High Galectin-7 and Low Galectin-8 Expression and the Combination of both are Negative Prognosticators for Breast Cancer Patients
Abstract
1. Introduction
2. Results
2.1. Gal-7 and Gal-8 Expression in Breast Cancer and Correlation to Different Clinical and Pathological Characteristics
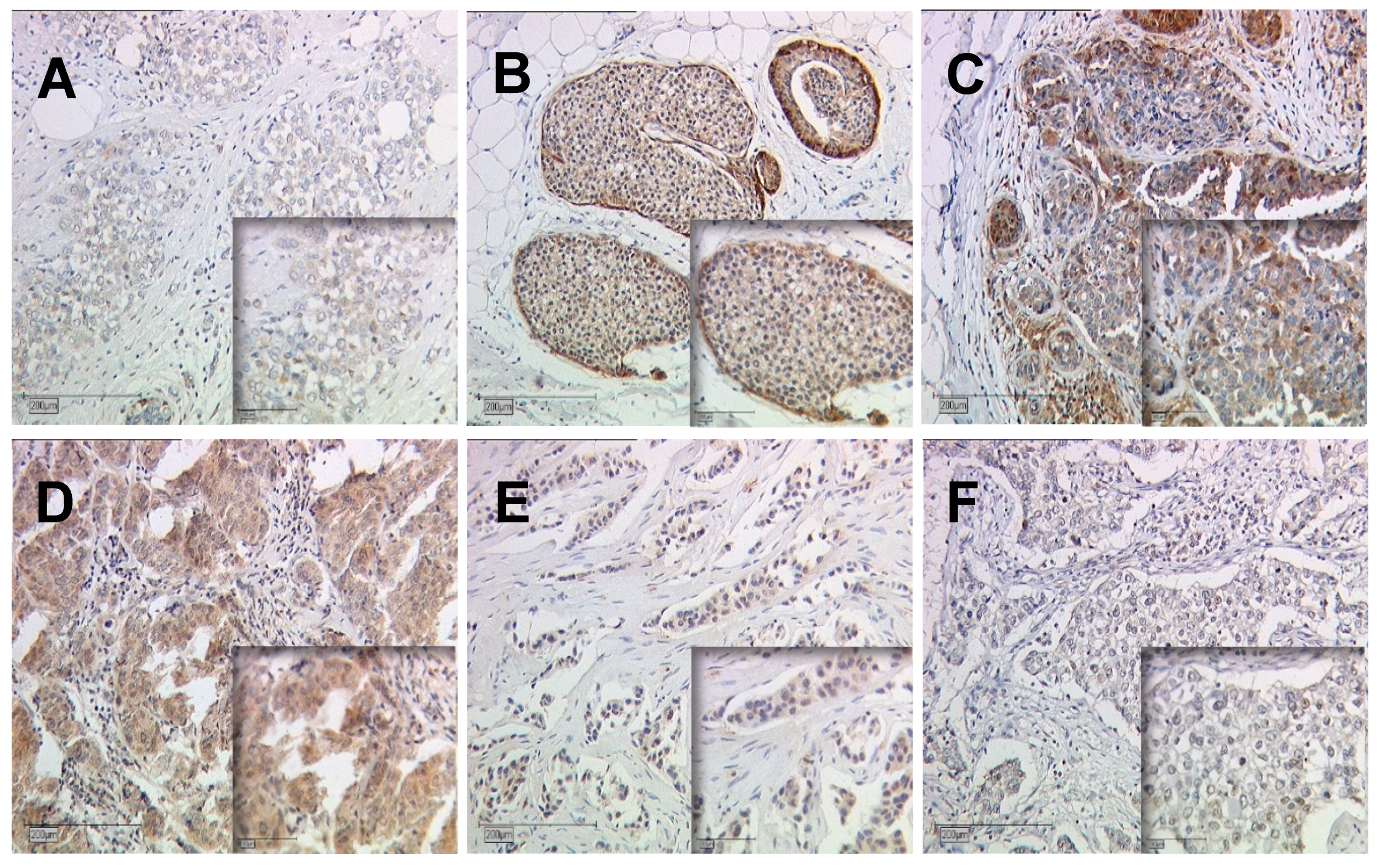
2.1.1. Gal-7 and Gal-8 Expression in Breast Cancer Cytoplasm and Nucleus
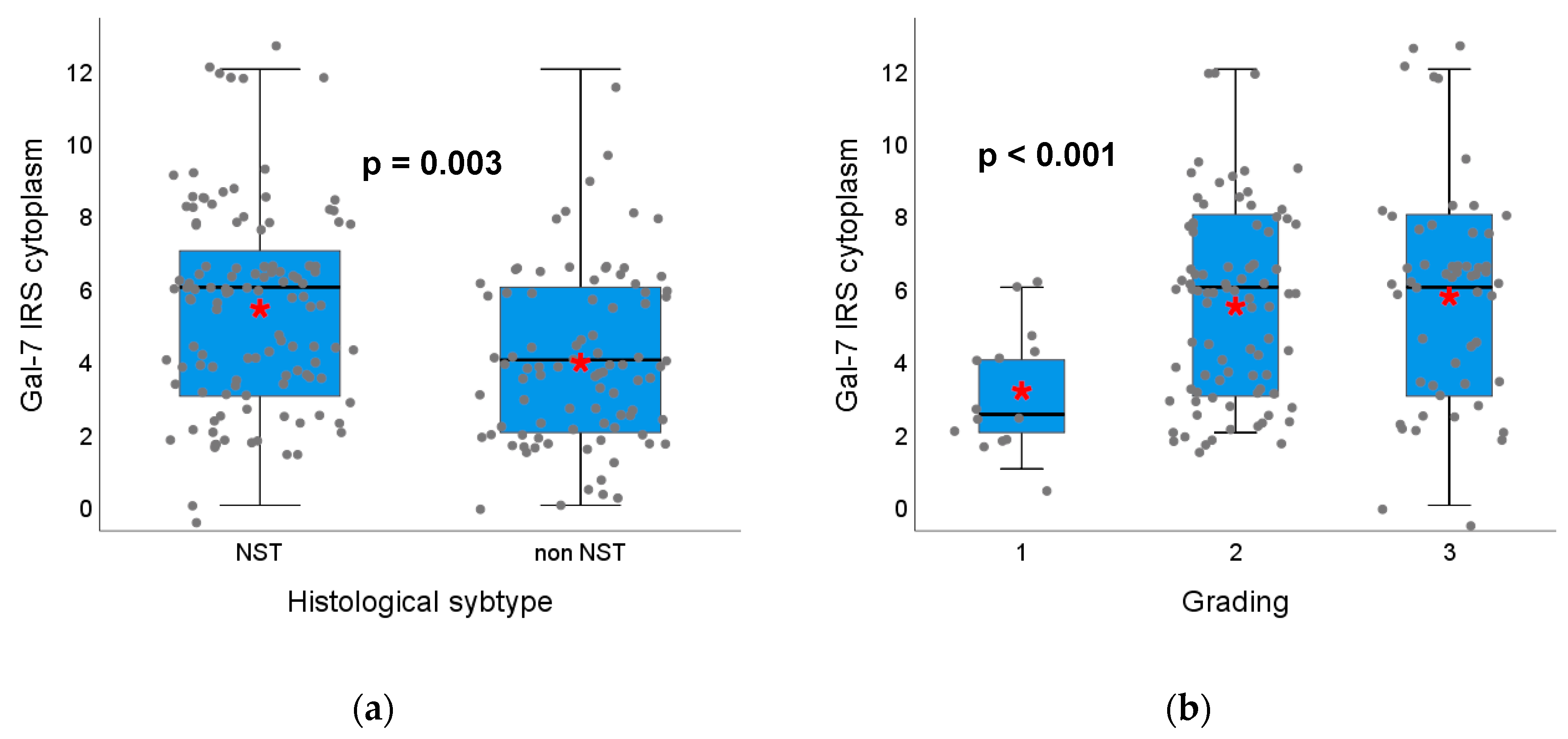
2.1.2. Gal-7 and Gal-8 Expression and Correlation with Clinical Characteristics, Histopathological Breast Cancer Subtypes and Grading
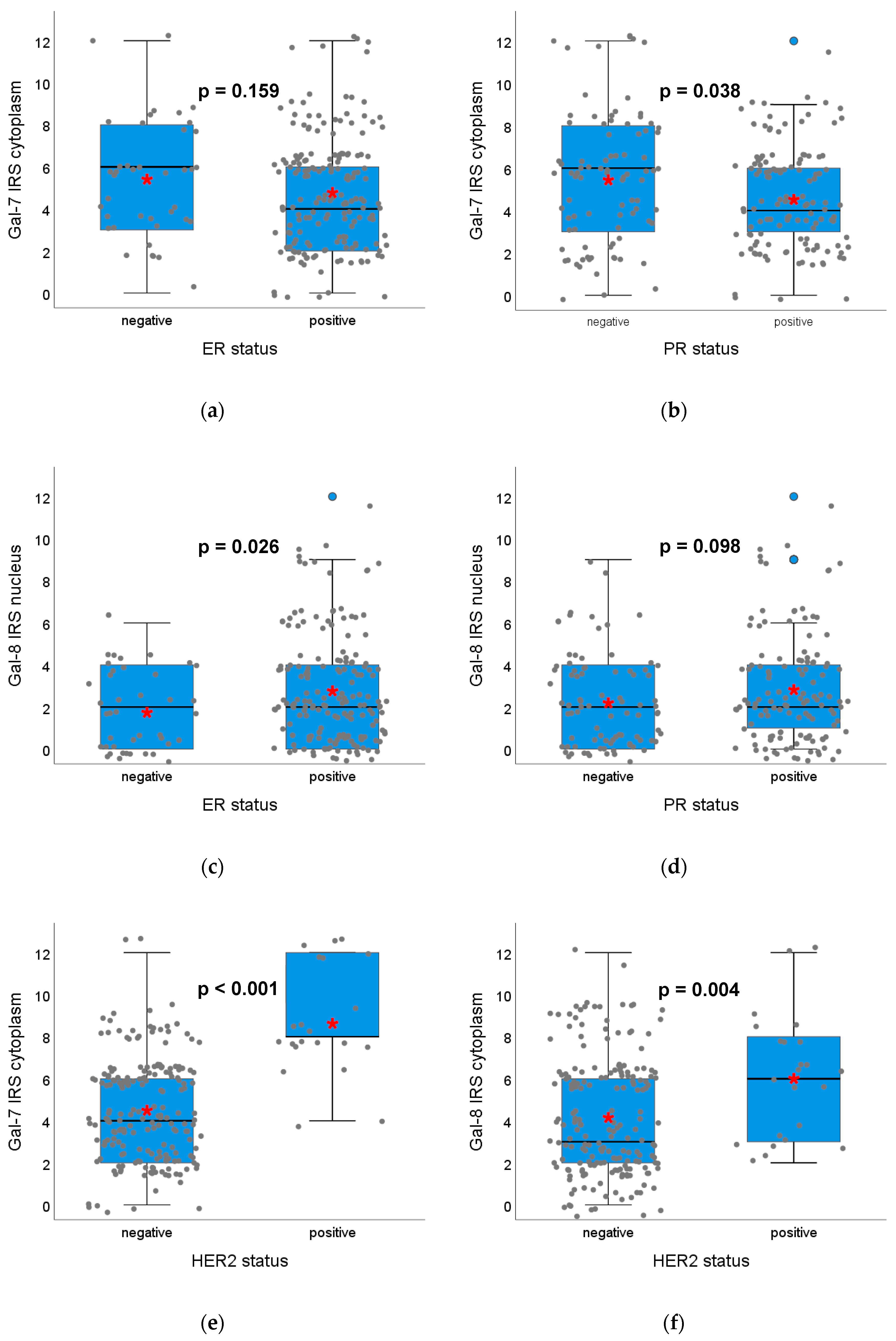
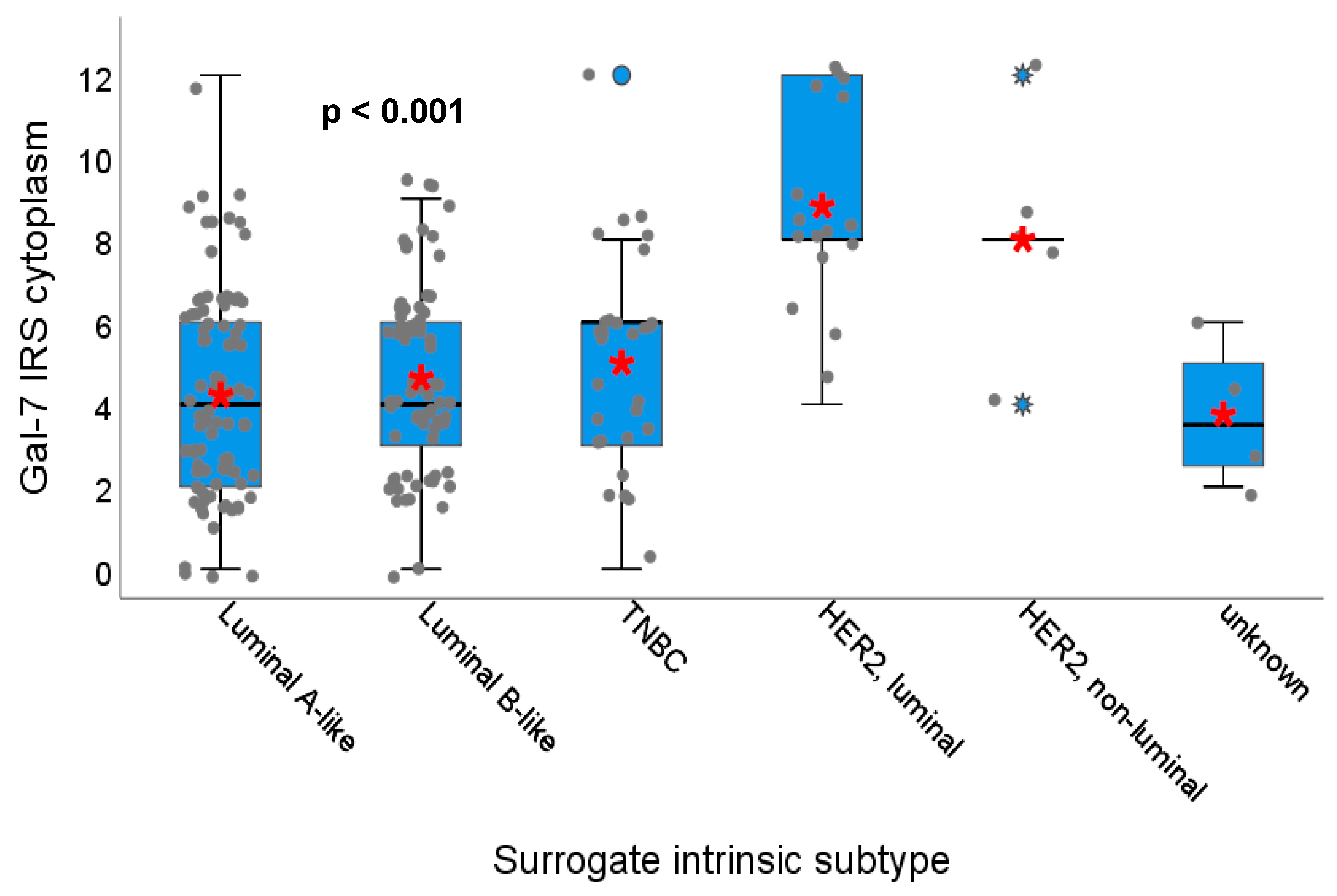
2.1.3. Gal-7 and Gal-8 Expression and Correlation with Hormone Receptor Status, HER2 Amplification and Surrogate Intrinsic Subtypes
2.1.4. Correlation of Gal-7 and Gal-8 Expression
2.2. Correlation of Gal-7 and Gal-8 Expression with Survival in Breast Cancer Patients
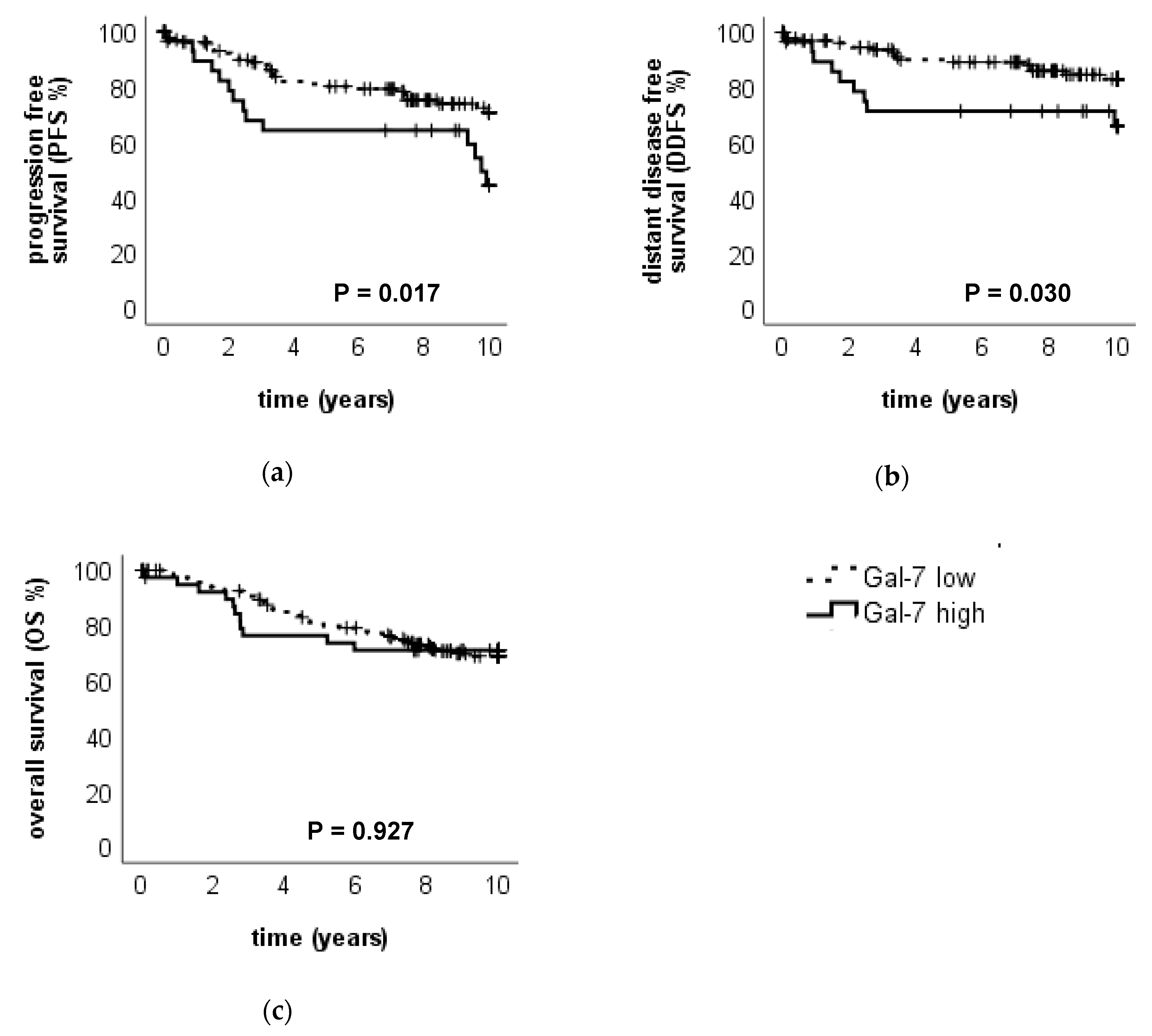
2.2.1. High Gal-7 Expression in the Cytoplasm is a Negative Prognosticator for Survival in Breast Cancer Patients
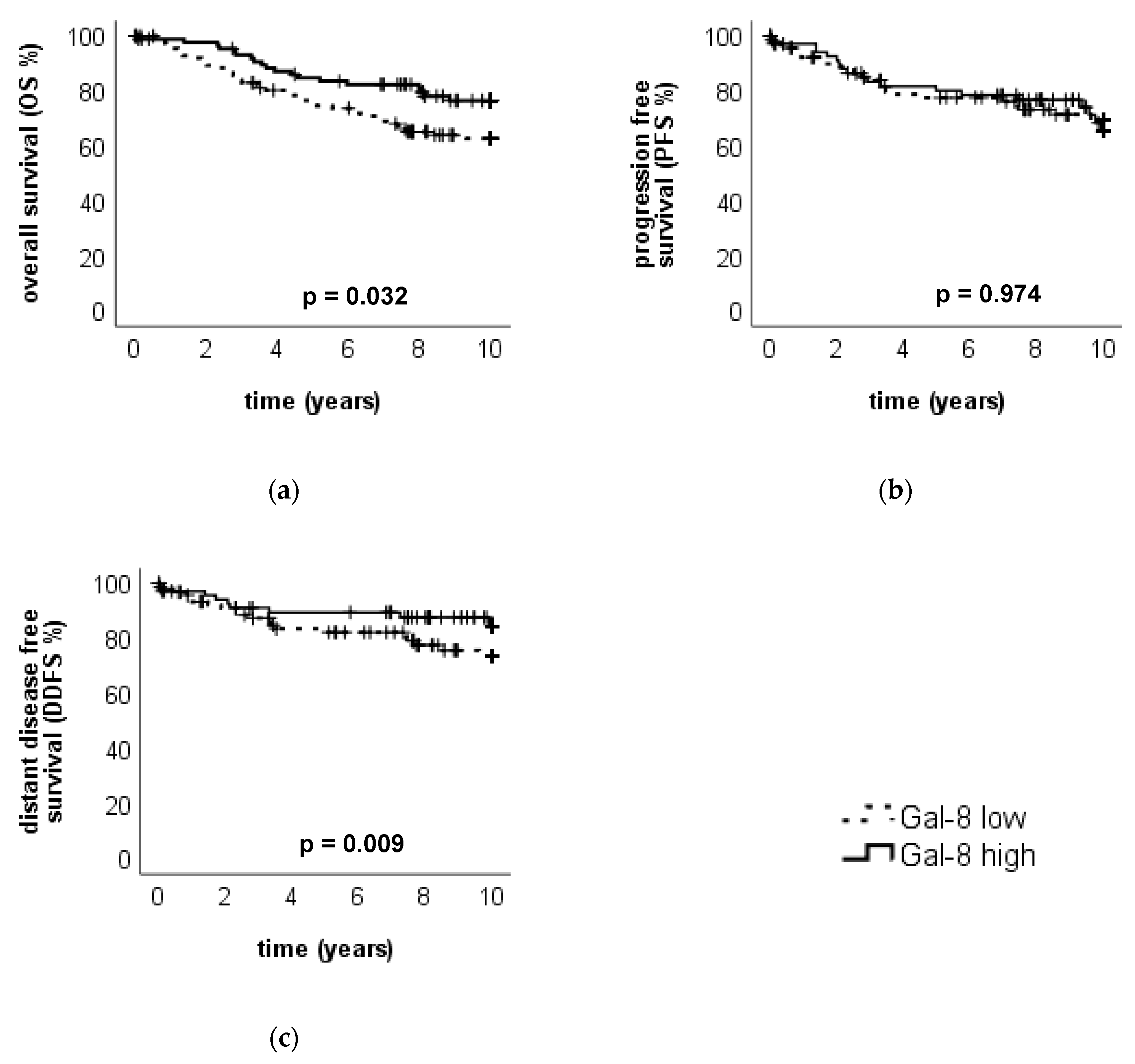
2.2.2. High Gal-8 Expression in the Cytoplasm is a Positive Prognosticator for Overall Survival in Breast Cancer Patients
2.2.3. Survival Analysis Using Combined Gal-7 and Gal-8 Staining
2.2.4. Immune Cell Infiltration Stained with Gal-7
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. Immunofluorescence
4.4. Statistical Analysis
4.5. Ethics Approval and Consent to Participate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Cho, S.H.; Jeon, J.; Kim, S.I. Personalized medicine in breast cancer: A systematic review. J. Breast Cancer 2012, 15, 265–272. [Google Scholar] [CrossRef]
- Harris, E.E.R. Precision medicine for breast cancer: The paths to truly individualized diagnosis and treatment. Int. J. Breast Cancer 2018, 2018, 4809183. [Google Scholar] [CrossRef]
