Targeted Assessment of the EGFR Status as Reflex Testing in Treatment-Naive Non-Squamous Cell Lung Carcinoma Patients: A Single Laboratory Experience (LPCE, Nice, France)
Abstract
1. Introduction
2. Results
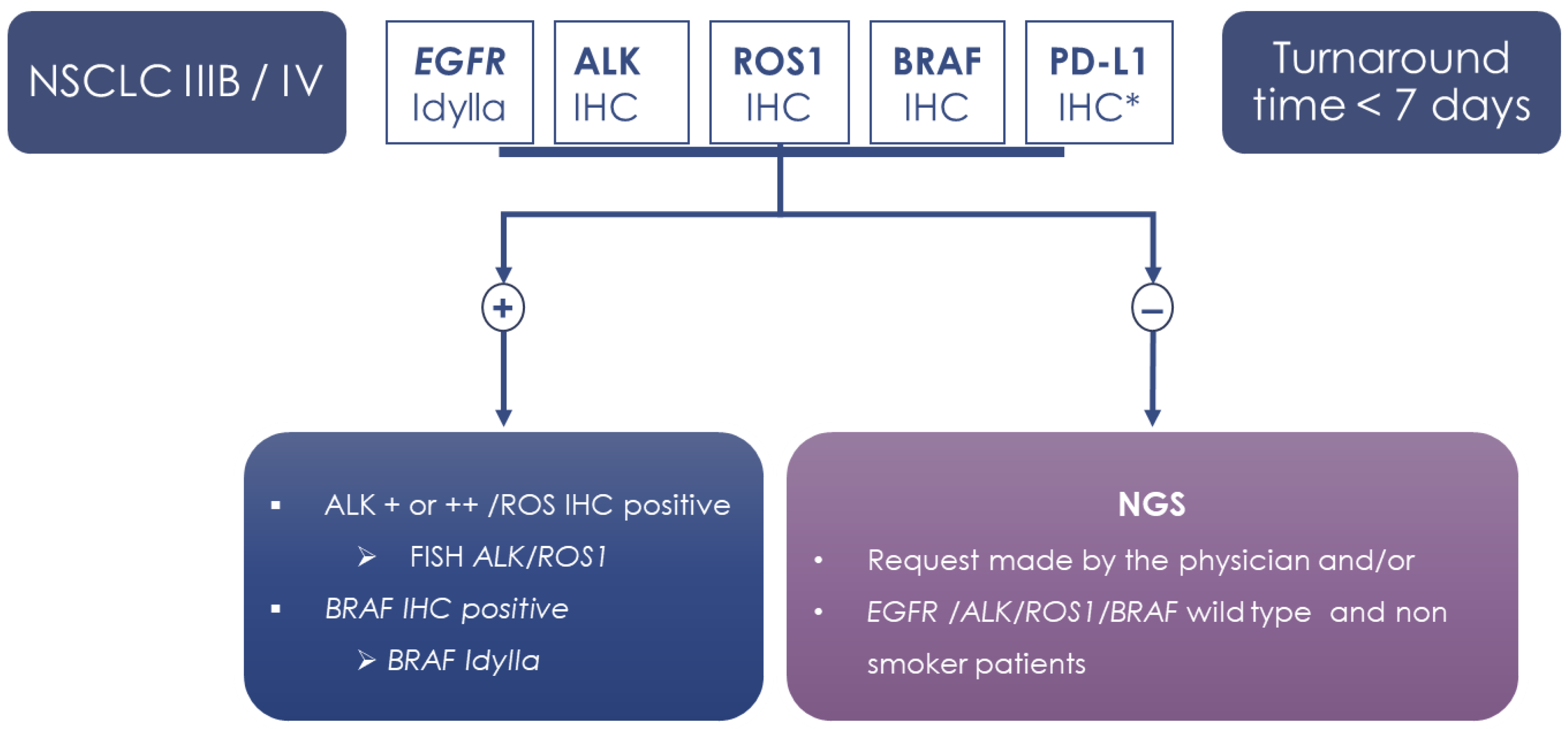
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.-L.; Yuan, J.-Q.; Wang, K.-F.; Fu, X.-H.; Han, X.-R.; Threapleton, D.; Yang, Z.-Y.; Mao, C.; Tang, J.-L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, E.; Feld, E.; Horn, L. Driven by Mutations: The Predictive Value of Mutation Subtype in EGFR -Mutated Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Nie, X.; Bie, Z.; Li, L. Impact of heavy smoking on the benefits from first-line EGFR-TKI therapy in patients with advanced lung adenocarcinoma. Medicine 2018, 97, e0006. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Wu, Y.-L.; Ding, P.N.; Lord, S.J.; Inoue, A.; Zhou, C.; Mitsudomi, T.; Rosell, R.; Pavlakis, N.; Links, M.; et al. Impact of Specific Epidermal Growth Factor Receptor (EGFR) Mutations and Clinical Characteristics on Outcomes After Treatment With EGFR Tyrosine Kinase Inhibitors Versus Chemotherapy in EGFR -Mutant Lung Cancer: A Meta-Analysis. J. Clin. Oncol. 2015, 33, 1958–1965. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef]
- Evrard, S.M.; Taranchon-Clermont, E.; Rouquette, I.; Murray, S.; Dintner, S.; Nam-Apostolopoulos, Y.-C.; Bellosillo, B.; Varela-Rodriguez, M.; Nadal, E.; Wiedorn, K.H.; et al. Multicenter Evaluation of the Fully Automated PCR-Based Idylla EGFR Mutation Assay on Formalin-Fixed, Paraffin-Embedded Tissue of Human Lung Cancer. J. Mol. Diagn. 2019, 21, 1010–1024. [Google Scholar] [CrossRef]
- Sheikine, Y.; Rangachari, D.; McDonald, D.C.; Huberman, M.S.; Folch, E.S.; VanderLaan, P.A.; Costa, D.B. EGFR Testing in Advanced Non-Small-Cell Lung Cancer, A Mini-Review. Clin. Lung Cancer 2016, 17, 483–492. [Google Scholar] [CrossRef]
- Séquençage à Haut Débit. Available online: https://lesdonnees.e-cancer.fr/Themes/Soins/Les-tests-de-genetique-somatique/Les-tests-de-genetique-somatique/Sequencage-a-haut-debit#graphique (accessed on 7 February 2020).
