Prognostic Value of SDF-1α Expression in Patients with Esophageal Squamous Cell Carcinoma Receiving Esophagectomy
Abstract
1. Introduction
2. Results
2.1. Patient Selection
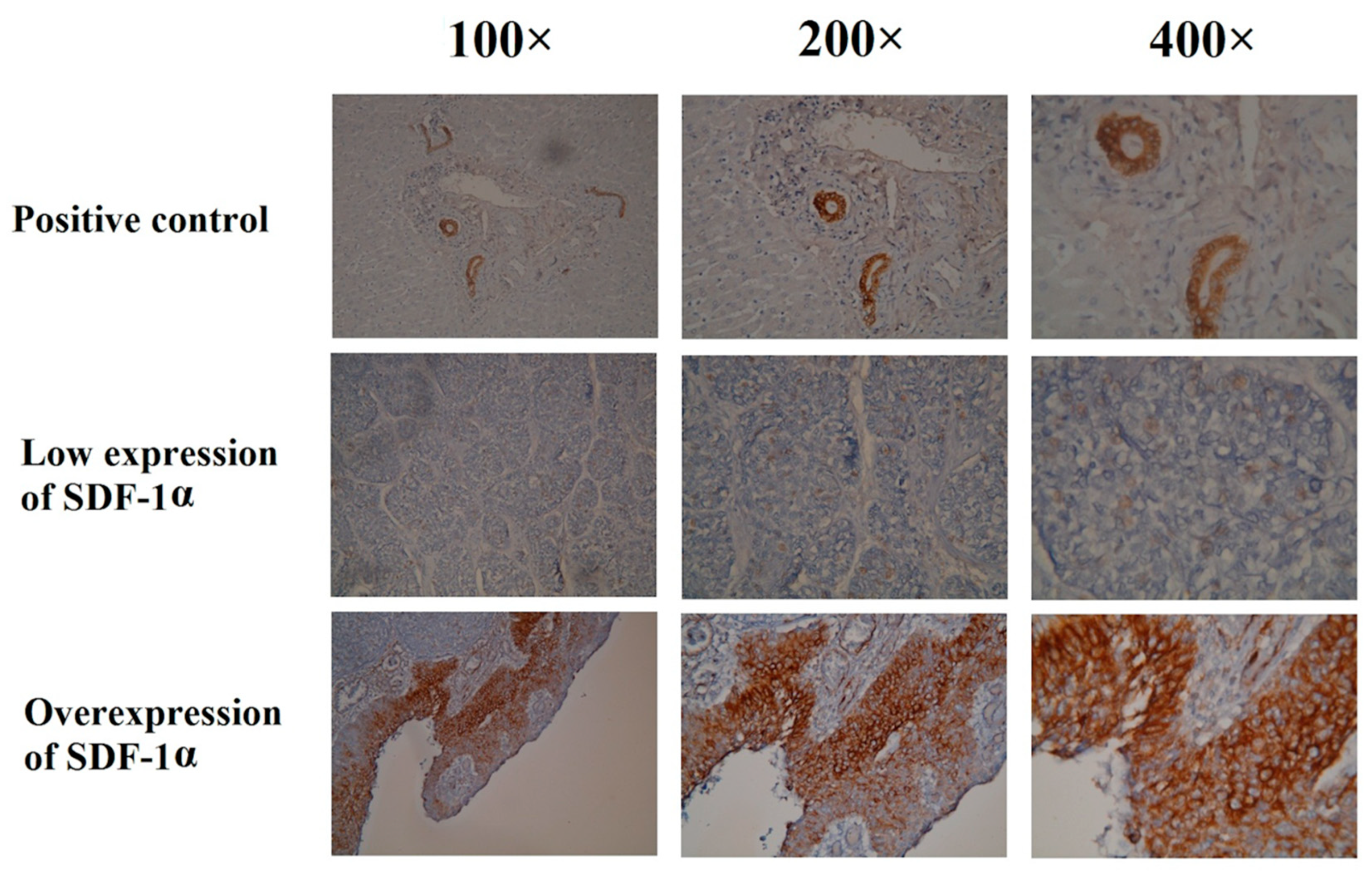
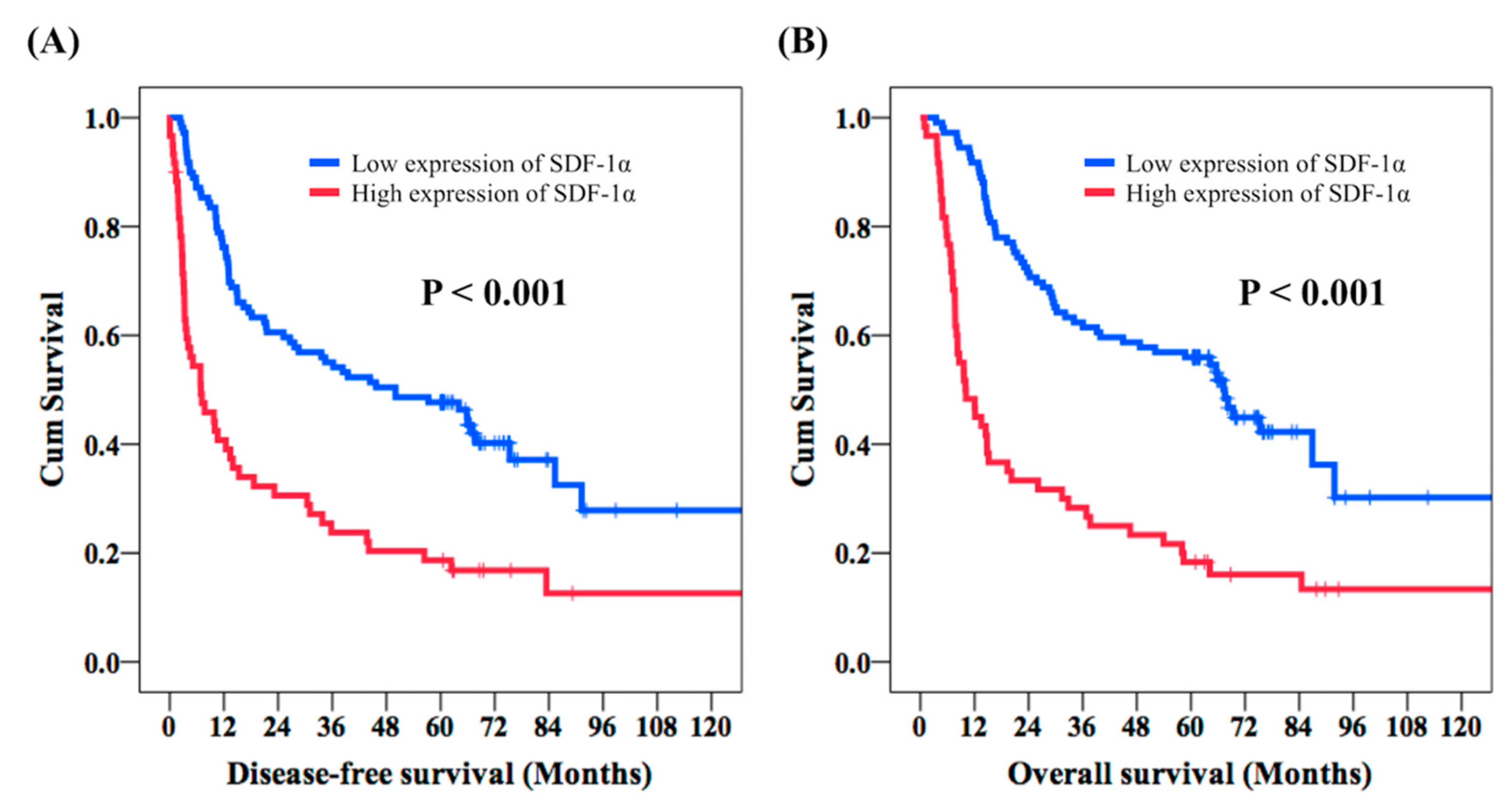
2.2. Clinical Outcomes of ESCC Patients Who Received Esophagectomy
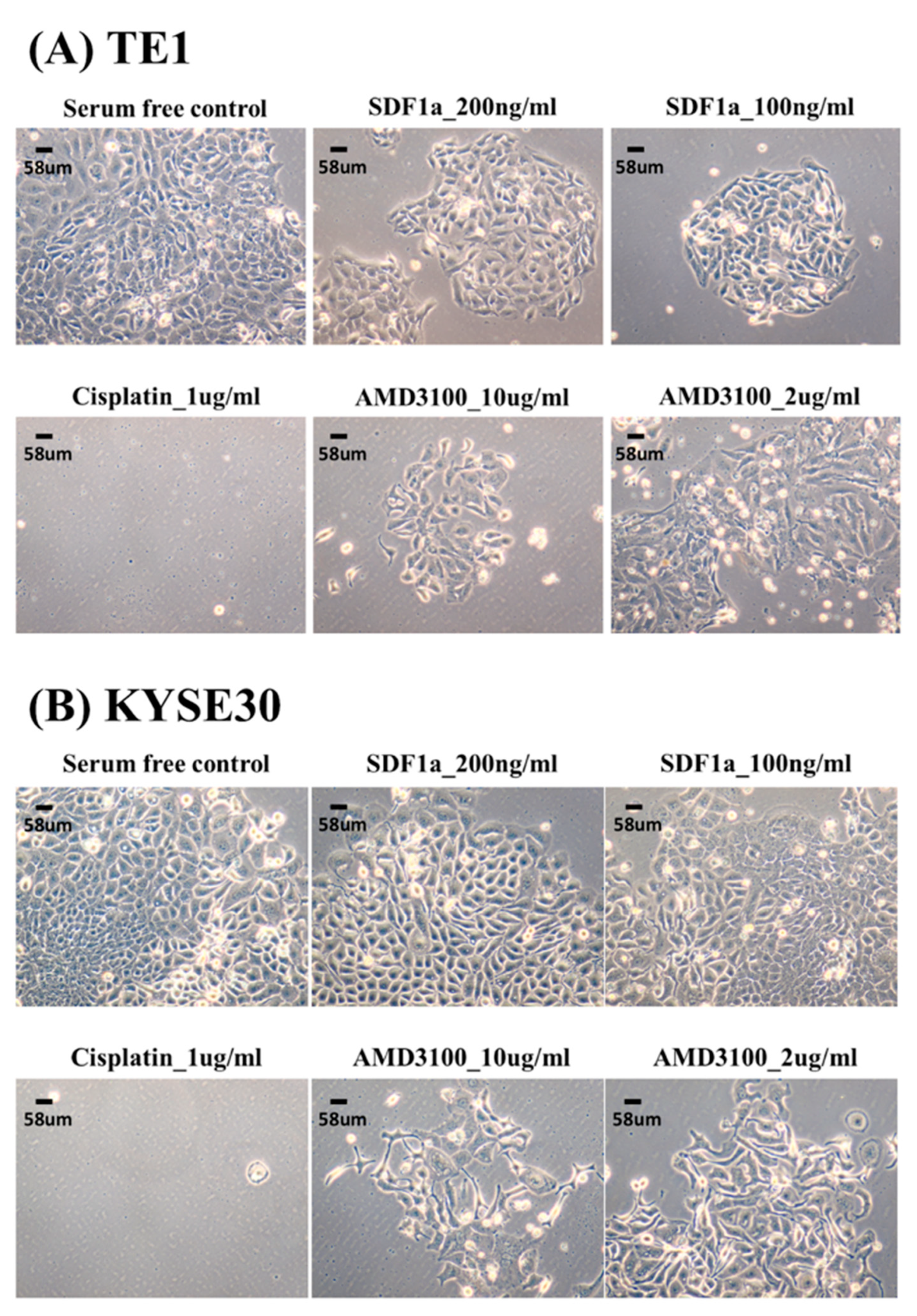
