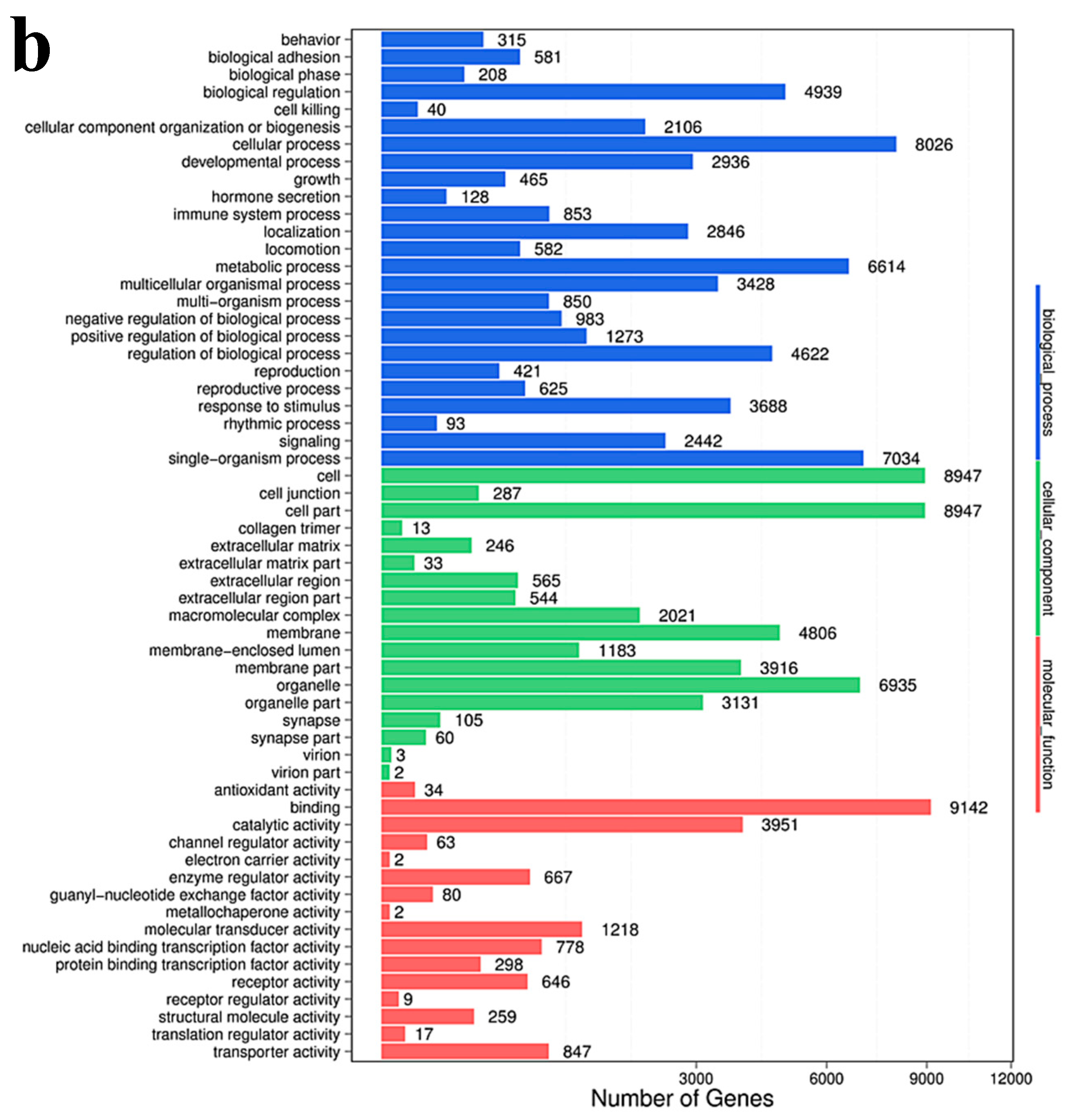
2.7. Gene Ontology Enrichment Analysis
Gene Ontology (GO) enrichment analysis finds all GO-terms that are significantly enriched in a list of differentially expressing small RNA’s (DESs) target genes, it also finds the genes that correspond to specific biological functions. To perform this analysis, BGI first maps all genes to GO-terms in the database, which calculates the gene numbers for every term. The hypergeometric test is then used to find significantly enriched GO-terms in the input gene list. This test is based on “GO-TermFinder”, using an algorithm developed by BGI to perform GO function analysis. The method used is described as follows:
In this algorithm, N is the number of all genes with GO annotation; n is the number of DES of the target gene in N; M is the number of all genes annotated to a particular GO term; m is the number of DES target genes in M.