- Trédan, O.; Wang, Q.; Pissaloux, D.; Cassier, P.; de la Fouchardière, A.; Fayette, J.; Desseigne, F.; Ray-Coquard, I.; de la Fouchardière, C.; Frappaz, D.; et al. Molecular screening program to select molecular-based recommended therapies for metastatic cancer patients: Analysis from the ProfiLER trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 757–765. [Google Scholar] [CrossRef]
- Rangachari, D.; Drake, L.; Huberman, M.S.; McDonald, D.C.; VanderLaan, P.A.; Folch, E.; Costa, D.B. Rapidly fatal advanced EGFR-mutated lung cancers and the need for rapid tumor genotyping in clinical practice. Cancer Treat. Commun. 2016, 9, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Azzolli, C.G.; Fintelmann, F.; Mino-Kenudson, M.; Farago, A.F.; Gainor, J.F.; Jiang, G.; Piotrowska, Z.; Heist, R.S.; Lennes, I.T.; et al. Clinical Utility of Rapid EGFR Genotyping in Advanced Lung Cancer. JCO Precis. Oncol. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Bocciarelli, C.; Cohen, J.; Pelletier, R.; Tran Van Nhieu, J.; Derman, J.; Favre, L.; Bourgogne, A.; Monnet, I.; Chouaid, C.; Pujals, A. Evaluation of the Idylla system to detect the EGFRT790M mutation using extracted DNA. Pathol. Res. Pract. 2020, 216, 152773. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Gragnano, G.; Pisapia, P.; Vigliar, E.; Malapelle, U.; Bellevicine, C.; Troncone, G. EGFR mutation detection on lung cancer cytological specimens by the novel fully automated PCR-based Idylla EGFR Mutation Assay. J. Clin. Pathol. 2017, 70, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Butori, C.; Lassalle, S.; Heeke, S.; Piton, N.; Sabourin, J.-C.; Tanga, V.; Washetine, K.; Long-Mira, E.; Maitre, P.; et al. Optimization ofEGFRmutation detection by the fully-automated qPCR-based Idylla system on tumor tissue from patients with non-small cell lung cancer. Oncotarget 2017, 8, 103055–103062. [Google Scholar] [CrossRef]
- Van Haele, M.; Vander Borght, S.; Ceulemans, A.; Wieërs, M.; Metsu, S.; Sagaert, X.; Weynand, B. Rapid clinical mutational testing of KRAS, BRAF and EGFR: A prospective comparative analysis of the Idylla technique with high-throughput next-generation sequencing. J. Clin. Pathol. 2020, 73, 35–41. [Google Scholar] [CrossRef]