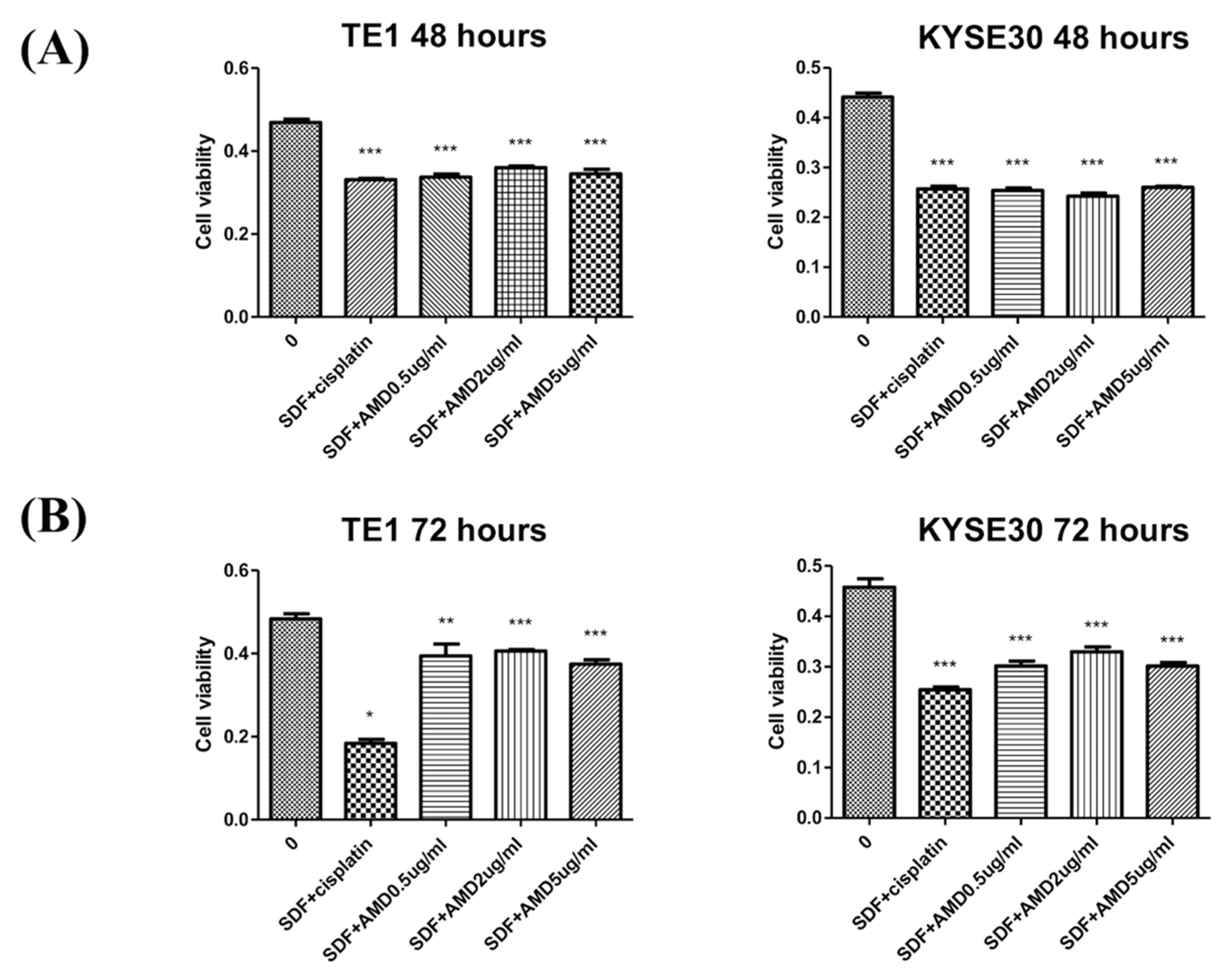
2.3. SDF-1α Promotes Tumor Cell Proliferation In Vitro
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Immunohistochemical Analysis
4.3. Cell Culture and MTT Assay
4.4. Statistical Analysis
4.5. Ethics Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cancer Registry Annual Report 1972–2015; National Department of Health: Beijing, China, 2015.