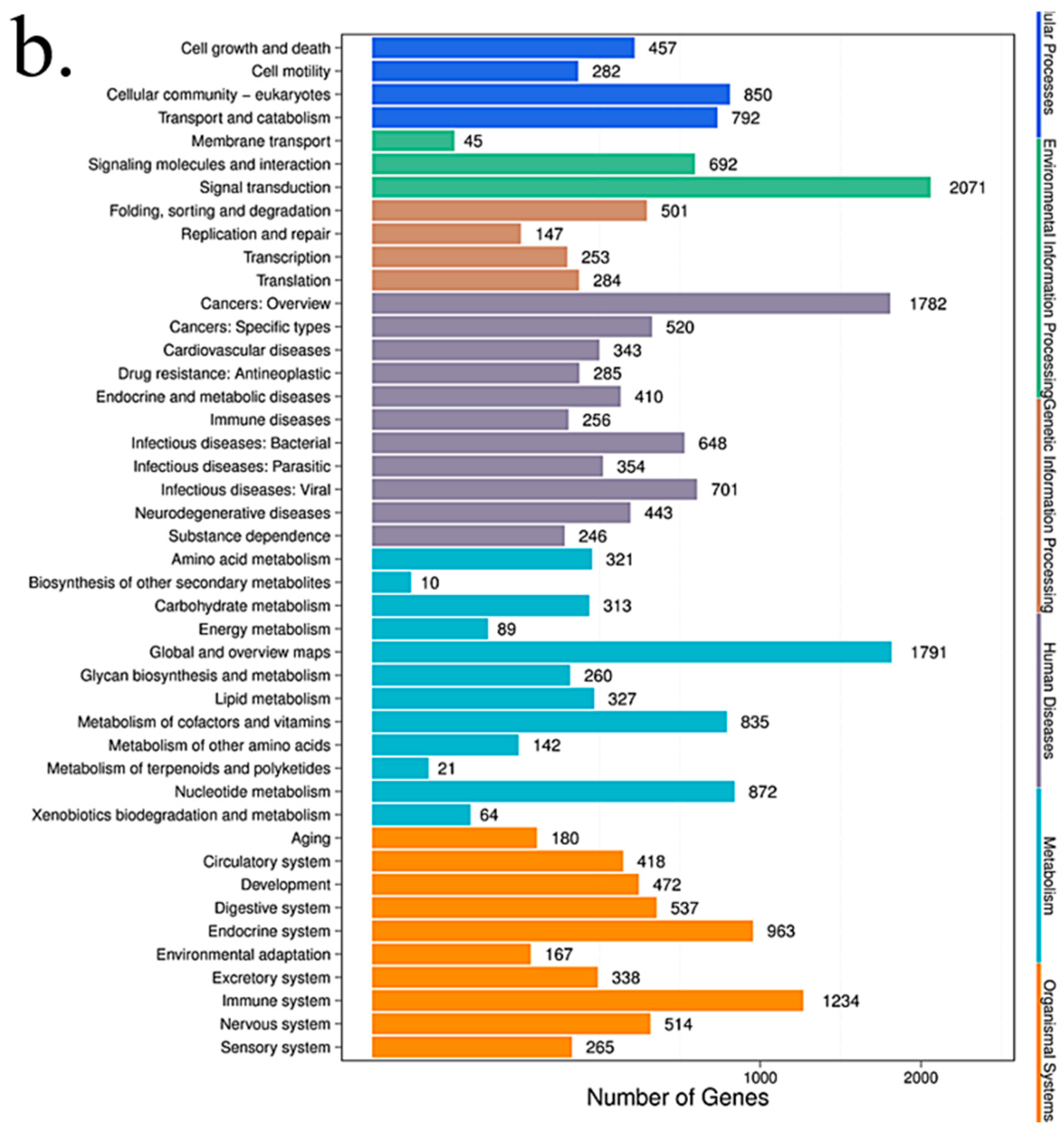
2.8. Pathway Analysis
Pathway-based analysis helps to further understand the biological functions of targeted genes that differentially express microRNAs. KEGG is used for pathway enrichment analysis. This analysis identifies significantly enriched metabolic pathways or signal transduction pathways in differential expressed microRNAs target genes when compared with the whole-genome background. The formula used is the same as that in GO analysis. However, N is the number of all genes with KEGG annotation; n is the number of DESs target genes in N; M is the number of all genes that are annotated to a specific pathway; m is the number of DESs target genes in M. The p-value is corrected by using the Bonferroni method, a corrected p-value < 0.05 is taken as a threshold. KEGG terms fulfilling this condition are defined as significantly enriched KEGG terms.
