LINC00963 Promotes Cancer Stemness, Metastasis, and Drug Resistance in Head and Neck Carcinomas via ABCB5 Regulation
Abstract
1. Introduction
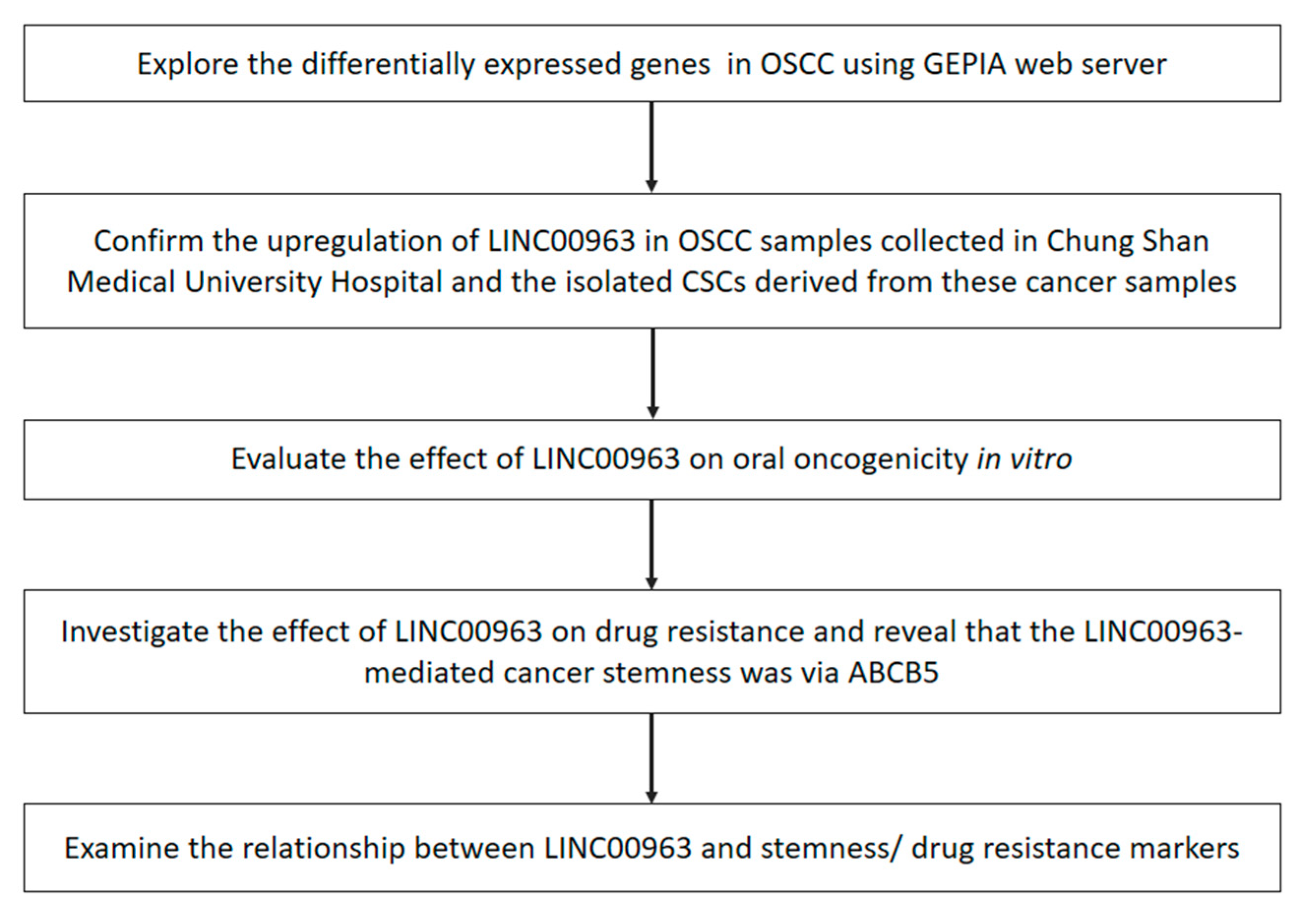
2. Results
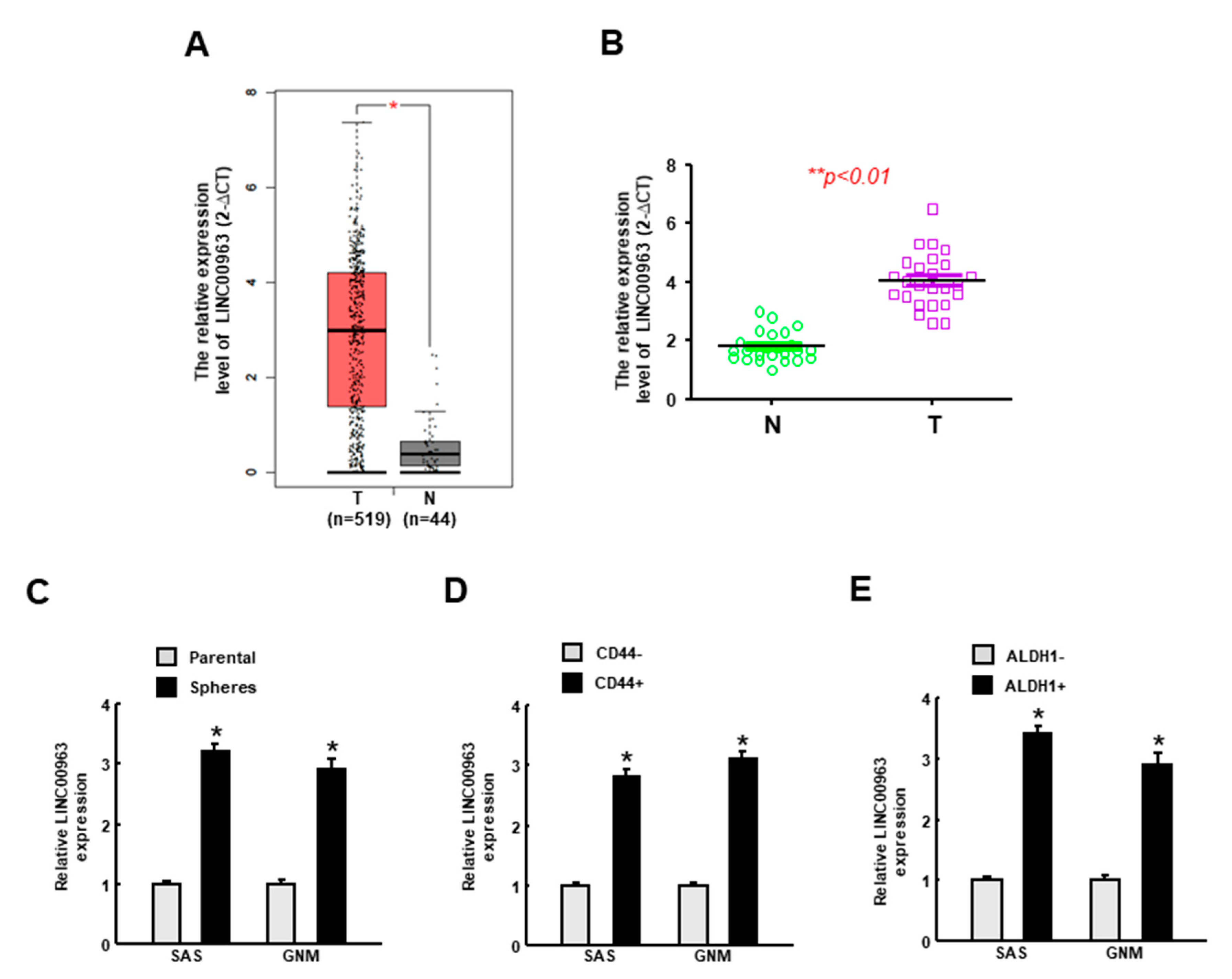
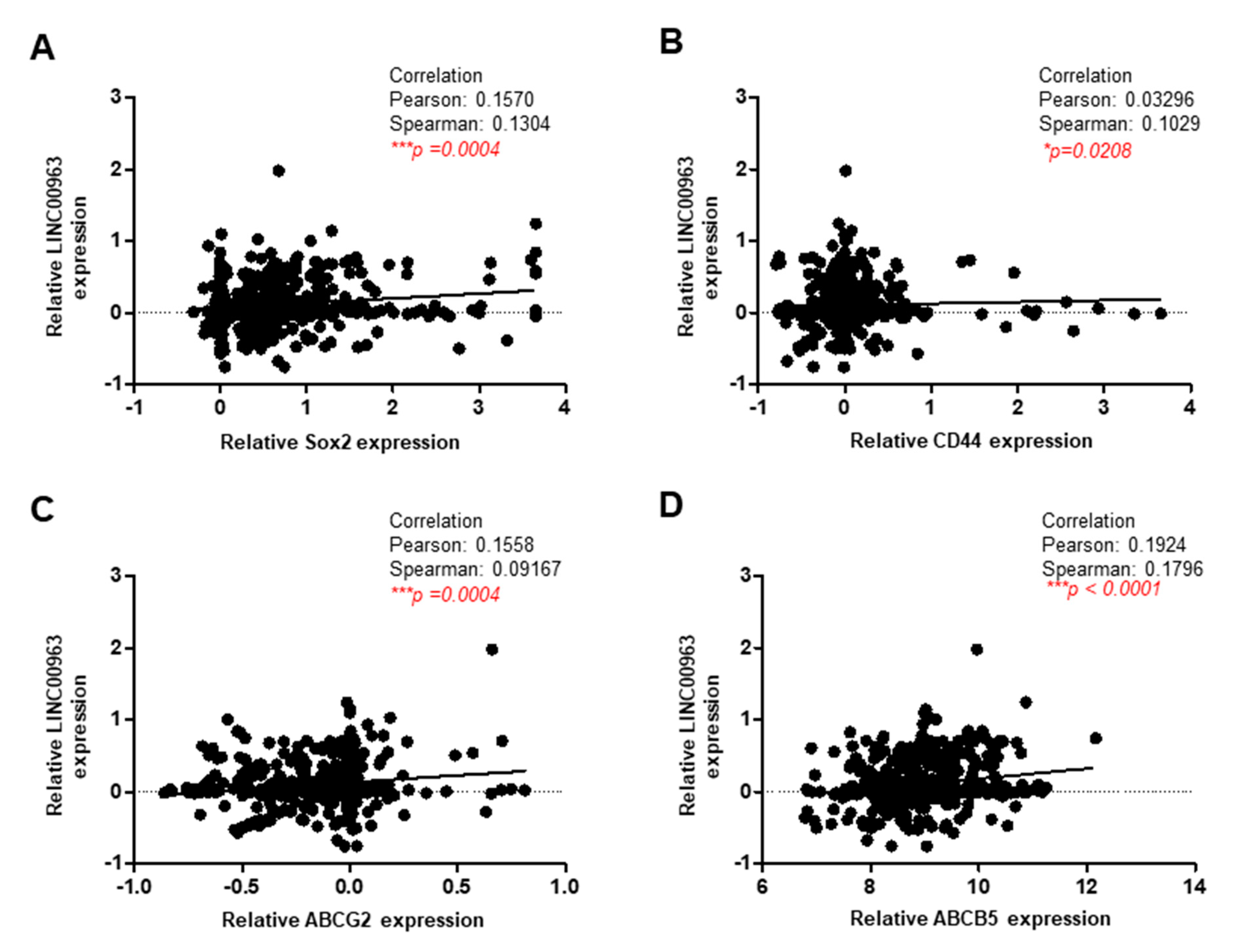
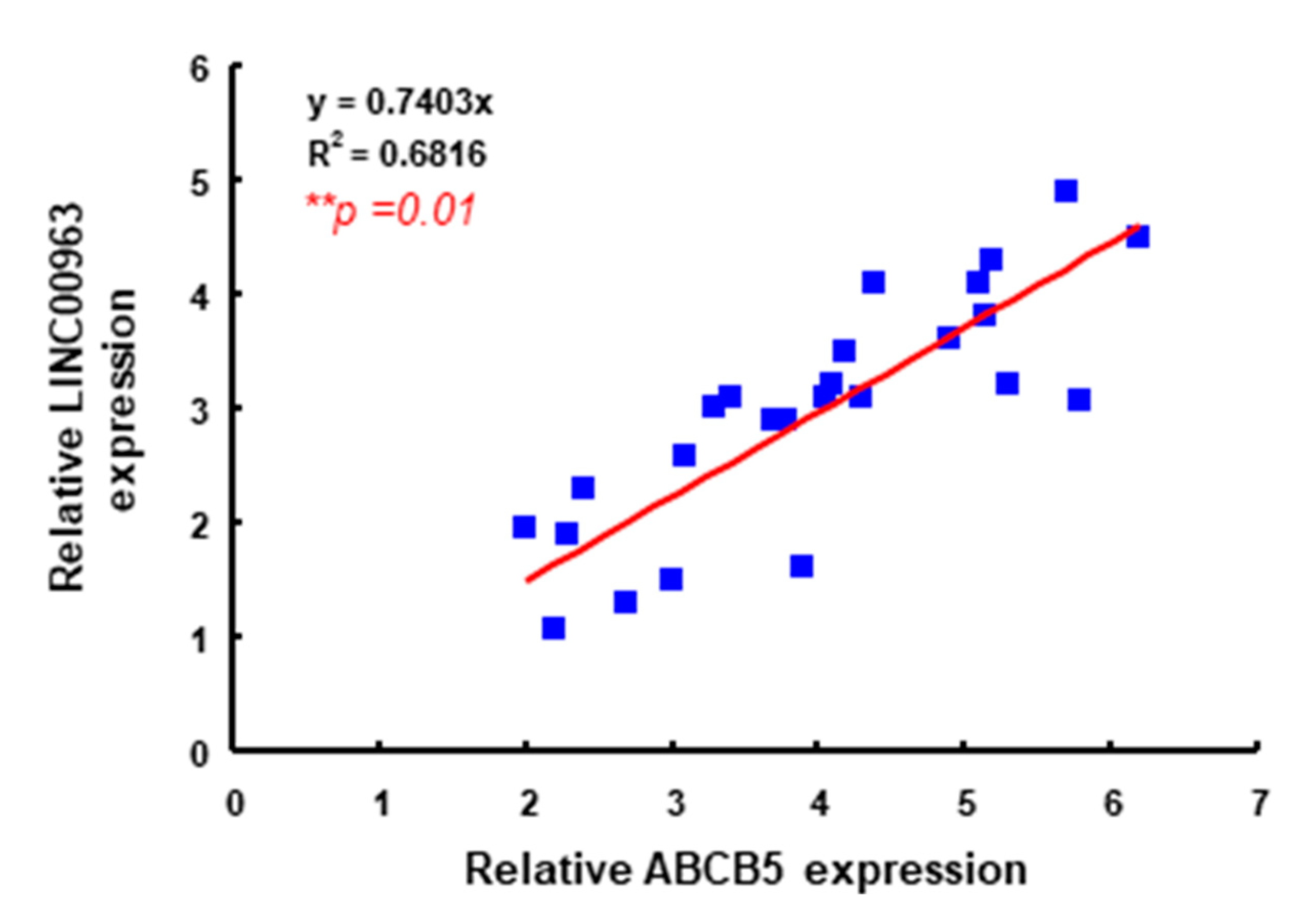
2.1. LINC00963 Is Upregulated in the Specimens of Oral Cancer and Oral CSC
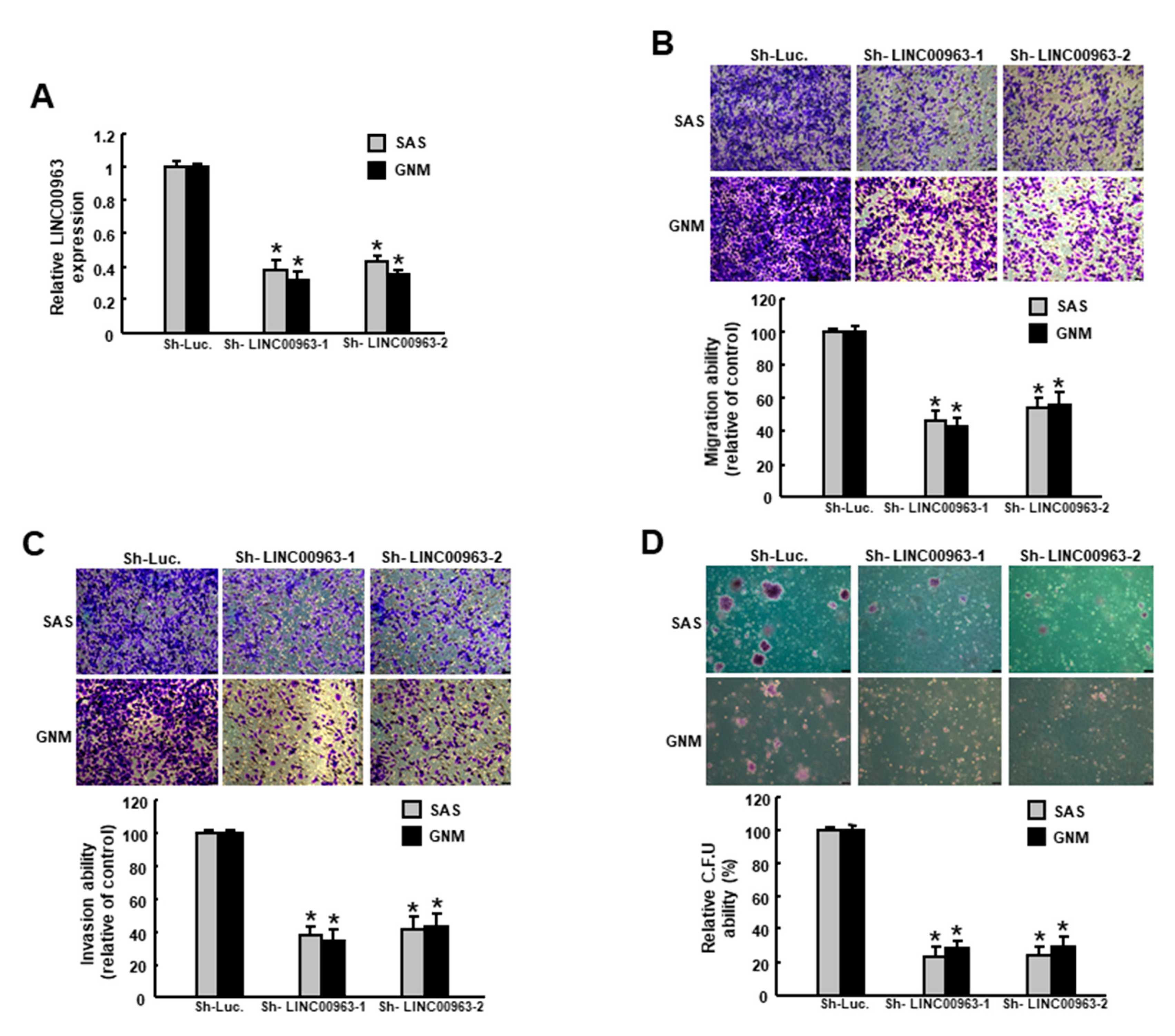
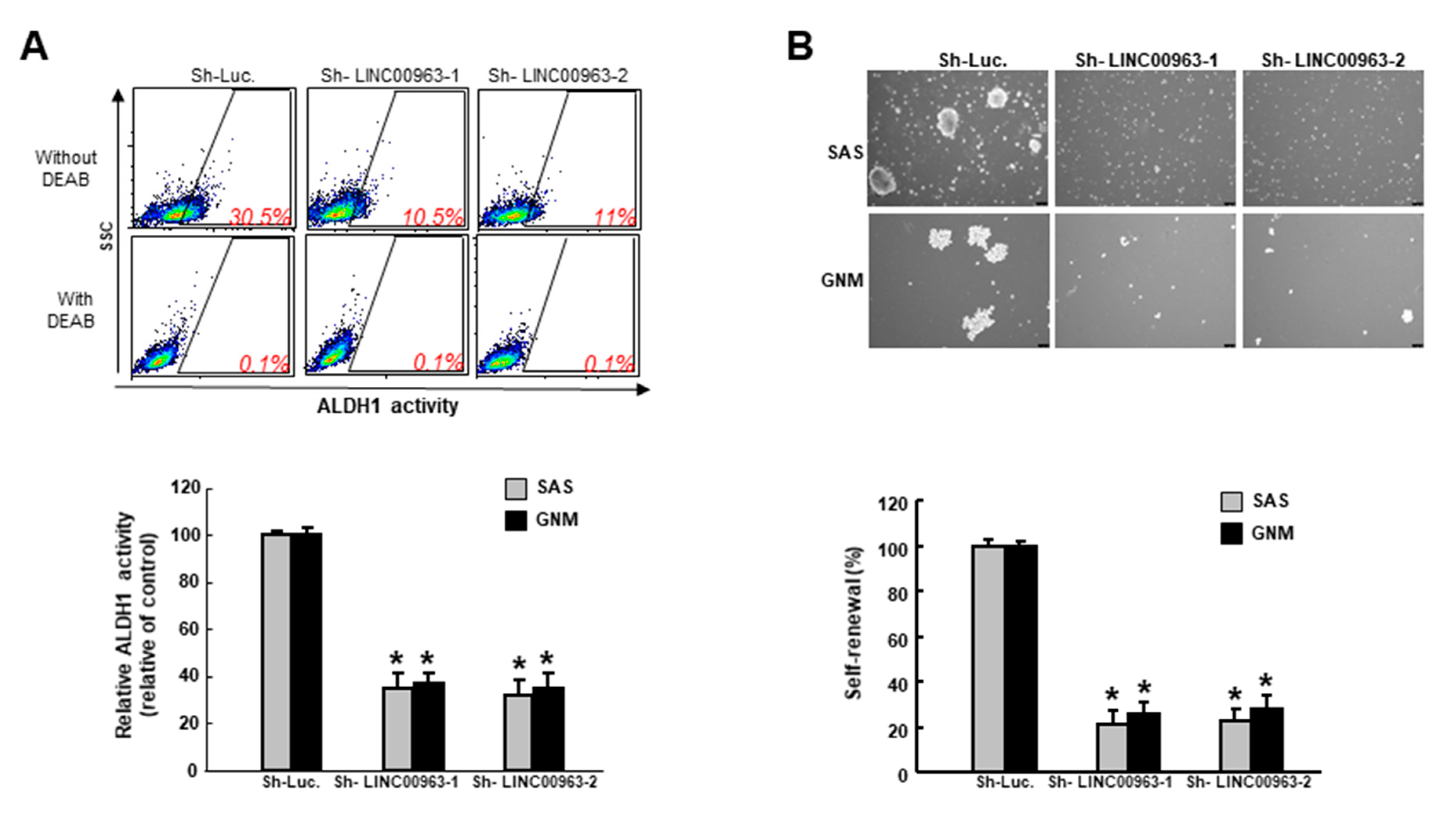
2.2. Silencing LINC00963 Inhibits the Tumorigenicity of Oral Cancer Cells
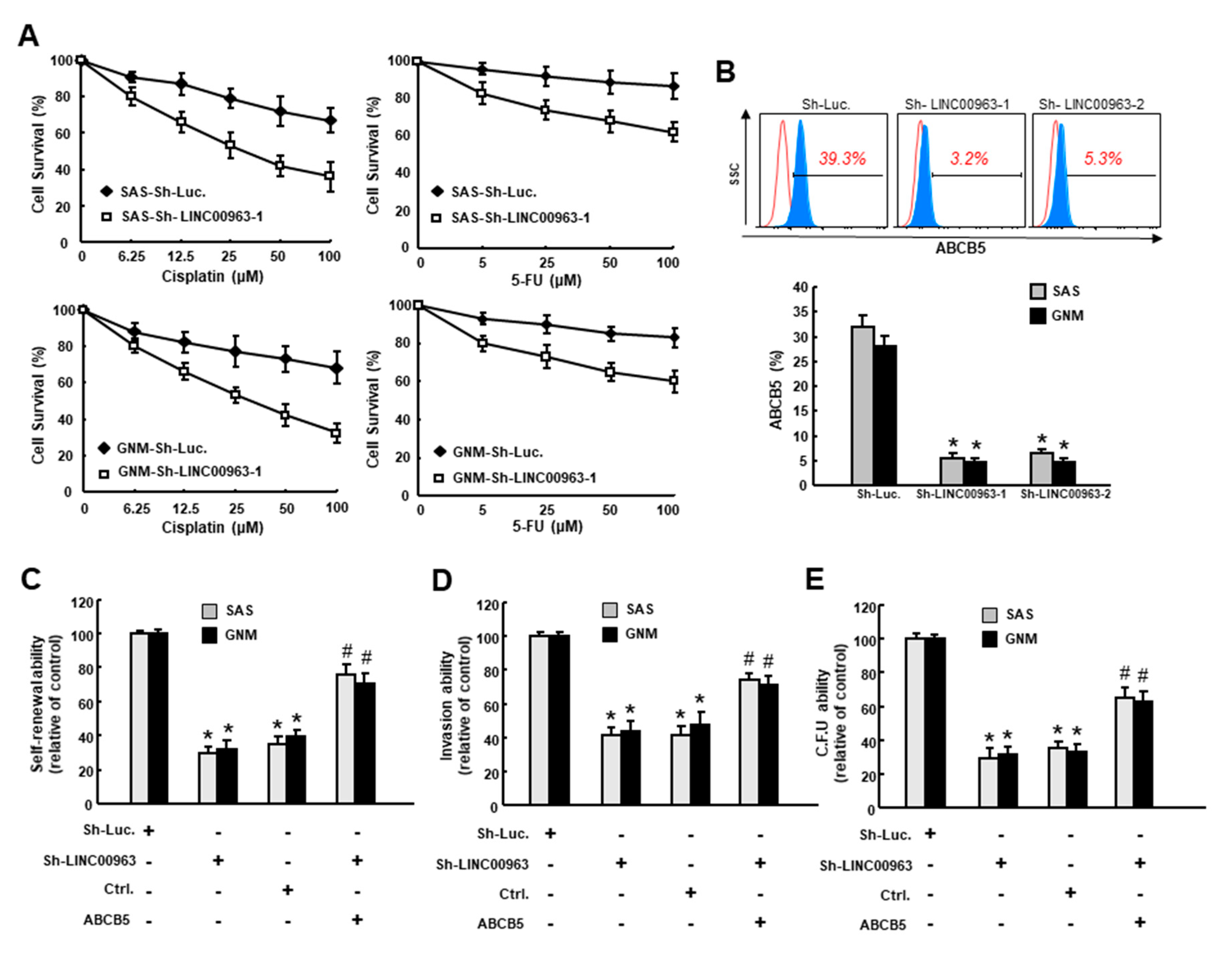
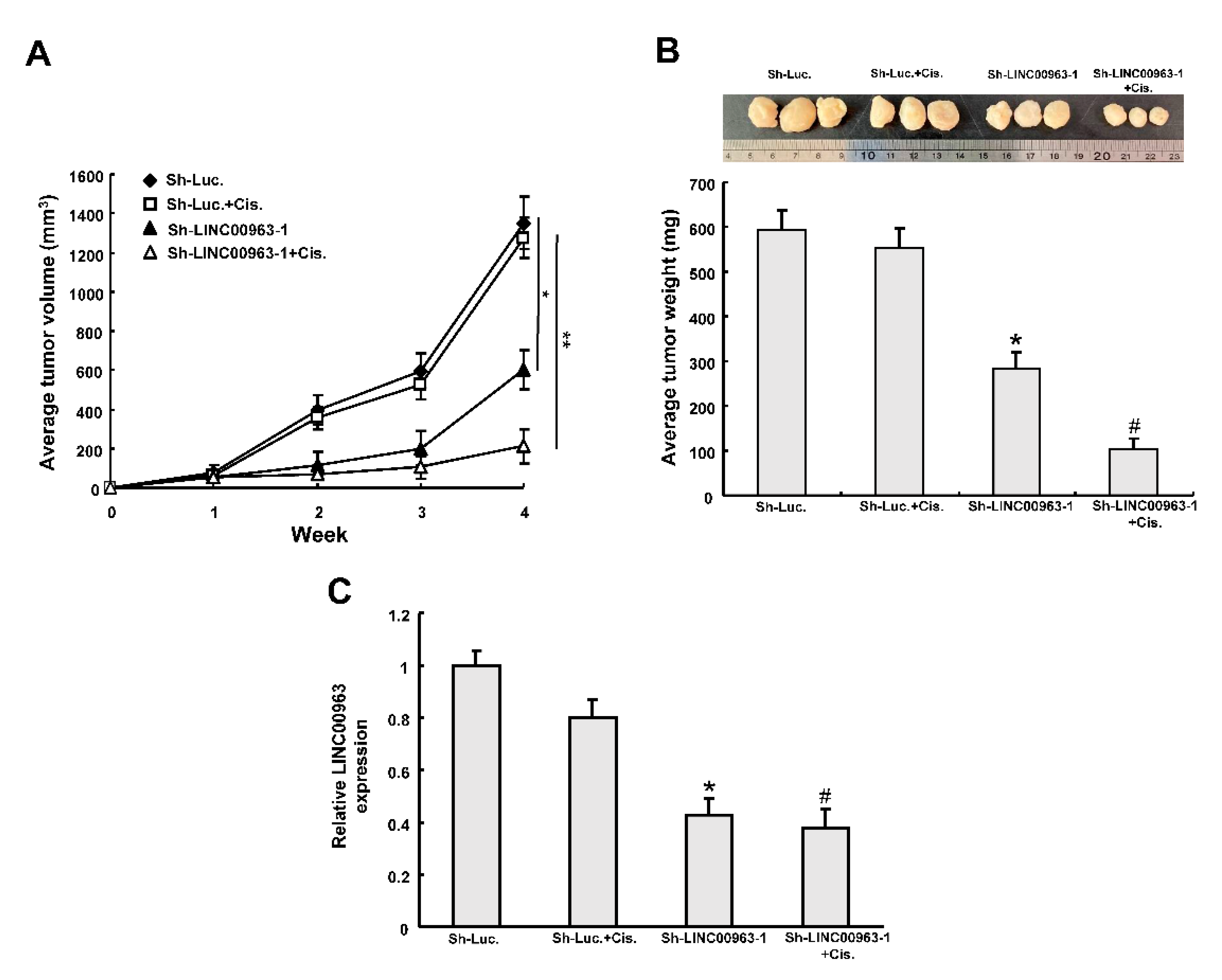
2.3. LINC00963 Modulates Chemosensitivity of Oral Cancer through ABCB5
3. Discussion
4. Materials and Methods
4.1. OSCC Tissues and Cell Culture
4.2. qRT-PCR Analysis
4.3. Flow Cytometry for Cancer Stem Cell Isolation and Drug Resistance Analysis
4.4. Tumorspheres Culture for Cancer Stem Cell Selection
4.5. Knockdown of LINC00963
4.6. Analyses of Cancer Stemness Phenotypes
4.7. Cell Survival Assay
4.8. Subcutaneous Xenografts in Nude Mice