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar]
- Meads, M.B.; Hazlehurst, L.A.; Dalton, W.S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin. Cancer Res. 2008, 14, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Riveiro, M.E.; Halimi, C.; Hourseau, M.; Couvelard, A.; Serova, M.; Barry, B.; Raymond, E.; Faivre, S. Focus on the role of the CXCL12/CXCR4 chemokine axis in head and neck squamous cell carcinoma. Head Neck 2013, 35, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Feng, Y.; Tian, X.; Wang, B.; Zhou, Y. Oncogenic roles and drug target of CXCR4/CXCL12 axis in lung cancer and cancer stem cell. Tumour Biol. 2016, 37, 8515–8528. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Chaar, I.; Khiari, M.; Ounissi, D.; Weslati, M.; Boughriba, R.; Hmida, A.B.; Bouraoui, S. Stromal cell derived factor-1 and CXCR4 expression in colorectal cancer promote liver metastasis. Cancer Biomark. 2015, 15, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.L.; Lu, Y.C.; Chen, H.Y.; Lee, C.C.; Chung, J.G.; Chen, S.S. Suppressing the formation of lipid raft-associated Rac1/PI3K/Akt signaling complexes by curcumin inhibits SDF-1alpha-induced invasion of human esophageal carcinoma cells. Mol. Carcinog. 2014, 53, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Lou, N.; Zeng, C.G.; Zhang, X.; Chen, F.J. Expression of CXCL12/CXCR4 and its correlation to prognosis in esophageal squamous cell carcinoma. Ai Zheng 2009, 28, 154–158. [Google Scholar] [PubMed]
- Otsuka, S.; Bebb, G. The CXCR4/SDF-1 chemokine receptor axis: A new target therapeutic for non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, Q.; Liu, Q.; Yu, H.; Zhao, L.; Shen, S.; Yao, J. Blockade of SDF-1/CXCR4 signalling inhibits pancreatic cancer progression in vitro via inactivation of canonical Wnt pathway. Br. J. Cancer 2008, 99, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.O.; Wang, C.Y. CXCL12/SDF-1 alpha activates NF-kappaB and promotes oral cancer invasion through the Carma3/Bcl10/Malt1 complex. Int. J. Oral Sci. 2009, 1, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.T.; Chu, C.Y.; Lu, Y.C.; Chang, C.C.; Lin, B.R.; Wu, H.H.; Liu, H.L.; Cha, S.T.; Prakash, E.; Ko, J.Y.; et al. CXCL12/CXCR4 promotes laryngeal and hypopharyngeal squamous cell carcinoma metastasis through MMP-13-dependent invasion via the ERK1/2/AP-1 pathway. Carcinogenesis 2008, 29, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wu, Y.; Huang, Y.; Yan, C.; Liu, Y.; Wang, Z.; Wang, X.; Wen, Y.; Wang, C.; Li, L. RNAi targeting CXCR4 inhibits tumor growth through inducing cell cycle