- Lambros, L.; Caumont, C.; Guibourg, B.; Barel, F.; Quintin-Roué, I.; Marcorelles, P.; Merlio, J.-P.; Uguen, A. Evaluation of a fast and fully automated platform to diagnose EGFR and KRAS mutations in formalin-fixed and paraffin-embedded non-small cell lung cancer samples in less than one day. J. Clin. Pathol. 2017, 70, 544–549. [Google Scholar] [CrossRef]
- Layfield, L.J.; Hammer, R.D.; White, S.K.; Furtado, L.V.; Schmidt, R.L. Molecular Testing Strategies for Pulmonary Adenocarcinoma: An Optimal Approach with Cost Analysis. Arch. Pathol. Lab. Med. 2019, 143, 628–633. [Google Scholar] [CrossRef]
- El-Deiry, W.S.; Goldberg, R.M.; Lenz, H.; Shields, A.F.; Gibney, G.T.; Tan, A.R.; Brown, J.; Eisenberg, B.; Heath, E.I.; Phuphanich, S.; et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J. Clin. 2019, 69, 305–343. [Google Scholar] [CrossRef]
- Charrier, M.; Mezquita, L.; Lueza, B.; Dupraz, L.; Planchard, D.; Remon, J.; Caramella, C.; Cassard, L.; Boselli, L.; Reiners, K.S.; et al. Circulating innate immune markers and outcomes in treatment-naïve advanced non–small cell lung cancer patients. Eur. J. Cancer 2019, 108, 88–96. [Google Scholar] [CrossRef]
- Chih-Hsin Yang, J.; Schuler, M.; Popat, S.; Miura, S.; Heeke, S.; Park, K.; Märten, A.; Kim, E.S. Afatinib for the Treatment of Non-Small Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J. Thorac. Oncol. 2020. [Google Scholar] [CrossRef]
- Vyse, S.; Huang, P.H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, G.M.; Bradbury, P.A.; Feld, R.; Leighl, N.B.; Liu, G.; Pisters, K.-M.; Kamel-Reid, S.; Tsao, M.S.; Shepherd, F.A. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer 2017, 109, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Springborn, S.; Haug, K.; Bartow, K.; Samra, H.; Menon, S.; Mackinnon, A.C. Evaluation, Validation, and Implementation of the Idylla System as Rapid Molecular Testing for Precision Medicine. J. Mol. Diagn. 2019, 21, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Heeke, S.; Hofman, P. EGFR Mutation Analysis in Non-small Cell Lung Carcinoma from Tissue Samples Using the Fully Automated IdyllaTM qPCR System. Methods Mol. Biol. 2019, 2054, 147–155. [Google Scholar] [CrossRef]