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Huang, T.Y.; Hsu, L.P.; Wen, Y.H.; Huang, T.T.; Chou, Y.F.; Lee, C.F.; Yang, M.C.; Chang, Y.K.; Chen, P.R. Predictors of locoregional recurrence in early stage oral cavity cancer with free surgical margins. Oral Oncol. 2010, 46, 49–55. [Google Scholar] [CrossRef]
- Larsen, S.R.; Johansen, J.; Sorensen, J.A.; Krogdahl, A. The prognostic significance of histological features in oral squamous cell carcinoma. J. Oral Pathol. Med. 2009, 38, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Nor, J.E. Head and neck cancer stem cells. J. Dent. Res. 2012, 91, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.J.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef]
- Clay, M.R.; Tabor, M.; Owen, J.H.; Carey, T.E.; Bradford, C.R.; Wolf, G.T.; Wicha, M.S.; Prince, M.E. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck 2010, 32, 1195–1201. [Google Scholar] [CrossRef]
- Grimm, M.; Krimmel, M.; Polligkeit, J.; Alexander, D.; Munz, A.; Kluba, S.; Keutel, C.; Hoffmann, J.; Reinert, S.; Hoefert, S. ABCB5 expression and cancer stem cell hypothesis in oral squamous cell carcinoma. Eur. J. Cancer 2012, 48, 3186–3197. [Google Scholar] [CrossRef]
