Merkel Cell Carcinoma Treatment in Finland in 1986–2016—A Real-World Data Study
Abstract
1. Introduction
2. Results
2.1. Patient Demographics and Survival
2.2. Treatment and Outcomes
2.3. Effect of Radiation Therapy
3. Discussion
4. Materials and Methods
- Date of diagnosis
- Age at diagnosis
- ICD-O-3 topography
- 440 Skin of lip, NOS, 441 Eyelid, 442 External ear, 443 Skin of other and unspecified parts of face, 444 Skin of scalp and neck, 445 Skin of trunk, 446 Skin of upper limb and shoulder, 447 Skin of lower limb and hip, 449 Skin, NOS
- Stage
- 0 Unknown, 1 Localized, 2 Non-localized, only regional lymph node metastases, 3 Metastasized farther than to regional lymph nodes or invades adjacent tissues, 4 Non-localized, no information on extent, 5 Non-localized, also distant lymph node metastases. Stage of disease is recorded in the cancer registry files at four months after diagnosis and is not updated later.
- Date of death
- Cause of death
- Deceased due to this cancer or due to other causes
- Pre-operative RT before the re-excision of the primary tumour
- Re-excision of primary tumour
- SLNB
- CLND, including partial and total parotidectomy
- Post-operative treatment
- i.
- adjuvant RT to the primary tumour
- ii.
- adjuvant RT to the regional lymph nodes
- iii.
- adjuvant cytostatic therapy
- Therapy for progressive malignancy
- i.
- RT of local recidive tumour
- ii.
- cytostatic therapy of local tumour
- iii.
- received RT of metastasis
- Non-specified RT
- Palliative treatment
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Becker, J.C. Merkel cell carcinoma. Ann. Oncol. 2010, 21, vii81–vii85. [Google Scholar] [CrossRef] [PubMed]
- Swann, M.H.; Yoon, J. Merkel cell carcinoma. Semin. Oncol. 2007, 34, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Erovic, I.; Erovic, B.M. Merkel cell carcinoma: The past, the present, and the future. J. Ski. Cancer 2013, 2013, 929364. [Google Scholar] [CrossRef]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Bohling, T.; Joensuu, H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J. Natl. Cancer Inst. 2009, 101, 938–945. [Google Scholar] [CrossRef]
- Erovic, B.M.; Al Habeeb, A.; Harris, L.; Goldstein, D.P.; Ghazarian, D.; Irish, J.C. Significant overexpression of the Merkel cell polyomavirus (MCPyV) large T antigen in Merkel cell carcinoma. Head Neck 2013, 35, 184–189. [Google Scholar] [CrossRef]
- Paik, J.Y.; Hall, G.; Clarkson, A.; Lee, L.; Toon, C.; Colebatch, A.; Chou, A.; Gill, A.J. Immunohistochemistry for Merkel cell polyomavirus is highly specific but not sensitive for the diagnosis of Merkel cell carcinoma in the Australian population. Hum. Pathol. 2011, 42, 1385–1390. [Google Scholar] [CrossRef]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Penas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef]
- Pellitteri, P.K.; Takes, R.P.; Lewis, J.S., Jr.; Devaney, K.O.; Harlor, E.J.; Strojan, P.; Rodrigo, J.P.; Suarez, C.; Rinaldo, A.; Medina, J.E.; et al. Merkel cell carcinoma of the head and neck. Head Neck 2012, 34, 1346–1354. [Google Scholar] [CrossRef]