- Gaudet, M.M.; Gierach, G.L.; Carter, B.D.; Luo, J.; Milne, R.L.; Weiderpass, E.; Giles, G.G.; Tamimi, R.M.; Eliassen, A.H.; Rosner, B.; et al. Pooled analysis of nine cohorts reveals breast cancer risk factors by tumor molecular subtype. Cancer Res. 2018, 78, 6011–6021. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.W.; Cummings, R.D.; Drickamer, K.; Felzi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.-I.; et al. Galectins: A family of animal β-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar]
- Ebrahim, A.H.; Alalawi, Z.; Mirandola, L.; Rakhshanda, R.; Dahlbeck, S.; Nguyen, D.; Jenkins, M.; Grizzi, F.; Cobos, E.; Figueroa, J.A.; et al. Galectins in cancer: Carcinogenesis, diagnosis and therapy. Ann. Transl. Med. 2014, 2, 88. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.; Liu, F.T. Galectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA; The Consortium of Glycobiology Editors: La Jolla, CA, USA, 2009. [Google Scholar]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Kolbl, A.C.; Andergassen, U.; Jeschke, U. The role of glycosylation in breast cancer metastasis and cancer control. Front. Oncol. 2015, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Mendez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, N.M.; Mohammadalipour, A.; Hall, C.R.; Asghari Adib, A.; Farnoud, A.M.; Burdick, M.M. Galectin-1 influences breast cancer cell adhesion to e-selectin via ligand intermediaries. Cell Mol. Bioeng. 2018, 11, 37–52. [Google Scholar] [CrossRef]
- Simone, G.; Malara, N.; Trunzo, V.; Renne, M.; Perozziello, G.; Di Fabrizio, E.; Manz, A. Galectin-3 coats the membrane of breast cells and makes a signature of tumours. Mol. Biosyst. 2014, 10, 258–265. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, M.; Liang, X.; Wang, D.; Gu, X.; Duan, C.; Gu, H.; Chen, G.; Zhao, X.; Zhao, Z.; et al. Galectin-3 as a marker and potential therapeutic target in breast cancer. PLoS ONE 2014, 9, e103482. [Google Scholar] [CrossRef]