- Yan, H.; Bu, P. Non-coding RNAs in cancer stem cells. Cancer Lett. 2018, 421, 121–126. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.Y.; Xin, H.W.; Wang, L.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Wang, H.; Tang, F.R.; Warrier, S.; et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int. J. Biochem. Cell Biol. 2019, 108, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.; Yu, C.C.; Chiou, G.Y.; Chen, Y.W.; Huang, P.I.; Chien, C.S.; Tseng, L.M.; Chu, P.Y.; Lu, K.H.; Chang, K.W.; et al. MicroRNA-200c attenuates tumour growth and metastasis of presumptive head and neck squamous cell carcinoma stem cells. J. Pathol. 2011, 223, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Y.; Hsieh, P.L.; Wang, T.H.; Yu, C.C.; Lu, M.Y.; Liao, Y.W.; Lee, T.H.; Peng, C.Y. Andrographolide impedes cancer stemness and enhances radio-sensitivity in oral carcinomas via miR-218 activation. Oncotarget 2017, 8, 4196–4207. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Peng, C.Y.; Liao, Y.W.; Chou, M.Y.; Hsieh, P.L.; Yu, C.C. miR-1246 Targets CCNG2 to Enhance Cancer Stemness and Chemoresistance in Oral Carcinomas. Cancers 2018, 10, 272. [Google Scholar] [CrossRef]