- Kukko, H.; Bohling, T.; Koljonen, V.; Tukiainen, E.; Haglund, C.; Pokhrel, A.; Sankila, R.; Pukkala, E. Merkel cell carcinoma—A population-based epidemiological study in Finland with a clinical series of 181 cases. Eur. J. Cancer 2012, 48, 737–742. [Google Scholar] [CrossRef]
- Ezaldein, H.H.; Ventura, A.; DeRuyter, N.P.; Yin, E.S.; Giunta, A. Understanding the influence of patient demographics on disease severity, treatment strategy, and survival outcomes in merkel cell carcinoma: A surveillance, epidemiology, and end-results study. Oncoscience 2017, 4, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Stang, A.; Hausen, A.Z.; Fischer, N.; DeCaprio, J.A.; Tothill, R.W.; Lyngaa, R.; Hansen, U.K.; Ritter, C.; Nghiem, P.; et al. Epidemiology, biology and therapy of Merkel cell carcinoma: Conclusions from the EU project IMMOMEC. Cancer Immunol. Immunother. CII 2018, 67, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Becker, J.C.; Nghiem, P.; Ferlay, J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: An international assessment. Eur. J. Cancer 2018, 94, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.; Carr, M.; Zager, J.S.; Naghavi, A.; Smith, F.O.; Cruse, C.W.; Messina, J.L.; Russell, J.; Rao, N.G.; Fulp, W.; et al. Radiation Therapy is Associated with Improved Outcomes in Merkel Cell Carcinoma. Ann. Surg. Oncol. 2016, 23, 3572–3578. [Google Scholar] [CrossRef]
- Iyer, J.G.; Parvathaneni, U.; Gooley, T.; Miller, N.J.; Markowitz, E.; Blom, A.; Lewis, C.W.; Doumani, R.F.; Parvathaneni, K.; Anderson, A.; et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med. 2015, 4, 1161–1170. [Google Scholar] [CrossRef]
- Lebbe, C.; Becker, J.C.; Grob, J.J.; Malvehy, J.; Del Marmol, V.; Pehamberger, H.; Peris, K.; Saiag, P.; Middleton, M.R.; Bastholt, L.; et al. Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 2396–2403. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Blitzblau, R.; Bowen, G.M.; Contreras, C.M.; Daniels, G.A.; Decker, R.; et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2018, 16, 742–774. [Google Scholar] [CrossRef]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbe, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17077. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- Garbutcheon-Singh, K.B.; Veness, M.J. The role of radiotherapy in the management of non-melanoma skin cancer. Australas. J. Dermatol. 2019, 60, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, A.; Torchio, M.; Prinzi, N.; Trevisan, F.; Dallera, P.; De Stefani, A.; Russo, A.; Vitali, E.; Bruschieri, L.; et al. Adjuvant radiotherapy for Merkel cell carcinoma: A systematic review and meta-analysis. Radiother. Oncol. 2019, 134, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Nghiem, P.; Bhatia, S.; Parvathaneni, U.; Schwartz, J.L. Merkel Cell Carcinoma Cell Death in Response to Single-Fraction Irradiation: Implications for Immune Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E714. [Google Scholar] [CrossRef]
- Xu, M.J.; Wu, S.; Daud, A.I.; Yu, S.S.; Yom, S.S. In-field and abscopal response after short-course radiation therapy in patients with metastatic Merkel cell carcinoma progressing on PD-1 checkpoint blockade: A case series. J. Immunother. Cancer 2018, 6, 43. [Google Scholar] [CrossRef]