- Heeke, S.; Hofman, V.; Long-Mira, E.; Lespinet, V.; Lalvée, S.; Bordone, O.; Ribeyre, C.; Tanga, V.; Benzaquen, J.; Leroy, S.; et al. Use of the ion PGM and the genereader NGS systems in daily routine practice for advanced lung adenocarcinoma patients: A practical point of view reporting a comparative study and assessment of 90 patients. Cancers 2018, 10, 88. [Google Scholar] [CrossRef]
- Heeke, S.; Benzaquen, J.; Hofman, V.; Ilié, M.; Allegra, M.; Long-Mira, E.; Lassalle, S.; Tanga, V.; Salacroup, C.; Bonnetaud, C.; et al. Critical Assessment in Routine Clinical Practice of Liquid Biopsy for EGFR Status Testing in Non-Small-Cell Lung Cancer: A Single-Laboratory Experience (LPCE, Nice, France). Clin. Lung Cancer 2020, 21, 56–65. [Google Scholar] [CrossRef]
- Hofman, V.; Rouquette, I.; Long-Mira, E.; Piton, N.; Chamorey, E.; Heeke, S.; Vignaud, J.M.; Yguel, C.; Mazières, J.; Lepage, A.-L.; et al. Multicenter Evaluation of a Novel ROS1 Immunohistochemistry Assay (SP384) for Detection of ROS1 Rearrangements in a Large Cohort of Lung Adenocarcinoma Patients. J. Thorac. Oncol. 2020, 14, 1204–1212. [Google Scholar] [CrossRef]
- Ilie, M.; Khambata-Ford, S.; Copie-Bergman, C.; Huang, L.; Juco, J.; Hofman, V.; Hofman, P. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS ONE 2017, 12, e0183023. [Google Scholar] [CrossRef]
- Ilie, M.; Long, E.; Hofman, V.; Dadone, B.; Marquette, C.H.; Mouroux, J.; Vignaud, J.M.; Begueret, H.; Merlio, J.P.; Capper, D.; et al. Diagnostic value of immunohistochemistry for the detection of the BRAF mutation in primary lung adenocarcinoma Caucasian patients. Ann. Oncol. 2013, 24, 742–748. [Google Scholar] [CrossRef]
- Ilie, M.; Long, E.; Butori, C.; Hofman, V.; Coelle, C.; Mauro, V.; Zahaf, K.; Marquette, C.H.; Mouroux, J.; Paterlini-Bréchot, P.; et al. ALK-gene rearrangement: A comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2907–2913. [Google Scholar] [CrossRef] [PubMed]