- Irie, A.; Yamauchi, A.; Kontani, K.; Kihara, M.; Liu, D.; Shirato, Y.; Seki, M.; Nishi, N.; Nakamura, T.; Yokomise, H.; et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin. Cancer Res. 2005, 11, 2962–2968. [Google Scholar] [CrossRef]
- Grosset, A.A.; Poirier, F.; Gaboury, L.; St-Pierre, Y. Galectin-7 expression potentiates her-2-positive phenotype in breast cancer. PLoS ONE 2016, 11, e0166731. [Google Scholar] [CrossRef]
- Demers, M.; Rose, A.A.; Grosset, A.A.; Biron-Pain, K.; Gaboury, L.; Siegel, P.M.; St-Pierre, Y. Overexpression of galectin-7, a myoepithelial cell marker, enhances spontaneous metastasis of breast cancer cells. Am. J. Pathol. 2010, 176, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Campion, C.G.; Labrie, M.; Lavoie, G.; St-Pierre, Y. Expression of galectin-7 is induced in breast cancer cells by mutant p53. PLoS ONE 2013, 8, e72468. [Google Scholar] [CrossRef] [PubMed]
- Ferragut, F.; Cagnoni, A.J.; Colombo, L.L.; Sanchez Terrero, C.; Wolfenstein-Todel, C.; Troncoso, M.F.; Vanzulli, S.I.; Rabinovich, G.A.; Marino, K.V.; Elola, M.T. Dual knockdown of Galectin-8 and its glycosylated ligand, the activated leukocyte cell adhesion molecule (ALCAM/CD166), synergistically delays in vivo breast cancer growth. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Grosset, A.A.; Labrie, M.; Vladoiu, M.C.; Yousef, E.M.; Gaboury, L.; St-Pierre, Y. Galectin signatures contribute to the heterogeneity of breast cancer and provide new prognostic information and therapeutic targets. Oncotarget 2016, 7, 18183–18203. [Google Scholar] [CrossRef]
- Piyush, T.; Chacko, A.R.; Sindrewicz, P.; Hilkens, J.; Rhodes, J.M.; Yu, L.G. Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ. 2017, 24, 1937–1947. [Google Scholar] [CrossRef]