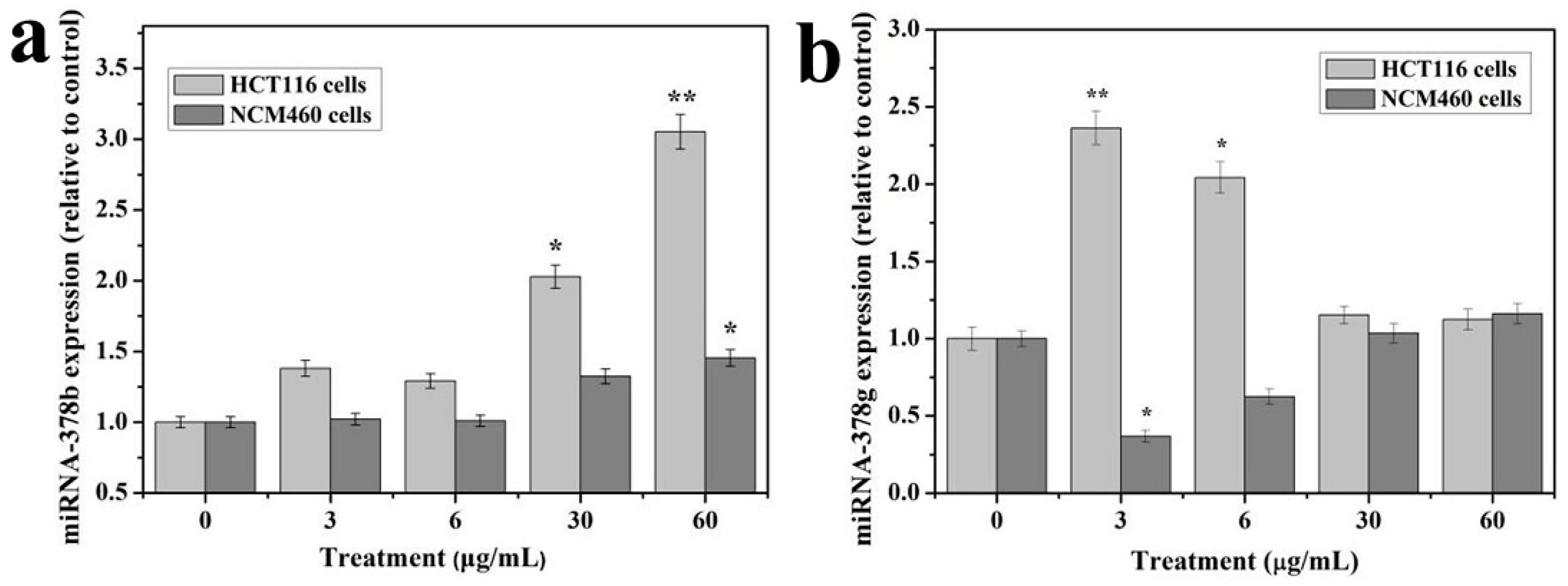
2.9. microRNA RT-qPCR
HCT116 cells and NCM460 cells were exposed to different concentrations of TiO2-NPs (0, 3, 15, 30, and 60 μg/mL) for 24 h, and the total RNA was extracted by SanPrep Column RNA Extraction Kit (Sangon Biotech, Shanghai, China) by following the manufacturer’s protocol. microRNA RT-PCR was performed by the microRNA first-strand cDNA Synthesis (Stem-loop method) from Sangon Biotech Co., Ltd (Shanghai, China) according to the manufacturer’s instructions. Briefly, 3 μg of total RNA was used to make the first-strand cDNA: 10 μL 2× microRNA L-RT solution mix, 1.5 μL microRNA L-RT enzyme mix, 1μL target gene’s stem-loop primer (10 μM), 1 μL U6 gene’s stem-loop primer (10 μM), 3 μg RNA sample and RNase free water to 20 μL. Gently mixed and centrifuged for 3~5 s. The reaction mixture was warmed at 16 °C for 30 min, then heated at 37 °C for 30 min, and at 85 °C for 5 min to inactivate the enzyme and then stored at 4 °C.