- Ezeife, D.A.; Morganstein, B.J.; Lau, S.; Law, J.H.; Le, L.W.; Bredle, J.; Cella, D.; Doherty, M.K.; Bradbury, P.; Liu, G.; et al. Financial Burden Among Patients with Lung Cancer in a Publically Funded Health Care System. Clin. Lung Cancer 2019, 20, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.M.; Morrison, C.; Gold, E.J.; Tradonsky, A.; Arnold, R.J.G. Budget Impact of Next-Generation Sequencing for Molecular Assessment of Advanced Non–Small Cell Lung Cancer. Value Health 2018, 21, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Offin, M.; Rizvi, H.; Tenet, M.; Ni, A.; Sanchez-Vega, F.; Li, B.T.; Drilon, A.; Kris, M.G.; Rudin, C.M.; Schultz, N.; et al. Tumor Mutation Burden and Efficacy of EGFR-Tyrosine Kinase Inhibitors in Patients with EGFR -Mutant Lung Cancers. Clin. Cancer Res. 2019, 25, 1063–1069. [Google Scholar] [CrossRef]
- Cheng, M.L.; Oxnard, G.R. Does TMB Impact the Effectiveness of TKIs in EGFR -Mutant NSCLC? Clin. Cancer Res. 2019, 25, 899–900. [Google Scholar] [CrossRef]
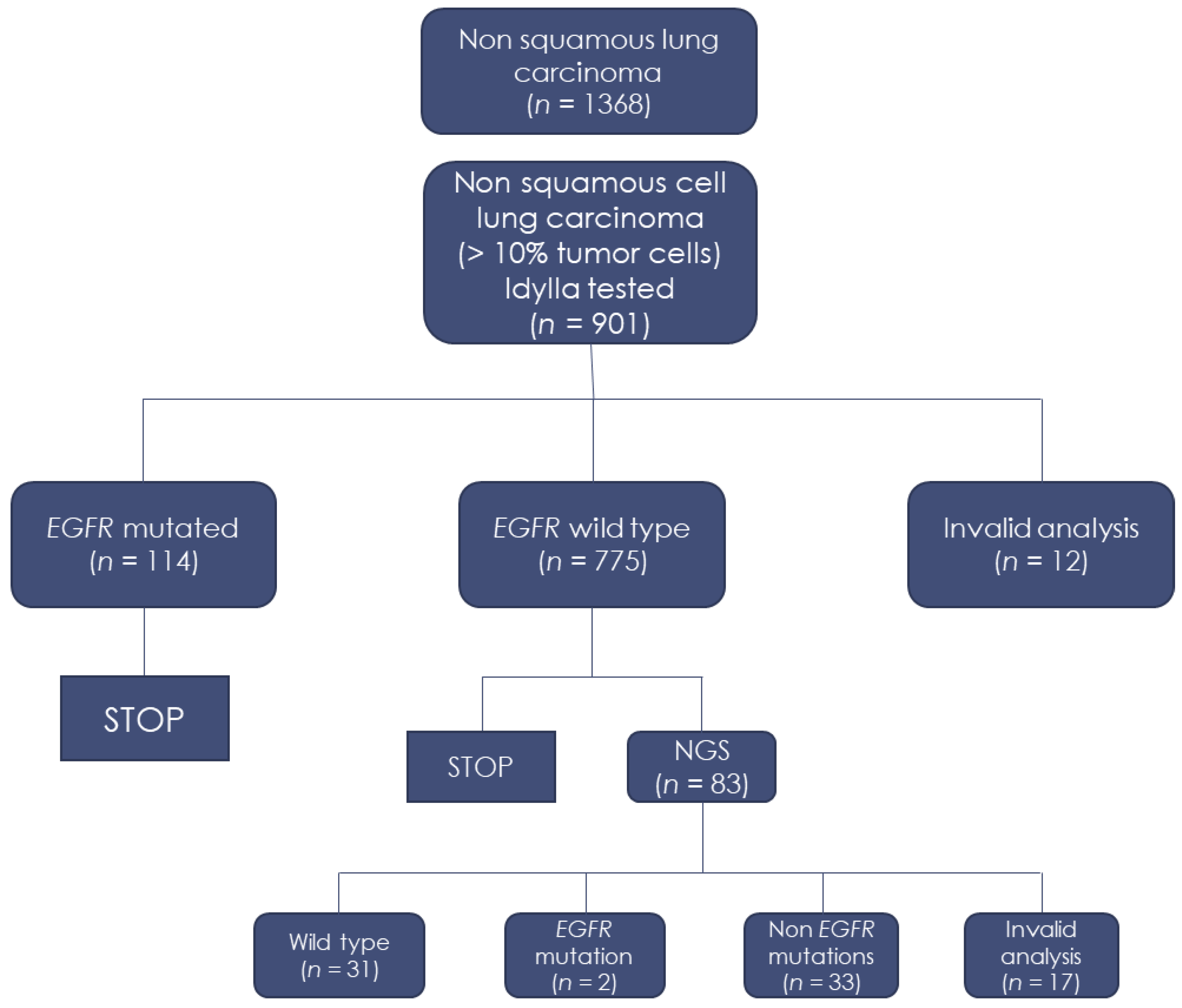
| Idylla System | Number (%) |
|---|---|
| Invalid | 12 (1.3%) |
| No mutation detected | 775 (86%) |
| Del exon 19 | 51 (5.6%) |
| Del exon 19 + T790M | 3 (0.3%) |
| L858R | 34 (3.9%) |
| L858R + T790M | 1 (0.1%) |
| L861Q | 10 (1.1%) |
| G719A/C/S | 5 (0.6%) |
| G719A/C/S + S768I | 4 (0.5%) |
| S768I | 3 (0.3%) |
| Ins exon 20 | 3 (0.3%) |
| Idylla Panel | Hotspot NGS Panel | Genes |
|---|---|---|
| Wild type | p.L747_S752 > Q, c.2239_2256 > CAA | EGFR Exon 19 |
| Wild type | p.P772_H773dup, c.2314_2319dup | EGFR Exon 20 |
| Not applicable | c.2325_2326insTCCGTGATGGCT; p.Ala775_Gly77linsSerValMetAla | HER2 |
| Not applicable | c.2325_2330delins18; p.Gly776_Val777delinsTyrValMetAlaGlyGly | HER2 |
| Not applicable | c.2585C > T; p.Thr862Ile | HER2 |
| Age (Years) | Mean (Range) | 66 (36–98) |
|---|---|---|
| Sex | F | 363 (40.3%) |
| M | 538 (59.7%) | |
| Smoking history | Smokers | 707 (78.5%) |
| Non-smokers | 95 (10.5%) | |
| Unknown | 99 (11%) | |
| Stage | I | 13 (1.4%) |
| II | 25 (2.8%) | |
| III | 115 (12.8%) | |
| IV | 465 (51.6%) | |
| Unknown (at the time of the histological diagnosis) | 283 (31.4%) | |
