Transport Metabolons and Acid/Base Balance in Tumor Cells
Abstract
1. Introduction
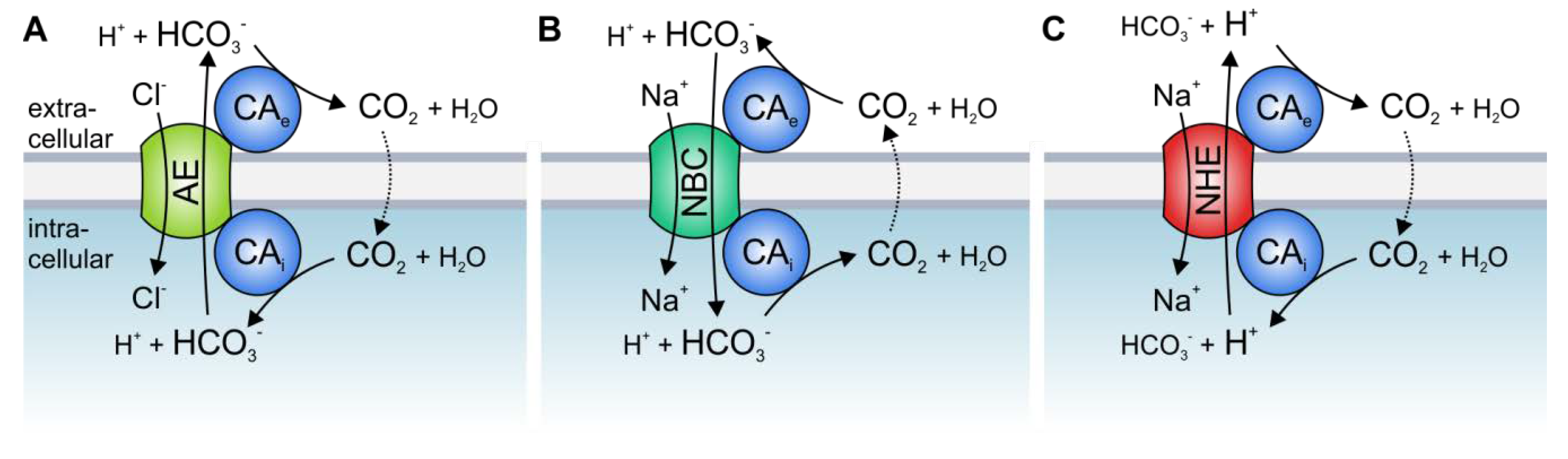
2. Acid/Base Transport Metabolons
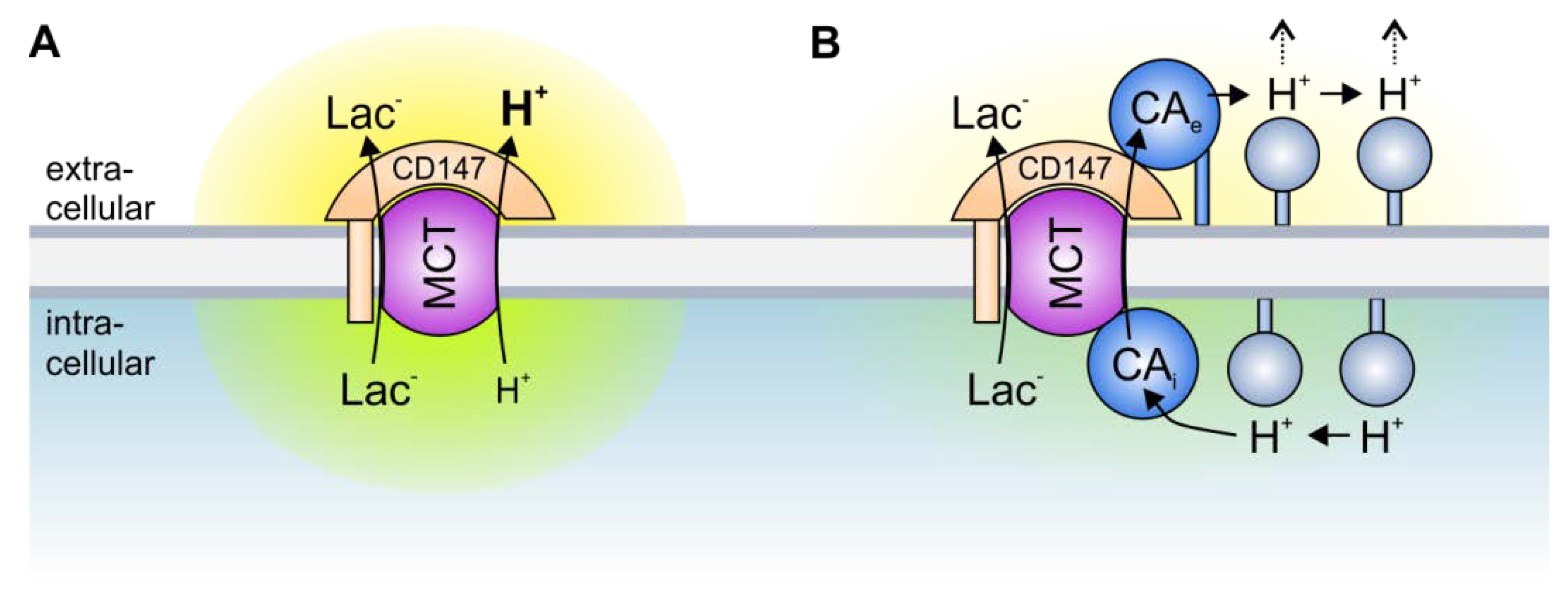
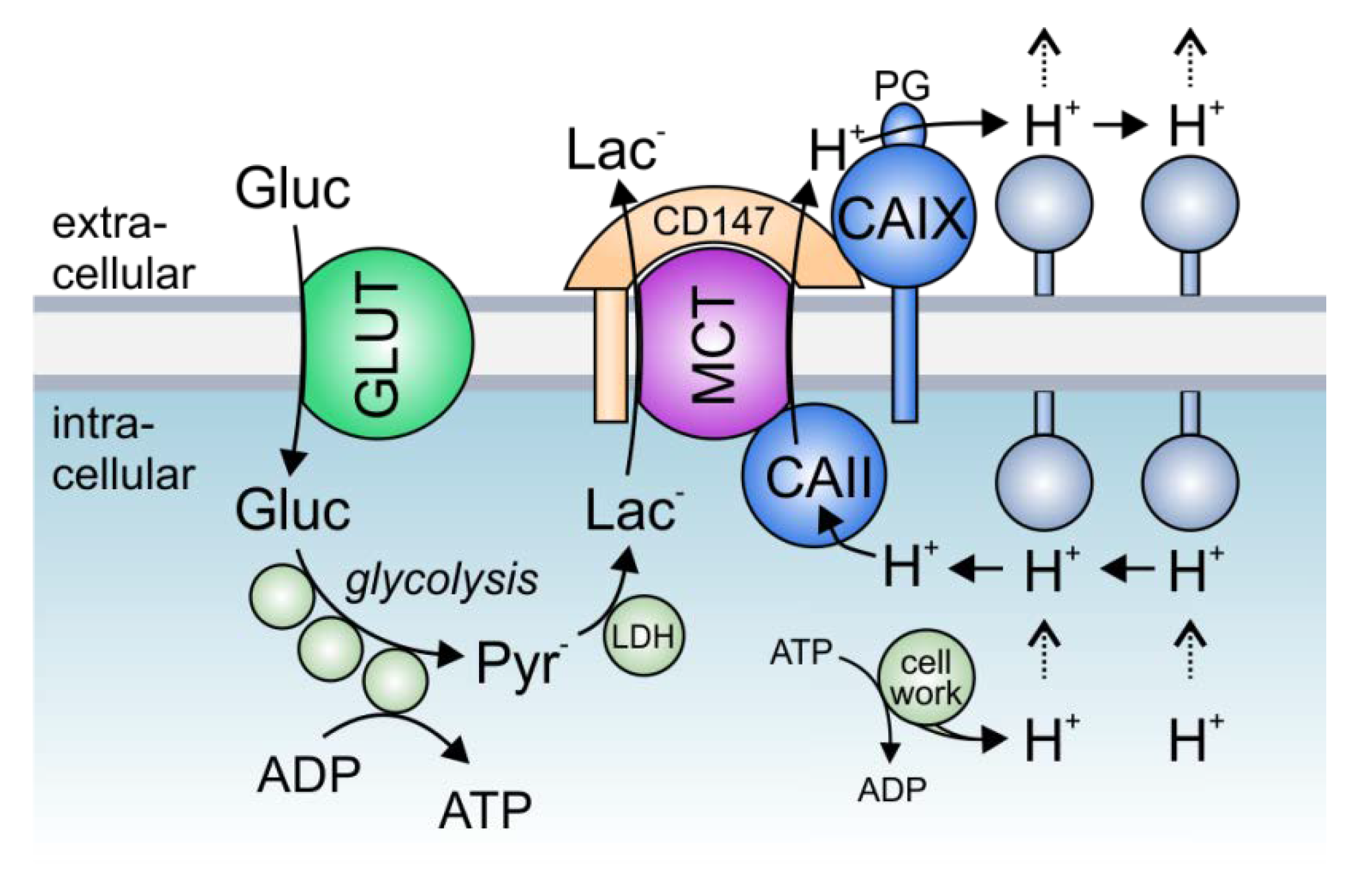
3. The Role of Transport Metabolons in Tumor Metabolism
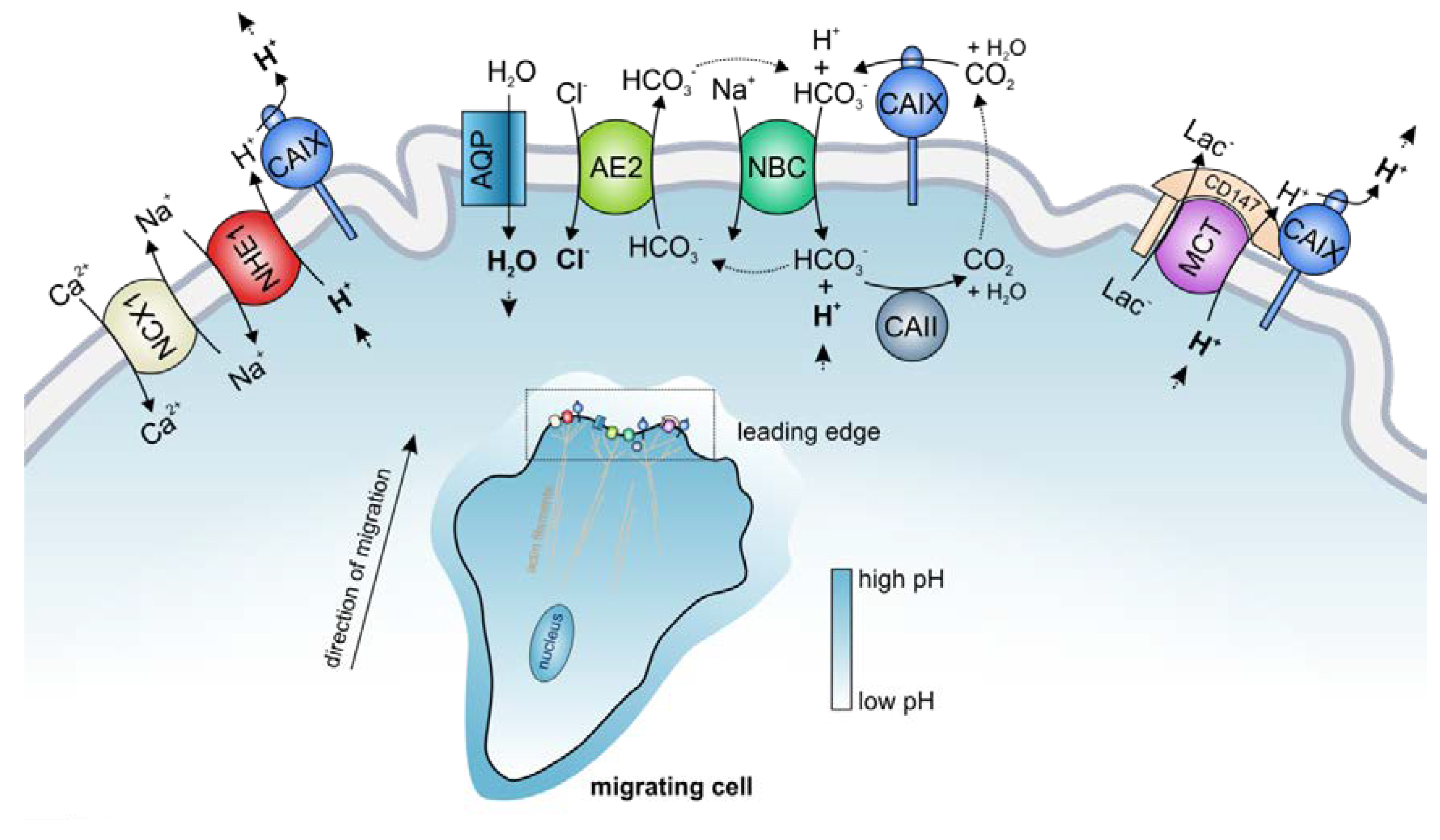
4. The Role of Transport Metabolons in Tumor pH Regulation and Cell Migration
5. Transport Metabolons as Drug Targets in Tumor Therapy
6. Conclusions
Funding
Conflicts of Interest
References
- Deitmer, J.W. Acid-base transport and pH regulation. In Handbook of Neurochemistry and Molecular Neurobiology—Brain Energetics. Integration of Molecular and Cellular Processes; Gibson, G.E., Dienel, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5, pp. 469–486. [Google Scholar]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Griffiths, J.R.; Stevens, A.N.; Iles, R.A.; Gordon, R.E.; Shaw, D. 31P-NMR investigation of solid tumours in the living rat. Biosci. Rep. 1981, 1, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Liu, Z.; Bhujwalla, Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am. J. Physiol. Cell Physiol. 1994, 267, C195–C203. [Google Scholar] [CrossRef]
- van Sluis, R.; Raghunand, N.; Bhujwalla, Z.M.; Cerdán, S.; Galons, J.; Ballesteros, P.; Gillies, R.J.; Alvarez, J. In vivo imaging of extracellular pH using 1H MRSI. Magn. Reson. Med. 2002, 41, 743–750. [Google Scholar] [CrossRef]
- Reshkin, S.J.; Bellizzi, A.; Caldeira, S.; Albarani, V.; Malanchi, I.; Poignee, M.; Alunni-Fabbroni, M.; Casavola, V.; Tommasino, M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000, 14, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Pouyssegur, J.; Sardet, C.; Franchi, A.; L’allemain, G.; Paris, S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH (H+-suicide selection/cytoplasmic pH/Na’ influx/growth control/somatic cell genetics). Cell Biol. 1984, 81, 4833–4837. [Google Scholar]
- Pouyssegur, J.; Franchi, A.; L’Allemain, G.; Paris, S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 1985, 190, 115–119. [Google Scholar] [CrossRef]
- Matsuyama, S.; Reed, J.C. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000, 7, 1155–1165. [Google Scholar] [CrossRef]
- Grillo-Hill, B.K.; Choi, C.; Jimenez-Vidal, M.; Barber, D.L. Increased H+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. Elife 2015, 2015, 1–31. [Google Scholar] [CrossRef]
- Frantz, C.; Barreiro, G.; Dominguez, L.; Chen, X.; Eddy, R.; Condeelis, J.; Kelly, M.J.S.; Jacobson, M.P.; Barber, D.L. Cofilin is a pH sensor for actin free barbed end formation: Role of phosphoinositide binding. J. Cell Biol. 2008, 183, 865–879. [Google Scholar] [CrossRef]
- Stock, C.; Schwab, A. Protons make tumor cells move like clockwork. Pflugers Arch. 2009, 458, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Lardner, A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001, 69, 522–530. [Google Scholar] [PubMed]