- Schadendorf, D.; Lebbe, C.; Zur Hausen, A.; Avril, M.F.; Hariharan, S.; Bharmal, M.; Becker, J.C. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur. J. Cancer 2017, 71, 53–69. [Google Scholar] [CrossRef]
- Toker, C. Trabecular carcinoma of the skin. Arch. Dermatol. 1972, 105, 107–110. [Google Scholar] [CrossRef]
- Agelli, M.; Clegg, L.X. Epidemiology of primary Merkel cell carcinoma in the United States. J. Am. Acad. Dermatol. 2003, 49, 832–841. [Google Scholar] [CrossRef]
- Agelli, M.; Clegg, L.X.; Becker, J.C.; Rollison, D.E. The etiology and epidemiology of merkel cell carcinoma. Curr. Probl. Cancer 2010, 34, 14–37. [Google Scholar] [CrossRef]
- Koljonen, V. Prognostic factors in primary merkel cell carcinoma. In Dissertationes Scholae Doctoralis ad Sanitatem Investigandam Universitatis Helsinkiensis; Univ. Printing House: Helsinki, Finland, 2004. [Google Scholar]
- Koljonen, V.; Tarkkanen, M.; Tukiainen, E.; Bohling, T. Merkel cell carcinoma in elderly. Duodecim 2005, 121, 2205–2213. [Google Scholar]
- Bichakjian, C.K.; Olencki, T.; Alam, M.; Andersen, J.S.; Berg, D.; Bowen, G.M.; Cheney, R.T.; Daniels, G.A.; Glass, L.F.; Grekin, R.C.; et al. Merkel cell carcinoma, version 1.2014. J. Natl. Compr. Cancer Netw. JNCCN 2014, 12, 410–424. [Google Scholar] [CrossRef]
- Miller, S.J.; Alam, M.; Andersen, J.; Berg, D.; Bichakjian, C.K.; Bowen, G.; Cheney, R.T.; Glass, L.F.; Grekin, R.C.; Hallahan, D.E.; et al. NCCN Clinical Practice Guidelines in Oncology: Merkel Cell Carcinoma. J. Natl. Compr. Cancer Netw. JNCCN 2009, 7, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Sahi, H.; Bohling, T.; Koljonen, V. Merkelinsolukarsinooma—Mitä uutta? Duodecim 2017, 133, 2365–2371. [Google Scholar]
- Steuten, L.; Garmo, V.; Phatak, H.; Sullivan, S.D.; Nghiem, P.; Ramsey, S.D. Treatment Patterns, Overall Survival, and Total Healthcare Costs of Advanced Merkel Cell Carcinoma in the USA. Appl. Health Econ. Health Policy 2019, 17, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Ciccolallo, L.; Kunkler, I.; Capocaccia, R.; Berrino, F.; Coleman, M.P.; De Angelis, R.; Faivre, J.; Lutz, J.M.; Martinez, C.; et al. Survival from rare cancer in adults: A population-based study. Lancet Oncol. 2006, 7, 132–140. [Google Scholar] [CrossRef]
- Schneider, S.; Thurnher, D.; Erovic, B.M. Merkel cell carcinoma: Interdisciplinary management of a rare disease. J. Ski. Cancer 2013, 2013, 189342. [Google Scholar] [CrossRef]
- Ghadjar, P.; Kaanders, J.H.; Poortmans, P.; Zaucha, R.; Krengli, M.; Lagrange, J.L.; Ozsoy, O.; Nguyen, T.D.; Miralbell, R.; Baize, A.; et al. The essential role of radiotherapy in the treatment of Merkel cell carcinoma: A study from the Rare Cancer Network. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e583–e591. [Google Scholar] [CrossRef]
- Howle, J.R.; Hughes, T.M.; Gebski, V.; Veness, M.J. Merkel cell carcinoma: An Australian perspective and the importance of addressing the regional lymph nodes in clinically node-negative patients. J. Am. Acad. Dermatol. 2012, 67, 33–40. [Google Scholar] [CrossRef]