| Samples | Surgical lymph node biopsy | 52 (5.8%) |
| Surgical pleural biopsy | 48 (5.3%) | |
| Core needle biopsy | 202 (22.5%) | |
| Bronchial biopsy | 599 (66.4%) | |
| Histological type | Lung adenocarcinoma | 795 (88.2%) |
| NSCLC NOS | 91 (10.1%) | |
| Large cell carcinoma | 15 (1.7%) | |
| Tumor cellularity | Mean (Range) | 45% (15–95%) |
| Type of pathological material | Formalin fixed paraffin embedded | 872 (97%) |
| Fresh tissue | 29 (3%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lassalle, S.; Hofman, V.; Heeke, S.; Benzaquen, J.; Long, E.; Poudenx, M.; Lantéri, E.; Boutros, J.; Tanga, V.; Zahaf, K.; et al. Targeted Assessment of the EGFR Status as Reflex Testing in Treatment-Naive Non-Squamous Cell Lung Carcinoma Patients: A Single Laboratory Experience (LPCE, Nice, France). Cancers 2020, 12, 955. https://doi.org/10.3390/cancers12040955
Lassalle S, Hofman V, Heeke S, Benzaquen J, Long E, Poudenx M, Lantéri E, Boutros J, Tanga V, Zahaf K, et al. Targeted Assessment of the EGFR Status as Reflex Testing in Treatment-Naive Non-Squamous Cell Lung Carcinoma Patients: A Single Laboratory Experience (LPCE, Nice, France). Cancers. 2020; 12(4):955. https://doi.org/10.3390/cancers12040955
Chicago/Turabian StyleLassalle, Sandra, Véronique Hofman, Simon Heeke, Jonathan Benzaquen, Elodie Long, Michel Poudenx, Elisabeth Lantéri, Jacques Boutros, Virginie Tanga, Katia Zahaf, and et al. 2020. "Targeted Assessment of the EGFR Status as Reflex Testing in Treatment-Naive Non-Squamous Cell Lung Carcinoma Patients: A Single Laboratory Experience (LPCE, Nice, France)" Cancers 12, no. 4: 955. https://doi.org/10.3390/cancers12040955
APA StyleLassalle, S., Hofman, V., Heeke, S., Benzaquen, J., Long, E., Poudenx, M., Lantéri, E., Boutros, J., Tanga, V., Zahaf, K., Lalvée, S., Lespinet, V., Bordone, O., Félix, J.-M., Bonnetaud, C., Marquette, C., Ilie, M., & Hofman, P. (2020). Targeted Assessment of the EGFR Status as Reflex Testing in Treatment-Naive Non-Squamous Cell Lung Carcinoma Patients: A Single Laboratory Experience (LPCE, Nice, France). Cancers, 12(4), 955. https://doi.org/10.3390/cancers12040955