- Pilon-Thomas, S.; Kodumudi, K.N.; El-Kenawi, A.E.; Russell, S.; Weber, A.M.; Luddy, K.; Damaghi, M.; Wojtkowiak, J.W.; Mulé, J.J.; Ibrahim-Hashim, A.; et al. Neutralization of Tumor Acidity Improves Antitumor Responses to Immunotherapy. Cancer Res. 2016, 76, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Monaco, S.; Gioia, M.; Rodriguez, J.; Fasciglione, G.F.; Di Pierro, D.; Lupidi, G.; Krippahl, L.; Marini, S.; Coletta, M. Modulation of the proteolytic activity of matrix metalloproteinase-2 (gelatinase A) on fibrinogen. Biochem. J. 2007, 402, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.P.; Samsøe-Petersen, J.; Oernbo, E.K.; Boedtkjer, E.; Moreira, J.M.A.; Kveiborg, M.; Pedersen, S.F. The net acid extruders NHE1, NBCn1 and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. Int. J. Cancer 2018, 1–14. [Google Scholar] [CrossRef]
- Grinstein, S.; Woodside, M.; Waddell, T.K.; Downey, G.P.; Orlowski, J.; Pouyssegur, J.; Wong, D.C.; Foskett, J.K. Focal localization of the NHE-1 isoform of the Na+/H+ antiport: Assessment of effects on intracellular pH. EMBO J. 1993, 12, 5209–5218. [Google Scholar] [CrossRef]
- Stock, C.; Mueller, M.; Kraehling, H.; Mally, S.; Noël, J.; Eder, C.; Schwab, A. pH nanoenvironment at the surface of single melanoma cells. Cell. Physiol. Biochem. 2007, 20, 679–686. [Google Scholar] [CrossRef]
- Stüwe, L.; Müller, M.; Fabian, A.; Waning, J.; Mally, S.; Noël, J.; Schwab, A.; Stock, C. pH dependence of melanoma cell migration: Protons extruded by NHE1 dominate protons of the bulk solution. J. Physiol. 2007, 585, 351–360. [Google Scholar] [CrossRef]
- Ludwig, F.T.; Schwab, A.; Stock, C. The Na+/H+-exchanger (NHE1) generates pH nanodomains at focal adhesions. J. Cell. Physiol. 2013, 228, 1351–1358. [Google Scholar] [CrossRef]
- Kondapalli, K.C.; Llongueras, J.P.; Capilla-González, V.; Prasad, H.; Hack, A.; Smith, C.; Guerrero-Cázares, H.; Quiñones-Hinojosa, A.; Rao, R. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, H.; Wen, J.; Luo, K.; Liu, Q.; Huang, Y.; Zheng, Y.; Tan, Z.; Huang, Q.; Fu, J. NHE9 induces chemoradiotherapy resistance in esophageal squamous cell carcinoma by upregulating the Src/Akt/β-catenin pathway and Bcl-2 expression. Oncotarget 2015, 6, 12405–12420. [Google Scholar] [CrossRef]
- Lucien, F.; Pelletier, P.P.; Lavoie, R.R.; Lacroix, J.M.; Roy, S.; Parent, J.L.; Arsenault, D.; Harper, K.; Dubois, C.M. Hypoxia-induced mobilization of NHE6 to the plasma membrane triggers endosome hyperacidification and chemoresistance. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.W.; Wood, I.S.; Ellis, A.; Shirazi-Beechey, S.P. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br. J. Cancer 2002, 86, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Longatto-Filho, A.; Scapulatempo, C.; Ferreira, L.; Martins, S.; Pellerin, L.; Rodrigues, M.; Alves, V.A.F.; Schmitt, F.; Baltazar, F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008, 452, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Reis, R.M.; Ricardo, S.; Longatto-Filho, A.; Schmitt, F.; Baltazar, F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J. Biomed. Biotechnol. 2010, 2010, 427694. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Longatto-Filho, A.; Azevedo-Silva, J.; Casal, M.; Schmitt, F.C.; Baltazar, F. Role of monocarboxylate transporters in human cancers: State of the art. J. Bioenerg. Biomembr. 2012, 44, 127–139. [Google Scholar] [CrossRef]