arrest and apoptosis. Mol. Ther. 2012, 20, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Natsugoe, S.; Ishigami, S.; Matsumoto, M.; Okumura, H.; Setoyama, T.; Uchikado, Y.; Kita, Y.; Tamotsu, K.; Hanazono, K.; et al. Expression of CXCL12 and its receptor CXCR4 in esophageal squamous cell carcinoma. Oncol. Rep. 2009, 21, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Uchi, Y.; Takeuchi, H.; Matsuda, S.; Saikawa, Y.; Kawakubo, H.; Wada, N.; Takahashi, T.; Nakamura, R.; Fukuda, K.; Omori, T.; et al. CXCL12 expression promotes esophageal squamous cell carcinoma proliferation and worsens the prognosis. BMC Cancer 2016, 16, 514. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
| Characteristics | Patient Numbers (%) |
|---|---|
| Age (years) | 55 years old (29–80) |
| Sex | |
| Male | 163 (96.4%) |
| Female | 6 (3.6%) |
| pT status | |
| 1 | 56 (33.1%) |
| 2 | 34 (20.1%) |
| 3 | 63 (37.3%) |
| 4 | 16 (9.5%) |
| pN status | |
| 0 | 116 (68.6%) |
| 1 | 33 (19.6%) |
| 2 | 13 (7.7%) |
| 3 | 7 (4.1%) |
| Tumor stage | |
| I | 51 (30.2%) |
| II | 63 (37.3%) |
| III | 34 (20.1%) |
| IVA | 21 (12.4%) |
| Location | |
| Upper | 28 (16.6%) |
| Middle | 63 (37.3%) |
| Lower | 78 (46.1%) |
| Grade | |
| 1 | 17 (10.1%) |
| 2 | 108 (63.9%) |
| 3 | 44 (26.0%) |
| Characteristics | Overexpression of SDF-1α (N = 60) | Low Expression of SDF-1α (N = 109) | p Value |
|---|---|---|---|
| Age | |||
| <60 years | 37 (61.7%) | 69 (63.3%) | 0.87 |
| ≥ 60 years | 23 (38.3%) | 40 (36.7%) | |
| Tumor stage | |||
| I + II | 27 (45.0%) | 87 (79.8%) | <0.001 * |
| III + IVA | 33 (55.0%) | 22 (20.2%) | |
| Location | |||
| Upper | 9 (15.0%) | 19 (17.4%) | 0.83 |
| Middle + Lower | 51 (85.0%) | 90 (82.6%) | |
| Grade | |||
| 1 + 2 | 40 (66.7%) | 85 (78.0%) | 0.14 |
| 3 | 20 (33.3%) | 24 (22.0%) | |
| Adjuvant treatment | |||
| Yes | 49 (81.7%) | 50 (45.9%) | <0.001 * |
| No | 11 (18.3%) | 59 (54.1%) |
| Parameters | Number of Patients | DFS (Months) | Univariate Analysis | Multivariate Analysis | |
|---|---|---|---|---|---|
| p-Value | HR (95% CI) | p-Value | |||
| Age | 0.007 * | ||||
| <60 years | 63 (37.3%) | 39.4 | |||
| ≥60 years | 106 (62.7%) | 14.8 | |||
| Sex | 0.14 | ||||
| Male | 163 (96.4%) | 21.5 | |||
| Female | 6 (3.6%) | NR | |||
| pT status | <0.001 * | ||||
| 1 + 2 | 90 (53.3%) | 56.4 | 0.50 (0.34–0.73) | <0.001 * | |
| 3 + 4 | 79 (46.7%) | 9.8 | |||
| pN status | <0.001 * | ||||
| 0 | 116 (68.6%) | 45.7 | 0.53 (0.36–0.79) | 0.002 * | |
| 1 + 2 + 3 | 53 (31.4%) | 7.1 | |||
| Tumor stage | <0.001 * | ||||
| I + II | 114 (67.5%) | 45.7 | |||