- Kuo, H.Y.; Hsu, H.T.; Chen, Y.C.; Chang, Y.W.; Liu, F.T.; Wu, C.W. Galectin-3 modulates the EGFR signalling-mediated regulation of Sox2 expression via c-Myc in lung cancer. Glycobiology 2016, 26, 155–165. [Google Scholar] [CrossRef]
- Wu, K.L.; Kuo, C.M.; Huang, E.Y.; Pan, H.M.; Huang, C.C.; Chen, Y.F.; Hsiao, C.C.; Yang, K.D. Extracellular galectin-3 facilitates colon cancer cell migration and is related to the epidermal growth factor receptor. Am. J. Transl. Res. 2018, 10, 2402–2412. [Google Scholar]
- Wang, Z. ErbB receptors and cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [CrossRef]
- Carlsson, M.C.; Balog, C.I.; Kilsgard, O.; Hellmark, T.; Bakoush, O.; Segelmark, M.; Ferno, M.; Olsson, H.; Malmstrom, J.; Wuhrer, M.; et al. Different fractions of human serum glycoproteins bind galectin-1 or galectin-8, and their ratio may provide a refined biomarker for pathophysiological conditions in cancer and inflammatory disease. Biochim. Biophys. Acta 2012, 1820, 1366–1372. [Google Scholar] [CrossRef]
- Huflejt, M.E.; Leffler, H. Galectin-4 in normal tissues and cancer. Glycoconj. J. 2004, 20, 247–255. [Google Scholar] [CrossRef]
- Bibens-Laulan, N.; St-Pierre, Y. Intracellular galectin-7 expression in cancer cells results from an autocrine transcriptional mechanism and endocytosis of extracellular galectin-7. PLoS ONE 2017, 12, e0187194. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Belardi, B.; Mori, H.; Kuo, P.; Tam, A.; Hines, W.C.; Le, Q.T.; Bertozzi, C.R.; Bissell, M.J. Nuclear repartitioning of galectin-1 by an extracellular glycan switch regulates mammary morphogenesis. Proc. Natl. Acad. Sci. USA 2016, 113, E4820–E4827. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Prognostic significance of tumor-associated macrophages in breast cancer: A meta-analysis of the literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef] [PubMed]