- Wang, L.; Han, S.; Jin, G.; Zhou, X.; Li, M.; Ying, X.; Wang, L.; Wu, H.; Zhu, Q. Linc00963: A novel, long non-coding RNA involved in the transition of prostate cancer from androgen-dependence to androgen-independence. Int. J. Oncol. 2014, 44, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhao, Y.; Hu, Z.; Li, J.; Chu, D.; Zhang, J.; Li, Z.; Chen, B.; Zhang, X.; Pan, H.; et al. MetaLnc9 Facilitates Lung Cancer Metastasis via a PGK1-Activated AKT/mTOR Pathway. Cancer Res. 2017, 77, 5782–5794. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, X.; Sun, C.; Guo, H.; Wang, T.; Wei, L.; Zhang, Y.; Zhao, J.; Ma, X. LncRNA LINC00963 Promotes Tumorigenesis and Radioresistance in Breast Cancer by Sponging miR-324-3p and Inducing ACK1 Expression. Mol. Ther. Nucleic Acids 2019, 18, 871–881. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhen, Q.; Fan, Y.K. LINC00963 predicts poor prognosis and promotes esophageal cancer cells invasion via targeting miR-214-5p/RAB14 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 164–173. [Google Scholar] [CrossRef]
- Guo, Q.; Grimmig, T.; Gonzalez, G.; Giobbie-Hurder, A.; Berg, G.; Carr, N.; Wilson, B.J.; Banerjee, P.; Ma, J.; Gold, J.S.; et al. ATP-binding cassette member B5 (ABCB5) promotes tumor cell invasiveness in human colorectal cancer. J. Biol. Chem. 2018, 293, 11166–11178. [Google Scholar] [CrossRef]
- Kawanobe, T.; Kogure, S.; Nakamura, S.; Sato, M.; Katayama, K.; Mitsuhashi, J.; Noguchi, K.; Sugimoto, Y. Expression of human ABCB5 confers resistance to taxanes and anthracyclines. Biochem. Biophys. Res. Commun. 2012, 418, 736–741. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, W.; Wang, Y.; Wang, X.; Sun, S.; Yao, Y.; Zhang, Y.; Ren, Z. Long noncoding RNA LINC00963 promotes breast cancer progression by functioning as a molecular sponge for microRNA-625 and thereby upregulating HMGA1. Cell Cycle 2020, 19, 610–624. [Google Scholar] [CrossRef]
- Frank, N.Y.; Pendse, S.S.; Lapchak, P.H.; Margaryan, A.; Shlain, D.; Doeing, C.; Sayegh, M.H.; Frank, M.H. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J. Biol. Chem. 2003, 278, 47156–47165. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.Y.; Margaryan, A.; Huang, Y.; Schatton, T.; Waaga-Gasser, A.M.; Gasser, M.; Sayegh, M.H.; Sadee, W.; Frank, M.H. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005, 65, 4320–4333. [Google Scholar] [CrossRef]