Fluorescence quantitative PCR was performed by using the prepared first strand cDNAs, were 1 μL/sample was used for one PCR reaction (in a 96-well plate) for each microRNA (three replicate wells per sample). Each PCR reaction (20 μL) contained 10 μL of 2× microRNA qPCR master mix, 0.5 μL forward primer (10 μM), 0.5μL of reverse primer (10 μM), 1 μL RT product and RNase-free water to 20 μL. The qPCR was performed with the CFX96 Touch (Bio-Rad, Hercules, CA, USA) and the reaction was set as follows: initial denaturation at 95 °C for 40 s followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 62 °C for 20 s and extension at 70 °C for 10 s. The primer sequences used are shown in
Table 1 and
Table 2.
2.10. Statistical Analysis
Results of at least three independent experiments are presented as mean ± SD. The comparison between treated and control groups was carried out by ANOVA analysis using the SPSS 23.0 software package (SPSS Company, Chicago, IL, USA). * p < 0.05 and ** p < 0.01 were considered as significant levels for all the analyses performed.
4. Discussion
Toxicological effects of nanoparticles are to reveal the basic laws of interaction between nanoparticles and living systems, which ensure the steady development of nanotechnology itself [
3,
4]. The TiO
2-NPs can easily enter the cell through the cell membrane because of the ultra-fine nature [
13,
14]. However, due to its large-specific surface area and high-reactivity, it may interact with many organelles and biological macromolecules in the cell, which can cause the intracellular microenvironment and cell function changes, and even cause genetic mutations and gene-level damage [
12,
13,
14,
15,
16,