- Grosset, A.A.; Labrie, M.; Gagne, D.; Vladoiu, M.C.; Gaboury, L.; Doucet, N.; St-Pierre, Y. Cytosolic galectin-7 impairs p53 functions and induces chemoresistance in breast cancer cells. BMC Cancer 2014, 14, 801. [Google Scholar] [CrossRef]
- Vuong, L.; Kouverianou, E.; Rooney, C.M.; McHugh, B.J.; Howie, S.E.M.; Gregory, C.D.; Forbes, S.J.; Henderson, N.C.; Zetterberg, F.R.; Nilsson, U.J.; et al. An orally active galectin-3 antagonist inhibits lung adenocarcinoma growth and augments response to pd-l1 blockade. Cancer Res. 2019, 79, 1480–1492. [Google Scholar] [CrossRef]
- Shih, T.C.; Liu, R.; Fung, G.; Bhardwaj, G.; Ghosh, P.M.; Lam, K.S. A novel galectin-1 inhibitor discovered through one-bead two-compound library potentiates the antitumor effects of paclitaxel in vivo. Mol. Cancer Ther. 2017, 16, 1212–1223. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Makki, J. Diversity of breast carcinoma: Histological subtypes and clinical relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The new TNM-based staging of breast cancer. Virchows Arch. 2018, 472, 697–703. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]


| Gal-7 Cytoplasm | Gal-7 Nucleus | ||||
|---|---|---|---|---|---|
| IRS | n | % | IRS | n | % |
| 0 | 7 | 3.0 | 0 | 35 | 14.9 |
| 1 | 2 | 0.9 | 1 | 17 | 7.2 |
| 2 | 43 | 18.3 | 2 | 64 | 27.2 |
| 3 | 21 | 8.9 | 3 | 51 | 21.7 |
| 4 | 40 | 17.0 | 4 | 25 | 10.6 |
| 6 | 60 | 25.5 | 6 | 24 | 10.2 |
| 8 | 27 | 11.5 | NA | 19 | 8.1 |
| 9 | 8 | 3.4 | total | 235 | 100.0 |
| 12 | 8 | 3.4 | |||
| NA | 19 | 8.1 | |||
| total | 235 | 100.0 | |||
| Gal-8 Cytoplasm | Gal-8 Nucleus | ||||
|---|---|---|---|---|---|
| IRS | n | % | IRS | n | % |
| 0 | 15 | 6.4 | 0 | 63 | 26.8 |
| 1 | 12 | 5.1 | 1 | 14 | 6.0 |