- Cheung, S.T.; Cheung, P.F.; Cheng, C.K.; Wong, N.C.; Fan, S.T. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology 2011, 140, 344–355. [Google Scholar] [CrossRef]
- Wilson, B.J.; Schatton, T.; Zhan, Q.; Gasser, M.; Ma, J.; Saab, K.R.; Schanche, R.; Waaga-Gasser, A.M.; Gold, J.S.; Huang, Q.; et al. ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients. Cancer Res. 2011, 71, 5307–5316. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.J.; Saab, K.R.; Ma, J.; Schatton, T.; Putz, P.; Zhan, Q.; Murphy, G.F.; Gasser, M.; Waaga-Gasser, A.M.; Frank, N.Y.; et al. ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res. 2014, 74, 4196–4207. [Google Scholar] [CrossRef]
- Grimm, M.; Cetindis, M.; Lehmann, M.; Biegner, T.; Munz, A.; Teriete, P.; Reinert, S. Apoptosis resistance-related ABCB5 and DNaseX (Apo10) expression in oral carcinogenesis. Acta Odontol. Scand. 2015, 73, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Curtarelli, R.B.; Goncalves, J.M.; Dos Santos, L.G.P.; Savi, M.G.; Nor, J.E.; Mezzomo, L.A.M.; Rodriguez Cordeiro, M.M. Expression of Cancer Stem Cell Biomarkers in Human Head and Neck Carcinomas: A