- Han, A.Y.; Patel, P.B.; Anderson, M.; Diaz, M.F.P.; Chin, R.; St John, M.A. Adjuvant radiation therapy improves patient survival in early-stage merkel cell carcinoma: A 15-year single-institution study. Laryngoscope 2018, 128, 1862–1866. [Google Scholar] [CrossRef]
- Fiedler, E.; Vordermark, D. Outcome of Combined Treatment of Surgery and Adjuvant Radiotherapy in Merkel Cell Carcinoma. Acta Derm. Venereol. 2018, 98, 699–703. [Google Scholar] [CrossRef]
- Jouary, T.; Leyral, C.; Dreno, B.; Doussau, A.; Sassolas, B.; Beylot-Barry, M.; Renaud-Vilmer, C.; Guillot, B.; Bernard, P.; Lok, C.; et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: A multicentric prospective randomized study. Ann. Oncol. 2012, 23, 1074–1080. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, A.H. Effect of radiation therapy on survival in patients with resected Merkel cell carcinoma: A propensity score surveillance, epidemiology, and end results database analysis. JAMA Dermatol. 2013, 149, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Lorenz, E.; Ugurel, S.; Eigentler, T.K.; Kiecker, F.; Pfohler, C.; Kellner, I.; Meier, F.; Kahler, K.; Mohr, P.; et al. Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget 2017, 8, 79731–79741. [Google Scholar] [CrossRef] [PubMed]
- Cowey, C.L.; Mahnke, L.; Espirito, J.; Helwig, C.; Oksen, D.; Bharmal, M. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017, 13, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Eigentler, T.; Frerich, B.; Gambichler, T.; Grabbe, S.; Holler, U.; Klumpp, B.; Loquai, C.; Krause-Bergmann, A.; Muller-Richter, U.; et al. S2k guidelines for Merkel cell carcinoma (MCC, neuroendocrine carcinoma of the skin)—Update 2018. J. Dtsch. Dermatol. Ges. 2019, 17, 562–576. [Google Scholar] [CrossRef]
- Bloom, B.C.; Augustyn, A.; Pezzi, T.A.; Menon, H.; Mayo, L.L.; Shah, S.J.; Schwartz, D.L.; Chmura, S.J.; Johnson, F.M.; Welsh, J.W.; et al. Rescue of Immunotherapy-Refractory Metastatic Merkel Cell Carcinoma With Conventionally Fractionated Radiotherapy and Concurrent Pembrolizumab. Front. Oncol. 2019, 9, 223. [Google Scholar] [CrossRef]
- Filatenkov, A.; Baker, J.; Mueller, A.M.; Kenkel, J.; Ahn, G.O.; Dutt, S.; Zhang, N.; Kohrt, H.; Jensen, K.; Dejbakhsh-Jones, S.; et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin. Cancer Res. 2015, 21, 3727–3739. [Google Scholar] [CrossRef]
- Garnett, C.T.; Palena, C.; Chakraborty, M.; Tsang, K.Y.; Schlom, J.; Hodge, J.W. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004, 64, 7985–7994. [Google Scholar] [CrossRef]
- Harrington, C.; Kwan, W. Outcomes of Merkel cell carcinoma treated with radiotherapy without radical surgical excision. Ann. Surg. Oncol. 2014, 21, 3401–3405. [Google Scholar] [CrossRef]
- Bajetta, E.; Celio, L.; Platania, M.; Lo Vullo, S.; Patuzzo, R.; Maurichi, A.; Santinami, M. Single-Institution Series of Early-Stage Merkel Cell Carcinoma: Long-Term Outcomes in 95 Patients Managed with Surgery Alone. Ann. Surg. Oncol. 2009, 16, 2985–2993. [Google Scholar] [CrossRef]
- Tello, T.L.; Coggshall, K.; Yom, S.S.; Yu, S.S. Merkel cell carcinoma: An update and review: Current and future therapy. J. Am. Acad. Dermatol. 2018, 78, 445–454. [Google Scholar] [CrossRef]