| 2 | 50 | 21.3 | 2 | 55 | 23.4 |
| 3 | 27 | 11.5 | 3 | 7 | 3.0 |
| 4 | 18 | 7.7 | 4 | 43 | 18.3 |
| 6 | 54 | 23.0 | 6 | 22 | 9.4 |
| 8 | 16 | 6.8 | 8 | 1 | 0.4 |
| 9 | 18 | 7.7 | 9 | 9 | 3.8 |
| 12 | 5 | 2.1 | 12 | 1 | 0.4 |
| NA | 20 | 8.5 | NA | 20 | 8.5 |
| total | 235 | 100.0 | total | 235 | 100.0 |
| Gal-7 IRS Cytoplasm | Gal-7 IRS Nucleus | Gal-8 IRS Cytoplasm | Gal-8 IRS Nucleus | ||
|---|---|---|---|---|---|
| Gal-7 IRS cytoplasm | Correlation Coefficient | 1.000 | 0.467 ** | 0.332 ** | 0.188 ** |
| Sig. (2-tailed) | <0.001 | <0.001 | 0.007 | ||
| N | 216 | 216 | 206 | 206 | |
| Gal-7 IRS nucleus | Correlation Coefficient | 0.467 ** | 1.000 | 0.185 ** | 0.301 ** |
| Sig. (2-tailed) | <0.001 | 0.008 | <0.001 | ||
| N | 216 | 216 | 206 | 206 | |
| Gal-8 IRS cytoplasm | Correlation Coefficient | 0.332 ** | 0.185 ** | 1.000 | 0.594 ** |
| Sig. (2-tailed) | <0.001 | 0.008 | 0.000 | ||
| N | 206 | 206 | 215 | 215 | |
| Gal-8 IRS nucleus | Correlation Coefficient | 0.188 ** | 0.301 ** | 0.594 ** | 1.000 |
| Sig. (2-tailed) | 0.007 | <0.001 | <0.001 | ||
| N | 206 | 206 | 215 | 215 |
| Prognostic Factor | B | SE | Wald | df | Sig. | Exp(B) | 95,0% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Histological subtype (NST vs. non-NST) | 0.245 | 0.459 | 0.286 | 1 | 0.593 | 1.278 | 0.520 | 3.140 |
| Grading (G1 vs. G2-3) | 0.312 | 0.817 | 0.146 | 1 | 0.702 | 1.367 | 0.276 | 6.773 |
| Tumor size (pT1 vs. pT2-4) | 0.284 | 0.377 | 0.564 | 1 | 0.453 | 1.328 | 0.634 | 2.783 |
| Nodal status (pN0 vs. pN1-3) | 0.553 | 0.397 | 1.935 | 1 | 0.164 | 1.738 | 0.798 | 3.788 |
| HER2 status (positive vs. negative) | −0.161 | 0.538 | 0.090 | 1 | 0.764 | 0.851 | 0.297 | 2.443 |
| ER (positive vs. negative) | −0.823 | 0.459 | 3.219 | 1 | 0.073 | 0.439 | 0.179 | 1.079 |
| PR (positive vs. negative) | −0.345 | 0.460 | 0.565 | 1 | 0.452 | 0.708 | 0.288 | 1.743 |