Systematic Review. Stem Cell Rev. Rep. 2018, 14, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Urrutia, E.; Bustamante Montes, L.P.; Ladron de Guevara Cervantes, D.; Perez-Plasencia, C.; Campos-Parra, A.D. Crosstalk Between Long Non-coding RNAs, Micro-RNAs and mRNAs: Deciphering Molecular Mechanisms of Master Regulators in Cancer. Front. Oncol. 2019, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Touil, Y.; Zuliani, T.; Wolowczuk, I.; Kuranda, K.; Prochazkova, J.; Andrieux, J.; Le Roy, H.; Mortier, L.; Vandomme, J.; Jouy, N.; et al. The PI3K/AKT signaling pathway controls the quiescence of the low-Rhodamine123-retention cell compartment enriched for melanoma stem cell activity. Stem Cells 2013, 31, 641–651. [Google Scholar] [CrossRef]
- Liu, C.M.; Peng, C.Y.; Liao, Y.W.; Lu, M.Y.; Tsai, M.L.; Yeh, J.C.; Yu, C.H.; Yu, C.C. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. J. Formos. Med. Assoc. 2017, 116, 41–48. [Google Scholar] [CrossRef]
- Tsai, L.L.; Yu, C.C.; Chang, Y.C.; Yu, C.H.; Chou, M.Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral Pathol. Med. 2011, 40, 621–628. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-P.; Hsieh, P.