- Santamaria-Barria, J.A.; Boland, G.M.; Yeap, B.Y.; Nardi, V.; Dias-Santagata, D.; Cusack, J.C., Jr. Merkel cell carcinoma: 30-year experience from a single institution. Ann. Surg. Oncol. 2013, 20, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Teppo, L.; Pukkala, E.; Lehtonen, M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994, 33, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Teppo, L.; Pukkala, E.; Saxen, E. Multiple cancer--an epidemiologic exercise in Finland. J. Natl. Cancer Inst. 1985, 75, 207–217. [Google Scholar] [PubMed]
- Sund, R. Quality of the Finnish Hospital Discharge Register: A systematic review. Scand. J. Public Health 2012, 40, 505–515. [Google Scholar] [CrossRef] [PubMed]
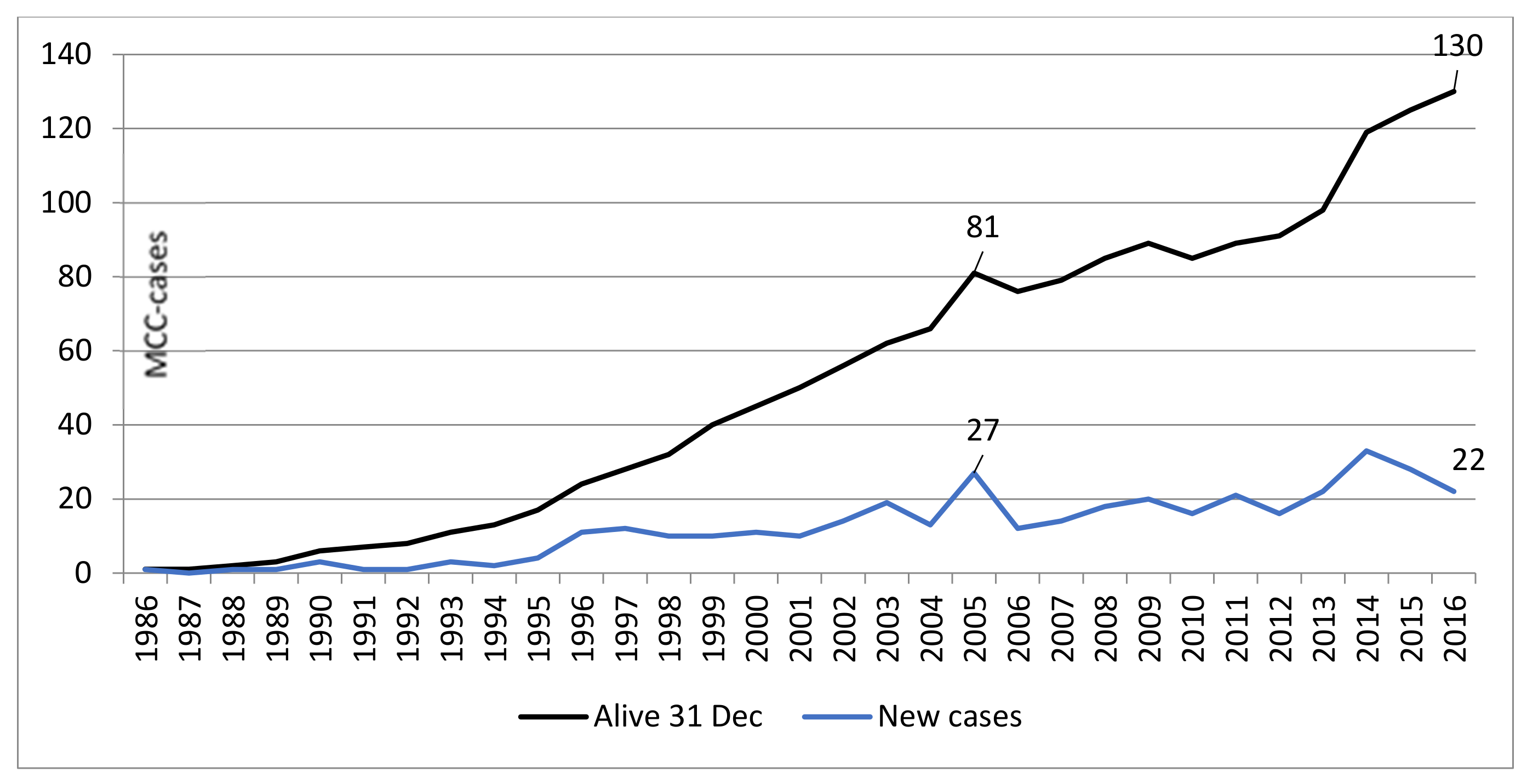
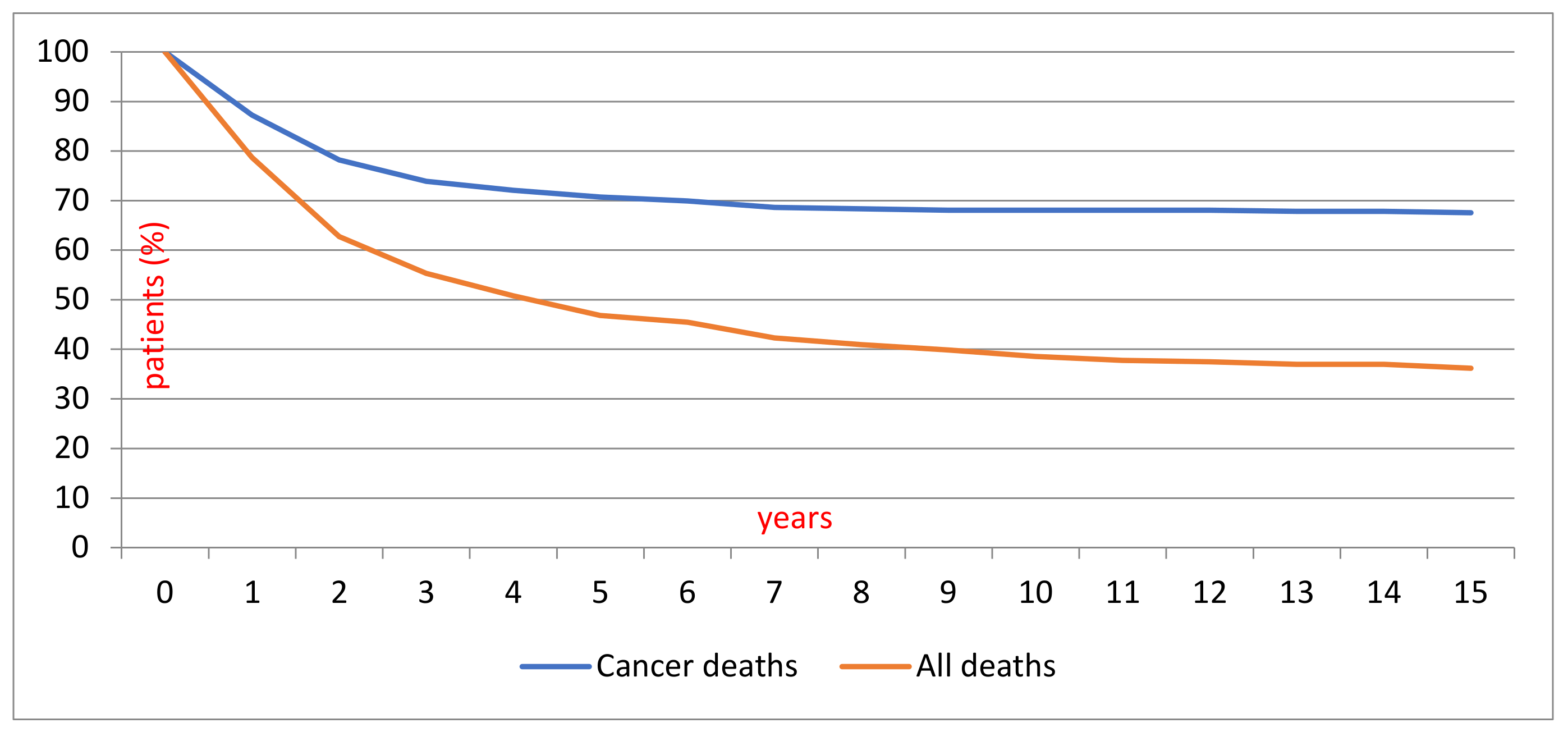
| Years 1986–2016 | Years 1986–2004 | Years 2005–2016 | |
|---|---|---|---|
| N 376 | N 127 | N 249 | |
| Male/female (%) | 132/244 (35/65) | 40/87 (31/69) | 92/157 (36/63) |
| Age at diagnosis, years | |||
| Range | 27–102 | 27–100 | 47–102 |
| Mean (SD) | 78.7 (10.6) | 76.6 (12.1) | 79.8 (9.6) |
| Tumour location | |||
| 440 Skin of lip, NOS | 4 (1.1) | - (0.0) | 4 (1.6) |
| 441 Eyelid | 11 (2.9) | 4 (3.1) | 7 (2.8) |
| 442 External ear | 18 (4.8) | 6 (4.7) | 12 (4.8) |
| 443 Skin of other and unspecified parts of face | 167 (44.4) | 57 (44.9) | 110 (44) |
| 444 Skin of scalp and neck | 24 (6.4) | 9 (7.1) | 15 (6) |
| 445 Skin of trunk | 28 (7.5) | 8 (6.3) | 20 (8) |
| 446 Skin of upper limb and shoulder | 53 (14.1) | 17 (13.4) | 36 (14) |
| 447 Skin of lower limb and hip | 47 (12.5) | 16 (12.6) | 31 (12.5) |
| 449 Skin, NOS | 24 (6.4) | 10 (7.9) | 14 (5.6) |
| Stage | |||
| 0 Unknown | 158 (42.0) | 42 (33) | 116 (46) |
| 1 Localized | 124 (33.0) | 64 (50) | 60 (24) |
| 2 Non-localised, only regional lymph node metastases | 28 (7.5) | 9 (7.1) | 19 (7.6) |
| 3 Metastasised farther than to regional lymph nodes or invades adjacent tissues | 23 (6.1) | 4 (3.1) | 19 (7.6) |
| 4 Non-localized, no information on extent | 40 (10.6) | 8 (6.3) | 32 (12.9) |
| 5 Non-localized, also distant lymph node metastases | 3 (0.8) | - (0.0) | 3 (1.2) |
| Survival years, cut off 31.12.2016 | |||
| Alive, n | 130 (34.6%) | 17 (13.4%) | 113 (45.4%) |
| dead due to this cancer, n | 103 (27.4%) | 36 (28.3%) | 67 (26.9%) |
| dead due to other cause, n | 143 (38.0%) | 74 (58.3%) | 69 (27.7%) |
| Mean overall survival years (SD) n | 376 | 127 | 249 |
| diagnose date to end of surveillance/death | 4.2 (4.9) | 6.6 (6.7) | 2.9 (2.9) |
| range | 0-27 | 0–27 | 0–12 |
| Deceased due to this cancer (SD) n | 103 | 36 | 67 |
| Mean survival, years | 1.8 (2.0) | 2.4 (2.6) | 1.5 (1.4) |
| range | 0–15 | 0–15 | 0.0–7 |
| Deceased due to other cause (SD) n | 143 | 74 | 69 |
| Mean survival, years | 4.1 (4.7) | 6.1 (5.8) | 2.1 (2.2) |
| range | 0–25 | 0–25 | 0–11 |
| Years 1986–2016 | Years 1986–2004 | Years 2005–2016 | ||
|---|---|---|---|---|
| N 376 (%) | N 127 (%) | N 249 (%) | ||
| No Treatment | No treatment | 68 (18) | 44 (34.6) | 24 (9.6) |
| Single treatment | Re-excision of the primary tumour (Re-ex) | 130 (34) | 55 (43) | 75 (30) |
| Sentinel lymph node biopsy (SLNB) | 1 (0.2) | - | 1 (0.4) | |
| Complete lymphnode dissection (CLND) | 5 (1.3) | 2 (1.6) | 3 (1.2) | |