| Patient age (continuous) | 0.010 | 0.017 | 0.329 | 1 | 0.566 | 1.010 | 0.977 | 1.043 |
| Gal-7 expression in the cytoplasm (high vs. low) | 0.806 | 0.410 | 3.860 | 1 | 0.049 | 2.239 | 1.002 | 5.003 |
| Prognostic Factor | B | SE | Wald | df | Sig. | Exp(B) | 95,0% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Histological subtype (NST vs. non-NST) | 0.199 | 0.456 | 0.190 | 1 | 0.663 | 1.220 | 0.499 | 2.983 |
| Grading (G1 vs. G2-3) | −0.321 | 0.591 | 0.295 | 1 | 0.587 | 0.725 | 0.228 | 2.311 |
| Tumor size (pT1 vs. pT2-4) | 0.237 | 0.385 | 0.378 | 1 | 0.539 | 1.267 | 0.596 | 2.693 |
| Nodal status (pN0 vs. pN1-3) | 1.144 | 0.406 | 7.927 | 1 | 0.005 | 3.140 | 1.416 | 6.964 |
| HER2 status (positive vs. negative) | 0.429 | 0.617 | 0.483 | 1 | 0.487 | 1.535 | 0.458 | 5.143 |
| ER (positive vs. negative) | −1.178 | 0.504 | 5.466 | 1 | 0.019 | 0.308 | 0.115 | 0.827 |
| PR (positive vs. negative) | 0.568 | 0.483 | 1.381 | 1 | 0.240 | 1.765 | 0.684 | 4.551 |
| Patient age (continuous) | 0.051 | 0.017 | 9.367 | 1 | 0.002 | 1.053 | 1.019 | 1.088 |
| Gal-8 expression in the cytoplasm (high vs. low) | −0.258 | 0.372 | 0.479 | 1 | 0.489 | 0.773 | 0.372 | 1.603 |
| Patients‘ Characteristics | Median | SD |
|---|---|---|
| Age | 58.2 | 13.3 |
| N | % | |
| Histological subtype | ||
| NST | 126 | 53.6 |
| Non-NST | 96 | 40.9 |
| NA | 13 | 5.5 |
| Intrinsic surrogate subtype | ||
| Luminal A-like | 103 | 43.8 |
| Luminal B-like | 73 | 31.1 |
| HER2-positive, luminal | 17 | 7.2 |
| HER2-positive, non-luminal | 7 | 3.0 |
| TNBC | 31 | 13.2 |
| NA | 4 | 1.7 |
| Grading | ||
| Grade 1 | 17 | 7.2 |
| Grade 2 | 90 | 38.3 |
| Grade 3 | 55 | 23.4 |
| NA | 73 | 31.1 |
| Lymph node involvement (pN) | ||
| pN0 | 128 | 54.5 |
| pN1 | 87 | 37.0 |
| pN2 | 10 | 4.3 |
| NA | 10 | 4.3 |
| Tumor size (pT) | ||