| III + IVA | 55 (32.5%) | 6.8 | |||
| Location | 0.1 | ||||
| Upper | 28 (16.6%) | 13.7 | 0.51 (0.31–0.82) | 0.006 * | |
| Middle + Lower | 141 (83.4%) | 33.8 | |||
| Grade | 0.16 | ||||
| 1 + 2 | 125 (74.0%) | 31.1 | |||
| 3 | 44 (26.0%) | 13.1 | |||
| Adjuvant treatment | <0.001 * | ||||
| Yes | 99 (58.6%) | 10.3 | |||
| No | 70 (41.4%) | 65.9 | |||
| SDF-1α expression | <0.001 * | ||||
| High | 60 (35.5%) | 6.9 | |||
| Low | 109 (64.5%) | 50 | 0.49 (0.33–0.72) | <0.001 * | |
| Parameters | Number of Patients | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| OS (Months) | p-Value | HR (95% CI) | p-Value | ||
| Age | 0.009 * | ||||
| <60 years | 63 (37.3%) | 64.2 | |||
| ≥60 years | 106 (62.7%) | 23.7 | |||
| Sex | 0.2 | ||||
| Male | 163 (96.4%) | 32.8 | |||
| Female | 6 (3.6%) | NR | |||
| pT status | <0.001 * | ||||
| 1 + 2 | 90 (53.3%) | 67.2 | 0.50 (0.34−0.75) | 0.001 * | |
| 3 + 4 | 79 (46.7%) | 15.1 | |||
| pN status | <0.001 * | ||||
| 0 | 116 (68.6%) | 65.6 | 0.51 (0.34−0.76) | 0.001 * | |
| 1 + 2 + 3 | 53 (31.4%) | 13 | |||
| Tumor stage | <0.001 * | ||||
| I + II | 114 (67.5%) | 65.8 | |||
| III + IVA | 55 (32.5%) | 12 | |||
| Location | 0.035 * | ||||
| Upper | 28 (16.6%) | 16.7 | 0.46 (0.29−0.75) | 0.002 * | |
| Middle + Lower | 141 (83.4%) | 45 | |||
| Grade | 0.07 | ||||
| 1 + 2 | 125 (74.0%) | 48.7 | |||
| 3 | 44 (26.0%) | 22.4 | |||
| Adjuvant treatment | <0.001 * | ||||
| Yes | 99 (58.6%) | 15.5 | |||
| No | 70 (41.4%) | 69.3 | |||
| SDF-1α expression | <0.001 * | ||||
| High | 60 (35.5%) | 10 | |||
| Low | 109 (64.5%) | 67.5 | 0.40 (0.27-0.60) | <0.001 * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Li, S.-H.; Lu, H.-I.; Lo, C.-M. Prognostic Value of SDF-1α Expression in Patients with Esophageal Squamous Cell Carcinoma Receiving Esophagectomy. Cancers 2020, 12, 1067. https://doi.org/10.3390/cancers12051067
Chen Y-H, Li S-H, Lu H-I, Lo C-M. Prognostic Value of SDF-1α Expression in Patients with Esophageal Squamous Cell Carcinoma Receiving Esophagectomy. Cancers. 2020; 12(5):1067. https://doi.org/10.3390/cancers12051067
Chicago/Turabian StyleChen, Yen-Hao, Shau-Hsuan Li, Hung-I Lu, and Chien-Ming Lo. 2020. "Prognostic Value of SDF-1α Expression in Patients with Esophageal Squamous Cell Carcinoma Receiving Esophagectomy" Cancers 12, no. 5: 1067. https://doi.org/10.3390/cancers12051067
APA StyleChen, Y.-H., Li, S.-H., Lu, H.-I., & Lo, C.-M. (2020). Prognostic Value of SDF-1α Expression in Patients with Esophageal Squamous Cell Carcinoma Receiving Esophagectomy. Cancers, 12(5), 1067. https://doi.org/10.3390/cancers12051067