17]. But so far, the mechanism of nanoparticle-induced tissue and cell damage is unclear, and the molecular level changes involved in this process are still unclear. Nevertheless, most researches have been focusing on the toxicity mechanism, exposure effects, toxicity effects, migration and transformation pathways, and risk prediction of TiO
2-NPs [
5,
6,
9,
16,
17].
Recently, studies on the interaction of biological macromolecules and molecular regulation mechanisms has just begun [
5,
17,
19]. At present, studies on various cell lines have shown that TiO
2 nanoparticles (NPs) induced the inflammatory response and cytotoxicity [
36,
37]. However, little is known about the potential mechanism of microRNA response in colon cells caused by TiO
2-NPs exposure. In this study, HCT116 and NCM460 cell lines were selected to evaluate the colonic cytotoxicity of TiO
2-NPs. Similarly, cell viability testing is a vital step in toxicology testing which shows the response of cells to drugs and provides information about cell death and survival [
5,
13,
14,
17,
18,
19,
36,
37]. In our study, when the concentration of TiO
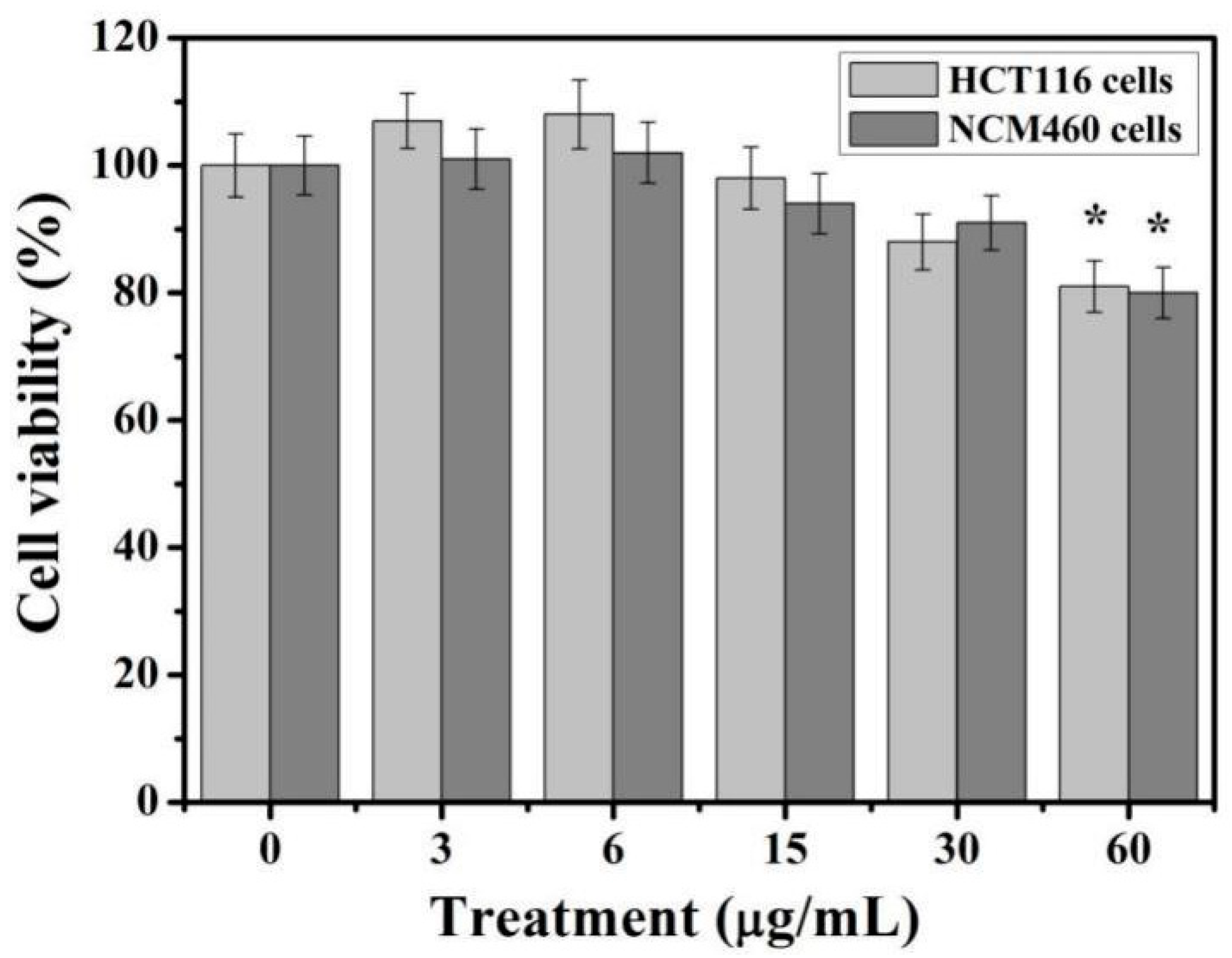
2-NPs reached 60 μg/mL, the viability of both cells decreased to about 80%. These results indicate that TiO
2-NPs are slight toxic to colon cells and inhibited cell viability.
The ROS mechanism is one of the most commonly accepted oxides for assessing the toxicity of nanomaterials [
9,
14]. Similarly, nanoparticles with active ingredients on the surface enhance their ability to produce ROS [
5,
6]. ROS mainly include superoxide ions, hydrogen peroxide, hydroxyl radicals, etc. Trace reactive oxygen species play an important role in the regulation of certain physiological phenomena, while excess reactive oxygen species can cause oxidative damage to cellular macromolecules [