| pT1 (≤2 cm) | 160 | 68.1 |
| pT2 (2–5 cm) | 68 | 28.9 |
| pT3 (>5 cm) | 1 | 0.4 |
| pT4 (with infiltration in the epidermis or the thoracic wall) | 5 | 2.1 |
| NA | 1 | 0.4 |
| HER2 status | ||
| Positive | 24 | 10.2 |
| Negative | 208 | 88.5 |
| NA | 3 | 1.3 |
| ER status | ||
| Positive | 192 | 81.7 |
| Negative | 43 | 18.3 |
| PR status | ||
| Positive | 141 | 60.0 |
| Negative | 94 | 40.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebo, A.; Ditsch, N.; Kuhn, C.; Heidegger, H.H.; Zeder-Goess, C.; Kolben, T.; Czogalla, B.; Schmoeckel, E.; Mahner, S.; Jeschke, U.; et al. High Galectin-7 and Low Galectin-8 Expression and the Combination of both are Negative Prognosticators for Breast Cancer Patients. Cancers 2020, 12, 953. https://doi.org/10.3390/cancers12040953
Trebo A, Ditsch N, Kuhn C, Heidegger HH, Zeder-Goess C, Kolben T, Czogalla B, Schmoeckel E, Mahner S, Jeschke U, et al. High Galectin-7 and Low Galectin-8 Expression and the Combination of both are Negative Prognosticators for Breast Cancer Patients. Cancers. 2020; 12(4):953. https://doi.org/10.3390/cancers12040953
Chicago/Turabian StyleTrebo, Anna, Nina Ditsch, Christina Kuhn, Helene Hildegard Heidegger, Christine Zeder-Goess, Thomas Kolben, Bastian Czogalla, Elisa Schmoeckel, Sven Mahner, Udo Jeschke, and et al. 2020. "High Galectin-7 and Low Galectin-8 Expression and the Combination of both are Negative Prognosticators for Breast Cancer Patients" Cancers 12, no. 4: 953. https://doi.org/10.3390/cancers12040953
APA StyleTrebo, A., Ditsch, N., Kuhn, C., Heidegger, H. H., Zeder-Goess, C., Kolben, T., Czogalla, B., Schmoeckel, E., Mahner, S., Jeschke, U., & Hester, A. (2020). High Galectin-7 and Low Galectin-8 Expression and the Combination of both are Negative Prognosticators for Breast Cancer Patients. Cancers, 12(4), 953. https://doi.org/10.3390/cancers12040953