| Pre-operative radiotherapy | 1 (0.2) | - | 1 (0.4) | |
| Adjuvant radiotherapy to the primary tumour | 4 (1.1) | 2 (1.6) | 2 (0.8) | |
| Adjuvant radiotherapy to the regional lymphnodes | 1 (0.2) | 1 (0.8) | - | |
| Radiotherapy to metastasis | 1 (0.2) | - | 1 (0.4) | |
| Non-specified radiation therapy—recorded as modality | 1(0.2) | - | 1 (0.4) | |
| Non-specified radiation therapy | 3 (0.8) | - | 3 (1.2) | |
| Palliative radiotherapy | 1 (0.2) | - | 1 (0.4) | |
| Radiotherapy of metastases | 2 (0.5) | - | 2 (0.8) | |
| Multiple treatments | Re-ex + SLNB | 37 (9.8) | 3 (2.4) | 34 (13.7) |
| Re-ex + CLND | 50 (13.3) | 16 (12.6) | 34 (13.7) | |
| Re-ex+ adjuvant radiotherapy to the primary tumour | 15 (4) | 1 (0.8) | 14 (5.6) | |
| Re-ex + chemotherapy to metastasised malignancy | 1 (0.2) | - | 1 (0.4) | |
| Re-ex + non-specified radiation therapy | 6 (1.6) | 1 (0.8) | 5 (2) | |
| Re-ex + SLNB + CLND | 7 (1.9) | 1 (0.8) | 6 (2.4) | |
| Re-ex + SLNB + adjuvant radiotherapy to the primary tumour | 8 (2.1) | - | 8 (3.2) | |
| Re-ex + CLND + adjuvant radiotherapy to the primary tumour | 10 (2.7) | - | 10 (4) | |
| Re-ex+ adjuvant radiotherapy to the primary tumour + radiotherapy to metastasis | 1 (0.2) | - | 1 (0.4) | |
| CLND + adjuvant radiotherapy to the regional lymphnodes | 1 (0.2) | - | 1 (0.4) | |
| CLND + radiotherapy to metastasis | 1 (0.2) | - | 1 (0.4) | |
| CLND + pre-operative chemotherapy | 2 (0.5) | - | 2 (0.8) | |
| Adjuvant radiotherapy to the primary tumour + pre-operative chemotherapy | 1 (0.2) | - | 1 (0.4) | |
| Radiotherapy to metastasis + pre-operative chemotherapy | 1 (0.2) | - | 1 (0.4) | |
| Re-ex + SLNB + non-specified radiation therapy | 2 (0.5) | - | 2 (0.8) | |
| Re-ex + SLNB + CLND + adjuvant radiotherapy to the primary tumour | 1 (0.2) | - | 1 (0.4) | |
| Re-ex + SLNB + CLND + chemotherapy to metastasized malignancy | 1 (0.2) | - | 1 (0.4) | |
| Re-ex + CLND + non-specified radiation therapy | 9 (2.4) | 1 (0.8) | 8 (3.2) | |
| Re-ex + SLNB + adjuvant radiotherapy to the primary tumour + palliative radiotherapy | 2 (0.5) | - | 2 (0.8) | |
| Re-ex + CLND + adjuvant radiotherapy to the primary tumour + radiotherapy to metastasis + pre-operative chemotherapy | 1 (0.2) | - | 1 (0.4) | |
| Re-ex + CLND + adjuvant radiotherapy to the primary tumour + radiotherapy to metastasis + chemotherapy to metastasized malignancy | 1 (0.2) | - | 1 (0.4) |
| Treatment | Years 1986–2016 | Years 1986–2004 | Years 2005–2016 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N 376 | N 127 | N 249 | |||||||||||||
| N (%) | Male/Female | Mean Age Years | Death to Cancer % | Survival Years Mean | N (%) | Male/Female | Mean age years | Death to cancer % | Survival, Years Mean | N (%) | Male/Female | Mean Age Years | Death to Cancer % | Survival, Years Mean | |
| No treatment | 68 (18) | 22/46 | 76.7 | 26.5 | 5.1 | 44 (34.6) | 15/30 | 75.2 | 27.3 | 6.4 | 24 (9.6) | 8/16 | 79.4 | 13.0 | 2.6 |
| Re-excision of the primary tumour (Re-ex) | 130 (34) | 33/97 | 80.9 | 20.0 | 4.9 | 55 (43) | 13/42 | 78.0 | 14.5 | 8.0 | 75 (30) | 20/55 | 83.1 | 29.0 | 2.7 |