37,
38]. If exogenous substances cause overactive oxygen in the body, or reduce the amount of antioxidants, the structure and function of cells may be destroyed when the balance of ROS in the body is destroyed [
38], and oxidative damage also causes apoptosis [
38,
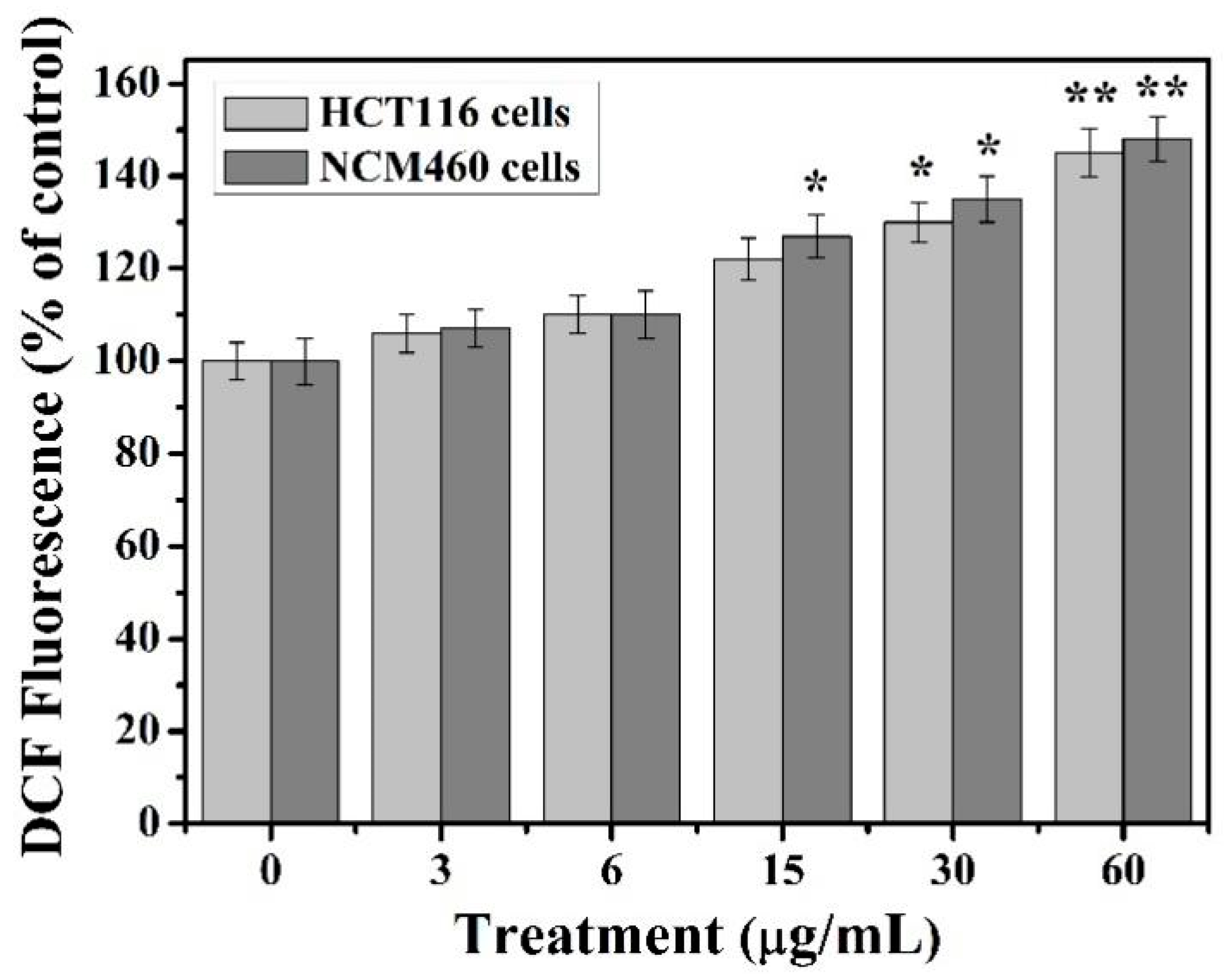
39]. Our results show that the content of reactive oxygen species in HCT116 and NCM460 cells increased with the increase of TiO
2-NPs concentration under the treatment of less than 60 μg/ml. Moreover, the intracellular ROS content considerably increased compared to control group at the concentration of 30–60 μg/mL TiO
2-NPs and reached the highest at 60 μg/mL concentration. This indicates that the excessive accumulation of ROS induced by TiO
2-NPs may be an important cause of cytotoxicity.
Due to unique physical and chemical properties of nanomaterials, traditional toxicological detection methods are not often completely applicable. Especially under low-dose exposure of nanomaterials (such as environmental exposure conditions), there is no significant oxidative stress damage, some indirect toxic effects such as secondary toxic reactions and compensatory damage effects are often ignored [
39]. In the maintenance and transmission of epigenetic information, RNA editing is an important carrier. RNA levels can change rapidly to alter the stress and metabolic state of cells under endogenous and exogenous stimuli [
23,
24,
40,
41,
42]. Therefore, microRNA expression levels are considered as sensitive early indicators of the cell/ body response to endogenous and exogenous stimuli [
23,
24,
42].