- Bröer, S.; Schneider, H.P.; Bröer, A.; Rahman, B.; Hamprecht, B.; Deitmer, J.W. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998, 333, 167–174. [Google Scholar] [CrossRef]
- Halestrap, A.P. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life 2012, 64, 1–9. [Google Scholar] [CrossRef]
- Forero-Quintero, L.S.; Deitmer, J.W.; Becker, H.M. Reduction of epileptiform activity in ketogenic mice: The role of monocarboxylate transporters. Sci. Rep. 2017, 7, 4900. [Google Scholar] [CrossRef]
- Silva, L.S.; Poschet, G.; Nonnenmacher, Y.; Becker, H.M.; Sapcariu, S.; Gaupel, A.; Schlotter, M.; Wu, Y.; Kneisel, N.; Seiffert, M.; et al. Branched-chain ketoacids secreted by glioblastoma cells via MCT1 modulate macrophage phenotype. EMBO Rep. 2017, e201744154. [Google Scholar]
- Parks, S.K.; Chiche, J.; Pouysségur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 2013, 13, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Deitmer, J.W.; Schlue, W.R. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J. Physiol. 1989, 411, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Aalkjaer, C.; Boulpaep, E.L.; Boron, W.F. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 2000, 405, 571–575. [Google Scholar] [CrossRef]
- Gross, E.; Hawkins, K.; Pushkin, A.; Sassani, P.; Dukkipati, R.; Abuladze, N.; Hopfer, U.; Kurtz, I. Phosphorylation of Ser (982) in the sodium bicarbonate cotransporter kNBC1 shifts the HCO(3)(-):Na(+) stoichiometry from 3:1 to 2:1 in murine proximal tubule cells. J. Physiol. 2001, 537, 659–665. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Moreira, J.M.A.; Mele, M.; Vahl, P.; Wielenga, V.T.; Christiansen, P.M.; Jensen, V.E.D.; Pedersen, S.F.; Aalkjaer, C. Contribution of Na+,HCO3−-cotransport to cellular pH control in human breast cancer: A role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int. J. Cancer 2013, 132, 1288–1299. [Google Scholar] [CrossRef]
- Boedtkjer, E. Na+,HCO3− cotransporter NBCn1 accelerates breast carcinogenesis. Cancer Metastasis Rev. 2019, 38, 165–178. [Google Scholar] [CrossRef]
- Svastova, E.; Witarski, W.; Csaderova, L.; Kosik, I.; Skvarkova, L.; Hulikova, A.; Zatovicova, M.; Barathova, M.; Kopacek, J.; Pastorek, J.; et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J. Biol. Chem. 2012, 287, 3392–3402. [Google Scholar] [CrossRef]
- Parks, S.K.; Chiche, J.; Pouyssegur, J. pH control mechanisms of tumor survival and growth. J. Cell. Physiol. 2011, 226, 299–308. [Google Scholar] [CrossRef]
- Gorbatenko, A.; Olesen, C.W.; Boedtkjer, E.; Pedersen, S.F. Regulation and roles of bicarbonate transporters in cancer. Front. Physiol. 2014, 5, 130. [Google Scholar] [CrossRef]