| Sentinel lymph node biopsy (SLNB) | 1 (0.2) | 0/1 | 79.2 | - | 11.5 | 1 (0.4) | 0/1 | 79.2 | 0.0 | 11.5 | |||||
| Complete lymph node dissection (CLND) | 5 (1.3) | 2/2 | 79.4 | 80.0 | 3.4 | 2 (1.6) | 1/2 | 73.6 | 100.0 | 3.5 | 3 (1.2) | 1/2 | 83.2 | 0.0 | 3.4 |
| Pre-operative radiotherapy | 1 (0.2) | 0/1 | 83.9 | 100.0 | 1.8 | 1 (0.4) | 0/1 | 83.9 | 0.0 | 1.8 | |||||
| Adjuvant radiotherapy to the primary tumour | 4 (1.1) | 1/3 | 82.3 | 0.0 | 3.7 | 2 (1.6) | 1/1 | 84.2 | 0.0 | 6.5 | 2 (0.8) | 0/2 | 80.4 | 1.0 | 0.8 |
| Adjuvant radiotherapy to the regional lymph nodes | 1 (0.2) | 0/1 | 87.0 | 0.0 | 12.6 | 1 (0.8) | 0/1 | 87.0 | 0.0 | 12.6 | - | -/- | - | - | - |
| Radiotherapy to metastasis | 1 (0.2) | 1/0 | 79.7 | 0.0 | 8.1 | 1 (0.4) | 1/0 | 79.7 | 0.0 | 8.1 | |||||
| Non-specified radiation therapy—recorded as modality | 1(0.2) | 0/1 | 86.2 | 100.0 | 1.7 | 1 (0.4) | 0/1 | 86.2 | 0.0 | 1.7 | |||||
| Non-specified radiation therapy | 3 (0.8) | 1/2 | 89.0 | 66.7 | 1.0 | 3 (1.2) | 1/2 | 89.0 | 1.0 | 1.0 | |||||
| Palliative radiotherapy | 1 (0.2) | 1/0 | 68.7 | 100.0 | 0.1 | 1 (0.4) | 1/0 | 68.7 | 0.0 | 0.1 | |||||
| Radiotherapy of metastases | 2 (0.5) | 2/0 | 89.8 | 0.0 | 1.2 | 2 (0.8) | 2/0 | 89.8 | 1.0 | 1.2 | |||||
| Re-ex + SLNB | 37 (9.8) | 18/19 | 77.8 | 27.0 | 3.6 | 3 (2.4) | 2/1 | 81.1 | 66.7 | 4.2 | 34 (13.7) | 16/18 | 77.5 | 4.0 | 3.5 |
| Re-ex + CLND | 50 (13.3) | 22/28 | 78.7 | 34.0 | 3.7 | 16 (12.6) | 7/9 | 75.6 | 56.3 | 3.9 | 34 (13.7) | 15/19 | 80.1 | 9.0 | 3.6 |
| Re-ex + adjuvant radiotherapy to the primary tumour | 15 (4) | 7/8 | 80.7 | 26.7 | 2.3 | 1 (0.8) | 0/1 | 90.9 | 100.0 | 1.4 | 14 (5.6) | 7/7 | 80.0 | 2.0 | 2.4 |
| Re-ex + chemotherapy to metastasized malignancy | 1 (0.2) | 0/1 | 84.1 | 0.0 | 6.8 | 1 (0.4) | 0/1 | 84.1 | 0.0 | 6.8 | |||||
| Re-ex + non-specified radiation therapy | 6 (1.6) | 1/5 | 78.7 | 33.3 | 3.7 | 1 (0.8) | 1/0 | 68.7 | 100.0 | 0.3 | 5 (2) | 0/5 | 80.7 | 2.0 | 4.3 |
| Re-ex + SLNB + CLND | 7 (1.9) | 3/4 | 70.2 | 14.3 | 3.9 | 1 (0.8) | 0/1 | 59.2 | 0.0 | 7.2 | 6 (2.4) | 3/3 | 72.0 | 2.0 | 3.4 |
| Re-ex + SLNB + adjuvant radiotherapy to the primary tumour | 8 (2.1) | 3/5 | 72.4 | 12.5 | 1.7 | 8 (3.2) | 3/5 | 72.4 | 1.0 | 1.7 | |||||
| Re-ex + CLND + adjuvant radiotherapy to the primary tumour | 10 (2.7) | 2/8 | 70.2 | 30.0 | 2.7 | 10 (4) | 2/8 | 70.2 | 0.0 | 2.7 | |||||
| Re-ex + adjuvant radiotherapy to the primary tumour + radiotherapy to metastasis | 1 (0.2) | 0/1 | 72.7 | 0.0 | 1.2 | 1 (0.4) | 0/1 | 72.7 | 0.0 | 1.2 | |||||
| CLND + adjuvant radiotherapy to the regional lymphnodes | 1 (0.2) | 1/0 | 67.2 | 0.0 | 11.3 | 1 (0.4) | 1/0 | 67.2 | 0.0 | 11.3 | |||||
| CLND + radiotherapy to metastasis | 1 (0.2) | 0/1 | 89.6 | 100.0 | 2.5 | 1 (0.4) | 0/1 | 89.6 | 0.0 | 2.5 | |||||
| CLND + pre-operative chemotherapy | 2 (0.5) | 1/1 | 84.8 | 100.0 | 1.1 | 2 (0.8) | 1/1 | 84.8 | 0.0 | 1.1 | |||||
| Adjuvant radiotherapy to the primary tumour + pre-operative chemotherapy | 1 (0.2) | 0/1 | 83.5 | 100.0 | 2.2 | 1 (0.4) | 0/1 | 83.5 | 0.0 | 2.2 | |||||
| Radiotherapy to metastasis+ pre-operative chemotherapy | 1 (0.2) | 1/0 | 72.6 | 100.0 | 1.1 | 1 (0.4) | 1/0 | 72.6 | 0.0 | 1.1 | |||||