-L.; Fang, C.-Y.; Chu, P.-M.; Liao, Y.-W.; Yu, C.-H.; Yu, C.-C.; Tsai, L.-L. LINC00963 Promotes Cancer Stemness, Metastasis, and Drug Resistance in Head and Neck Carcinomas via ABCB5 Regulation. Cancers 2020, 12, 1073. https://doi.org/10.3390/cancers12051073
Lee S-P, Hsieh P-L, Fang C-Y, Chu P-M, Liao Y-W, Yu C-H, Yu C-C, Tsai L-L. LINC00963 Promotes Cancer Stemness, Metastasis, and Drug Resistance in Head and Neck Carcinomas via ABCB5 Regulation. Cancers. 2020; 12(5):1073. https://doi.org/10.3390/cancers12051073
Chicago/Turabian StyleLee, Shiao-Pieng, Pei-Ling Hsieh, Chih-Yuan Fang, Pei-Ming Chu, Yi-Wen Liao, Chuan-Hang Yu, Cheng-Chia Yu, and Lo-Lin Tsai. 2020. "LINC00963 Promotes Cancer Stemness, Metastasis, and Drug Resistance in Head and Neck Carcinomas via ABCB5 Regulation" Cancers 12, no. 5: 1073. https://doi.org/10.3390/cancers12051073
APA StyleLee, S.-P., Hsieh, P.-L., Fang, C.-Y., Chu, P.-M., Liao, Y.-W., Yu, C.-H., Yu, C.-C., & Tsai, L.-L. (2020). LINC00963 Promotes Cancer Stemness, Metastasis, and Drug Resistance in Head and Neck Carcinomas via ABCB5 Regulation. Cancers, 12(5), 1073. https://doi.org/10.3390/cancers12051073