- Loncaster, J.; Harris, A.L.; Davidson, S.; Logue, J.; Hunter, R.; Wycoff, C.; Pastorek, J.; Ratcliffe, P.; Stratford, I.; West, C. CAIX expression, a potential new intrinsic marker of hypoxia: Correlations with tumour oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001, 61, 6394–6399. [Google Scholar] [PubMed]
- Tan, E.Y.; Yan, M.; Campo, L.; Han, C.; Takano, E.; Turley, H.; Candiloro, I.; Pezzella, F.; Gatter, K.C.; Millar, E.K.A.; et al. The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br. J. Cancer 2009, 100, 405–411. [Google Scholar] [CrossRef] [PubMed]
- van Kuijk, S.J.A.; Yaromina, A.; Houben, R.; Niemans, R.; Lambin, P.; Dubois, L.J. Prognostic Significance of Carbonic Anhydrase IX Expression in Cancer Patients: A Meta-Analysis. Front. Oncol. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Závada, J.; Závadová, Z.; Pastorek, J.; Biesová, Z.; Jez, J.; Jezek, J.; Velek, J. Human tumour-associated cell adhesion protein MN/CA IX: Identification of M75 epitope and of the region mediating cell adhesion. Br. J. Cancer 2000, 82, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Csaderova, L.; Debreova, M.; Radvak, P.; Stano, M.; Vrestiakova, M.; Kopacek, J.; Pastorekova, S.; Svastova, E. The effect of carbonic anhydrase IX on focal contacts during cell spreading and migration. Front. Physiol. 2013, 4, 271. [Google Scholar] [CrossRef]
- Lee, S.-H.; McIntyre, D.; Honess, D.; Hulikova, A.; Pacheco-Torres, J.; Cerdán, S.; Swietach, P.; Harris, A.L.; Griffiths, J.R. Carbonic anhydrase IX is a pH-stat that sets an acidic tumour extracellular pH in vivo. Br. J. Cancer 2018, 1–9. [Google Scholar] [CrossRef]
- Ilie, M.I.; Hofman, V.; Ortholan, C.; Ammadi, R.E.; Bonnetaud, C.; Havet, K.; Venissac, N.; Mouroux, J.; Mazure, N.M.; Pouysségur, J.; et al. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int. J. Cancer 2011, 128, 1614–1623. [Google Scholar] [CrossRef]
- Chien, M.H.; Ying, T.H.; Hsieh, Y.H.; Lin, C.H.; Shih, C.H.; Wei, L.H.; Yang, S.F. Tumor-associated carbonic anhydrase XII is linked to the growth of primary oral squamous cell carcinoma and its poor prognosis. Oral Oncol. 2012, 48, 417–423. [Google Scholar] [CrossRef]
- Kopecka, J.; Campia, I.; Jacobs, A.; Frei, A.P.; Ghigo, D.; Wollscheid, B.; Riganti, C. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget 2015, 6, 6776–6793. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Mujumdar, P.; Annovazzi, L.; Kopecka, J.; Mellai, M.; Schiffer, D.; Poulsen, S.-A.; Riganti, C. Carbonic Anhydrase XII Inhibitors Overcome P-Glycoprotein–Mediated Resistance to Temozolomide in Glioblastoma. Mol. Cancer Ther. 2018, 17, 2598–2609. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Srere, P.A. The metabolon. Trends Biochem. Sci. 1985, 10, 109–110. [Google Scholar] [CrossRef]
- Srere, P.A. Complexes Of Sequential Metabolic Enzymes. Annu. Rev. Biochem. 1987, 56, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Deitmer, J.W.; Becker, H.M. Transport metabolons with carbonic anhydrases. Front. Physiol. 2013, 4, 291. [Google Scholar] [CrossRef]