| Re-ex + SLNB+ non-specified radiation therapy | 2 (0.5) | 0/2 | 83.6 | 0.0 | 3.1 | 2 (0.8) | 0/2 | 83.6 | 1.0 | 3.1 | |||||
| Re-ex+SLNB+CLND+ adjuvant radiotherapy to the primary tumour | 1 (0.2) | 1/0 | 69.9 | 0.0 | 2.6 | 1 (0.4) | 1/0 | 69.9 | 0.0 | 2.6 | |||||
| Re-ex + SLNB + CLND + chemotherapy to metastasized malignanacy | 1 (0.2) | 1/0 | 74.4 | 0.0 | 1.2 | 1 (0.4) | 1/0 | 74.4 | 1.0 | 1.2 | |||||
| Re-ex + CLND + non-specified radiation therapy | 9 (2.4) | 5/4 | 75.3 | 66.7 | 2.1 | 1 (0.8) | 1/0 | 49.8 | 100.0 | 2.5 | 8 (3.2) | 4/4 | 78.5 | 2.0 | 2.0 |
| Re-ex + SLNB+ adjuvant radiotherapy to the primary tumour + Palliative radiotherapy | 2 (0.5) | 1/1 | 71.6 | 50.0 | 0.8 | 2 (0.8) | 1/1 | 71.6 | 0.0 | 0.8 | |||||
| Re-ex + CLND + adjuvant radiotherapy to the primary tumour + radiotherapy to metastasis+ pre-operative chemotherapy | 1 (0.2) | 1/0 | 76.0 | 0.0 | 1.9 | 1 (0.4) | 1/0 | 76.0 | 0.0 | 1.9 | |||||
| Re-ex + CLND + adjuvant radiotherapy to the primary tumour + chemotherapy to metastasized malignancy | 1 (0.2) | 1/0 | 76.7 | 0.0 | 1.2 | 1 (0.4) | 1/0 | 76.7 | 0.0 | 1.2 | |||||
| Treatment Categories | N | Male/Female | Mean Age Years | Statistical Difference, Age, p | Head and Neck | Trunk | Upper Limb and Shoulder | Lower Limb and Hip | Skin, not specified | Statistical Difference, Location, p | Death to Cancer (%) | Statistical Difference, Death to Cancer, p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excision | Re-excision of the primary tumour | 75 | 20/55 | 83.1 | NS | 48 | 8 | 12 | 4 | 3 | NS | 29 (38.7) | 0.005 |
| Re-ex + adjuvant radiotherapy to the primary tumour | 14 | 7/7 | 80.0 | 11 | 1 | 0 | 2 | 0 | 0 | ||||
| Excision and SLNB | Re-ex + SLNB | 34 | 16/18 | 77.5 | NS | 18 | 3 | 5 | 5 | 3 | NS | 1 (2.9) | NS |
| Re-ex + SLNB + adjuvant radiotherapy to the primary tumour | 8 | 3/5 | 72.4 | 2 | 0 | 1 | 5 | 0 | 0 | ||||
| Excision and SLNB and CLND | Re-ex + SLNB + CLND | 6 | 3/3 | 72.0 | NA | 3 | 0 | 2 | 1 | 0 | NA | 1 (16.7) | NA |
| Re-ex + SLNB + CLND + adjuvant radiotherapy to the primary tumour | 1 | 1/0 | 69.9 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| Excision and CLND | Re-ex + CLND | 34 | 15/19 | 80.1 | <0.001 | 22 | 1 | 5 | 3 | 3 | NS | 9 (26.5) | NS |
| Re-ex + CLND + adjuvant radiotherapy to the primary tumour | 10 | 2/8 | 70.2 | 7 | 1 | 2 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahi, H.; Their, J.; Gissler, M.; Koljonen, V. Merkel Cell Carcinoma Treatment in Finland in 1986–2016—A Real-World Data Study. Cancers 2020, 12, 1224. https://doi.org/10.3390/cancers12051224
Sahi H, Their J, Gissler M, Koljonen V. Merkel Cell Carcinoma Treatment in Finland in 1986–2016—A Real-World Data Study. Cancers. 2020; 12(5):1224. https://doi.org/10.3390/cancers12051224
Chicago/Turabian StyleSahi, Helka, Jenny Their, Mika Gissler, and Virve Koljonen. 2020. "Merkel Cell Carcinoma Treatment in Finland in 1986–2016—A Real-World Data Study" Cancers 12, no. 5: 1224. https://doi.org/10.3390/cancers12051224
APA StyleSahi, H., Their, J., Gissler, M., & Koljonen, V. (2020). Merkel Cell Carcinoma Treatment in Finland in 1986–2016—A Real-World Data Study. Cancers, 12(5), 1224. https://doi.org/10.3390/cancers12051224