At present, there are many unknowns about the changing rules and related mechanisms of the changes in microRNA levels caused by nanomaterial exposures [
43,
44]. There are many regulatory factors in the development and progression of cancer. However, in vivo studies have demonstrated that titanium dioxide facilitates growth of chemically induced colorectal tumors and induce transcriptomic changes suggestive of an impaired immune system and cancer development [
35,
36,
43,
44]. MicroRNA regulates cell proliferation, differentiation, and apoptosis, such as cytokines, transcription factors, growth factors, pro-apoptotic and anti-apoptotic genes, by regulating the expression of signaling molecules, indicating that microRNAs are closely related to tumorigenesis development [
40,
41]. Deregulation of microRNA expression may be an important factor in tumorigenesis and cancer progression [
42,
45]. New researches suggest that certain expression changes in oncogenes or tumor suppressor genes may also lead to colon cancer [
43,
44,
46]. Moreover,
in vitro studies showed that TiO
2-NPs induced oxidative stress responses, DNA damage, and micronuclei formation, which showed that TiO
2-NPs induce gene expression changes related to signaling, the olfactory/GPCR receptor family, oxidative stress, inflammation, immune system, transport and cancer. The gene expression changes associated with the immune system and inflammation induced by TiO
2-NPs suggest the creation of a favorable environment for colon cancer development [
37,
43,
44].
Furthermore, using nuclear factor (NF)-kappa B reporter gene assays, TiO
2-NPs-induced IL8 mRNA expression occurs in part through activation of NF-kappa B and p38 mitogen-activated protein kinase pathways in the human colon adenocarcinoma cell line Caco-2 [
36] and those results demonstrate that TiO
2-NPs cause an activation of inflammatory pathways in the human colon adenocarcinoma cell line Caco-2. Nevertheless, studying changes in microRNA expression may help to more clearly understand the mechanisms of colon cancer development, progression, migration, and recurrence, as well as contribute to the early diagnosis and treatment of colon cancer. The detailed function of microRNA in colon cancer development has not been clearly defined. However, it has been found that the target pathways of microRNAs in colon tissue include cAMP, Wnt, TGF-β, MAPK, and mTOR signaling pathways [
47,
48,
49]. MicroRNAs may control genes in these pathways to regulate colon tissue development and pathology [
50].