- Vince, J.W.; Reithmeier, R.A.F. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1−/HCO3− exchanger. J. Biol. Chem. 1998, 273, 28430–28437. [Google Scholar] [CrossRef]
- Vince, J.W.; Reithmeier, R.A.F. Identification of the carbonic anhydrase II binding site in the Cl(-)/HCO(3)(-) anion exchanger AE1. Biochemistry 2000, 39, 5527–5533. [Google Scholar] [CrossRef]
- Dahl, N.K.; Jiang, L.; Chernova, M.N.; Stuart-Tilley, A.K.; Shmukler, B.E.; Alper, S.L. Deficient HCO3− transport in an AE1 mutant with normal Cl− transport can be rescued by carbonic anhydrase II presented on an adjacent AE1 protomer. J. Biol. Chem. 2003, 278, 44949–44958. [Google Scholar] [CrossRef]
- Shen, W.W.; Wu, J.; Cai, L.; Liuz, B.Y.; Gao, Y.; Chen, G.Q.; Fu, G.H. Expression of anion exchanger 1 sequestrates p16 in the cytoplasm in gastric and colonic adenocarcinoma. Neoplasia 2007, 9, 812–819. [Google Scholar] [CrossRef]
- Shiozaki, A.; Kudou, M.; Ichikawa, D.; Shimizu, H.; Arita, T.; Kosuga, T.; Konishi, H.; Komatsu, S.; Fujiwara, H.; Okamoto, K.; et al. Expression and role of anion exchanger 1 in esophageal squamous cell carcinoma. Oncotarget 2017, 8, 17921–17935. [Google Scholar] [CrossRef]
- Sterling, D.; Reithmeier, R.A.F.; Casey, J.R. A transport metabolon: Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J. Biol. Chem. 2001, 276, 47886–47894. [Google Scholar] [CrossRef]
- Gross, E.; Pushkin, A.; Abuladze, N.; Fedotoff, O.; Kurtz, I. Regulation of the sodium bicarbonate cotransporter kNBC1 function: Role of Asp986, Asp988 and kNBC1-carbonic anhydrase II binding. J. Physiol. 2002, 544, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Pushkin, A.; Abuladze, N.; Gross, E.; Newman, D.; Tatishchev, S.; Lee, I.; Fedotoff, O.; Bondar, G.; Azimov, R.; Ngyuen, M.; et al. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. J. Physiol. 2004, 559, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, F.B.; Morgan, P.E.; Alvarez, B.V.; Casey, J.R. Regulation of the human NBC3 Na+/HCO3− cotransporter by carbonic anhydrase II and PKA. Am. J. Physiol. Cell Physiol. 2004, 286, C1423–C1433. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.M.; Deitmer, J.W. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3− cotransporter. J. Biol. Chem. 2007, 282, 13508–13521. [Google Scholar] [CrossRef]
- Schüler, C.; Becker, H.M.; McKenna, R.; Deitmer, J.W. Transport activity of the sodium bicarbonate cotransporter NBCe1 is enhanced by different isoforms of carbonic anhydrase. PLoS ONE 2011, 6, e27167. [Google Scholar] [CrossRef]
- Li, X.; Alvarez, B.V.; Casey, J.R.; Reithmeier, R.A.F.; Fliegel, L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J. Biol. Chem. 2002, 277, 36085–36091. [Google Scholar] [CrossRef]
- Krishnan, D.; Liu, L.; Wiebe, S.A.; Casey, J.R.; Cordat, E.; Alexander, R.T. Carbonic anhydrase II binds to and increases the activity of the epithelial sodium-proton exchanger, NHE3. Am. J. Physiol.-Ren. Physiol. 2015, 309, F383–F392. [Google Scholar] [CrossRef]
- Jaquenod De Giusti, C.; Blanco, P.G.; Lamas, P.A.; Carrizo Velasquez, F.; Lofeudo, J.M.; Portiansky, E.L.; Alvarez, B. V Carbonic anhydrase II/sodium-proton exchanger 1 metabolon complex in cardiomyopathy of ob−/− type 2 diabetic mice. J. Mol. Cell. Cardiol. 2019, 136, 53–63. [Google Scholar] [CrossRef]
- Sterling, D.; Alvarez, B.V.; Casey, J.R. The extracellular component of a transport metabolon: Extracellular loop 4 of the human AE1 Cl−/HCO3− exchanger binds carbonic anhydrase IV. J. Biol. Chem. 2002, 277, 25239–25246. [Google Scholar] [CrossRef]
- Alvarez, B.V.; Loiselle, F.B.; Supuran, C.T.; Schwartz, G.J.; Casey, J.R. Direct Extracellular Interaction between Carbonic Anhydrase IV and the Human NBC1 Sodium/Bicarbonate Co-Transporter. Biochemistry 2003, 42, 12321–12329. [Google Scholar] [CrossRef]
- Morgan, P.E.; Pastorekova, S.; Stuart-Tilley, A.K.; Alper, S.L.; Casey, J.R. Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. AJP Cell Physiol. 2007, 293, C738–C748. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, A.; De Giusti, V.C.; Morgan, P.E.; Aiello, E.A.; Alvarez, B.V. Binding of carbonic anhydrase IX to extracellular loop 4 of the NBCe1 Na+/HCO3− cotransporter enhances NBCe1-mediated HCO3− influx in the rat heart. Am. J. Physiol. Cell Physiol. 2012, 303, C69–C80. [Google Scholar] [CrossRef]
- Kifor, G.; Toon, M.R.; Janoshazi, A.; Solomon, A.K. Interaction between red cell membrane band 3 and cytosolic carbonic anhydrase. J. Membr. Biol. 1993, 134, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.W.; Carlsson, U.; Reithmeier, R.A.F. Localization of the Cl−/HCO3− anion exchanger binding site to the amino-terminal region of carbonic anhydrase II. Biochemistry 2000, 39, 13344–13349. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.B.; Fujinaga, J.; Kopito, R.; Casey, J.R. Topology of the region surrounding Glu681 of human AE1 protein, the erythrocyte anion exchanger. J. Biol. Chem. 1998, 273, 22545–22553. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Sly, W.S.; Shah, G.N.; Alvarez, B.V. Bicarbonate homeostasis in excitable tissues: Role of AE3 Cl−/HCO3− exchanger and carbonic anhydrase XIV interaction. Am. J. Physiol. Cell Physiol. 2009, 297, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.V.; Vilas, G.L.; Casey, J.R. Metabolon disruption: A mechanism that regulates bicarbonate transport. EMBO J. 2005, 24, 2499–2511. [Google Scholar] [CrossRef]
- Ro, H.; Carson, J.H. pH microdomains in oligodendrocytes. J. Biol. Chem. 2004, 279, 37115–37123. [Google Scholar] [CrossRef]
- Debreova, M.; Csaderova, L.; Burikova, M.; Lukacikova, L.; Kajanova, I.; Sedlakova, O.; Kery, M.; Kopacek, J.; Zatovicova, M.; Bizik, J.; et al. CAIX regulates invadopodia formation through both a pH-dependent mechanism and interplay with actin regulatory proteins. Int. J. Mol. Sci. 2019, 20, 2745. [Google Scholar] [CrossRef]