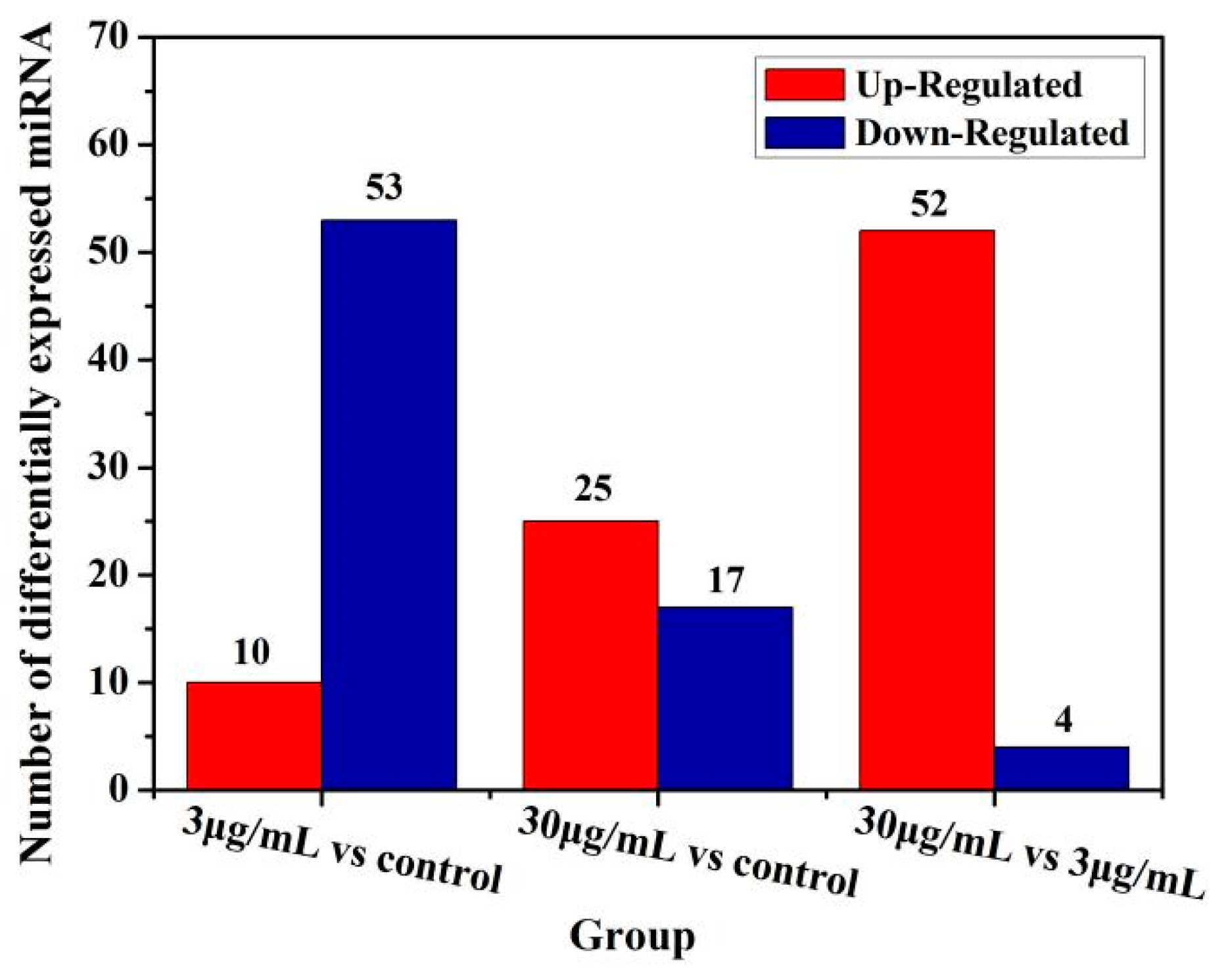
Moreover, function of some microRNAs has been extensively studied. Although the test dose of TiO2-NPs was not significantly cytotoxic to HCT116 cells, the expression of microRNA changed significantly. The expression of microRNA changes was examined in GO function analysis and ingenuity pathway analysis to delineate associated canonical/signaling pathways. The purpose was to analyze microRNA profiling of NCM460 and HCT116 cells after exposure to TiO2-NPs. Comparative analysis identified 34 and 24 microRNAs were significantly altered in the TiO2-NPs treated cells at concentrations of 3 and 30 μg/mL, respectively. Additionally, functional classification demonstrated that a large proportion of genes involved in thiamine metabolism, purine metabolism, glycosaminoglycan biosynthesis—chondroitin sulfate/dermatan sulfate, metabolic pathways, notch signaling pathway, glycosaminoglycan biosynthesis—heparan sulfate/heparin, transcriptional misregulation in cancer, lysine degradation, ECM-receptor interaction, amoebiasis, cAMP pathway and mTOR signaling pathway were significantly upregulated by TiO2-NPs.
In addition, bioinformatics analysis suggested that microRNA 378 may be an early indicator of cellular response to exogenous stimuli with apoptosis signals. Contrary, many genes are known to be regulated by microRNAs, and the expression of microRNAs is altered by nano-titanium dioxide treatment in these pathways. Hence, the microRNA 378 family that is involved in metabolism, transcriptional regulation, protein modification, cell proliferation, differentiation, and apoptosis were altered by TiO
2-NPs treatments. However, studies have shown that microRNA 378 has biological functions that regulate a variety of tumor cells, including cell proliferation, migration and invasion, and drug resistance [
51,
52,
53,
54]. In gastric cancer, microRNA 378 affects cell proliferation and cell cycle by targeting CDK6 and VEGF genes [
52]. Also, microRNA 378 inhibits the growth and proliferation of tumor cells in hepatocellular carcinoma [
54]. MicroRNA 378 inhibits proliferation and migration of colon cancer cells by targeting SDAD1 [
55]. Specifically, TiO
2-NPs altered the expression of certain microRNAs and also regulate relevant microRNAs to their target genes [
56,
57]. This suggests that nanoparticles may disrupt the expression of genes in cells, and as the regulatory systems, microRNAs may correct perturbations by enhancing transcriptional programs or attenuating aberrant transcripts.