- Liskova, V.; Hudecova, S.; Lencesova, L.; Iuliano, F.; Sirova, M.; Ondrias, K.; Pastorekova, S.; Krizanova, O. Type 1 Sodium Calcium Exchanger Forms a Complex with Carbonic Anhydrase IX and Via Reverse Mode Activity Contributes to pH Control in Hypoxic Tumors. Cancers (Basel). 2019, 11, 1139. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Alvarez, B.V.; Casey, J.R.; Fliegel, L. A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry 2006, 45, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Pierce, W.M.; Delamere, N.A. Cytoplasmic pH responses to carbonic anhydrase inhibitors in cultured rabbit nonpigmented ciliary epithelium. J. Membr. Biol. 1998, 162, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Weise, A.; Becker, H.M.; Deitmer, J.W. Enzymatic suppression of the membrane conductance associated with the glutamine transporter SNAT3 expressed in Xenopus oocytes by carbonic anhydrase II. J. Gen. Physiol. 2007, 130, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Weise, A.; Schneider, H.-P.; McKenna, R.; Deitmer, J.W. Substrate-dependent interference of carbonic anhydrases with the glutamine transporter SNAT3-induced conductance. Cell. Physiol. Biochem. 2011, 27, 79–90. [Google Scholar] [CrossRef]
- Becker, H.M.; Hirnet, D.; Fecher-Trost, C.; Sültemeyer, D.; Deitmer, J.W. Transport activity of MCT1 expressed in Xenopus oocytes is increased by interaction with carbonic anhydrase. J. Biol. Chem. 2005, 280, 39882–39889. [Google Scholar] [CrossRef]
- Becker, H.M.; Deitmer, J.W. Nonenzymatic proton handling by carbonic anhydrase II during H+-lactate cotransport via monocarboxylate transporter 1. J. Biol. Chem. 2008, 283, 21655–21667. [Google Scholar] [CrossRef]
- Becker, H.M.; Klier, M.; Schüler, C.; McKenna, R.; Deitmer, J.W. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc. Natl. Acad. Sci. USA 2011, 108, 3071–3076. [Google Scholar] [CrossRef]
- Stridh, M.H.; Alt, M.D.; Wittmann, S.; Heidtmann, H.; Aggarwal, M.; Riederer, B.; Seidler, U.; Wennemuth, G.; McKenna, R.; Deitmer, J.W.; et al. Lactate flux in astrocytes is enhanced by a non-catalytic action of carbonic anhydrase II. J. Physiol. 2012, 590, 2333–2351. [Google Scholar] [CrossRef]
- Klier, M.; Andes, F.T.; Deitmer, J.W.; Becker, H.M. Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters. J. Biol. Chem. 2014, 289, 2765–2775. [Google Scholar] [CrossRef]
- Jamali, S.; Klier, M.; Ames, S.; Barros, L.F.; McKenna, R.; Deitmer, J.W.; Becker, H.M. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci. Rep. 2015, 5, 13605. [Google Scholar] [CrossRef]
- Noor, S.I.; Jamali, S.; Ames, S.; Langer, S.; Deitmer, J.W.; Becker, H.M. A surface proton antenna in carbonic anhydrase II supports lactate transport in cancer cells. Elife 2018, 7, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, S.A.; Surulescu, C.; Jamali, S.; Ames, S.; Deitmer, J.W.; Becker, H.M. Modeling of pH regulation in tumor cells: Direct interaction between proton-coupled lactate transporters and cancer-associated carbonic anhydrase. Math. Biosci. Eng. 2018, 16, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Forero-Quintero, L.S.; Ames, S.; Schneider, H.-P.; Thyssen, A.; Boone, C.D.; Andring, J.T.; McKenna, R.; Casey, J.R.; Deitmer, J.W.; Becker, H.M. Membrane-anchored carbonic anhydrase IV interacts with monocarboxylate transporters via their chaperones CD147 and GP70. J. Biol. Chem. 2018, 294, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Ames, S.; Pastorekova, S.; Becker, H.M. The proteoglycan-like domain of carbonic anhydrase IX mediates non-catalytic facilitation of lactate transport in cancer cells. Oncotarget 2018, 9, 27940–27957. [Google Scholar] [CrossRef] [PubMed]
- Ames, S.; Andring, J.T.; McKenna, R.; Becker, H.M. CAIX forms a transport metabolon with monocarboxylate transporters in human breast cancer cells. Oncogene 2019, 39, 1710–1723. [Google Scholar] [CrossRef]
- Klier, M.; Schüler, C.; Halestrap, A.P.; Sly, W.S.; Deitmer, J.W.; Becker, H.M. Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II. J. Biol. Chem. 2011, 286, 27781–27791. [Google Scholar] [CrossRef]
- Noor, S.I.; Dietz, S.; Heidtmann, H.; Boone, C.D.; McKenna, R.; Deitmer, J.W.; Becker, H.M. Analysis of the binding moiety mediating the interaction between monocarboxylate transporters and carbonic anhydrase II. J. Biol. Chem. 2015, 290, 4476–4486. [Google Scholar] [CrossRef]
- Becker, H.M.; Klier, M.; Deitmer, J.W. Nonenzymatic augmentation of lactate transport via monocarboxylate transporter isoform 4 by carbonic anhydrase II. J. Membr. Biol. 2010, 234, 125–135. [Google Scholar] [CrossRef]
- Noor, S.I.; Pouyssegur, J.; Deitmer, J.W.; Becker, H.M. Integration of a ‘proton antenna’ facilitates transport activity of the monocarboxylate transporter MCT4. FEBS J. 2017, 284, 149–162. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Chen, Z.; Khokhar, D.; Wolff, A.; Ai, L.; Heldermon, C.D.; Bozdag, M.; Carta, F.; Supuran, C.T.; Brown, K.D.; et al. A non-catalytic function of carbonic anhydrase IX contributes to the glycolytic phenotype and pH regulation in human breast cancer cells. Biochem. J. 2019, 476, 1497–1513. [Google Scholar] [CrossRef]
- Piermarini, P.M.; Kim, E.Y.; Boron, W.F. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J. Biol. Chem. 2007, 282, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Horita, S.; Suzuki, M.; Fujita, T.; Seki, G. Functional role of a putative carbonic anhydrase II-binding domain in the electrogenic Na+-HCO₃− cotransporter NBCe1 expressed in Xenopus oocytes. Channels (Austin). 2011, 5, 106–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villafuerte, F.C.; Swietach, P.; Youm, J.-B.; Ford, K.; Cardenas, R.; Supuran, C.T.; Cobden, P.M.; Rohling, M.; Vaughan-Jones, R.D. Facilitation by intracellular carbonic anhydrase of Na+-HCO3− co-transport but not Na+/H+ exchange activity in the mammalian ventricular myocyte. J. Physiol. 2014, 592, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Al-Samir, S.; Papadopoulos, S.; Scheibe, R.J.; Meißner, J.D.; Cartron, J.-P.; Sly, W.S.; Alper, S.L.; Gros, G.; Endeward, V. Activity and distribution of intracellular carbonic anhydrase II and their effects on the transport activity of anion exchanger AE1/SLC4A1. J. Physiol. 2013, 591, 4963–4982. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Casey, J.R. Bicarbonate Transport Metabolons. In Drug Design of Zinc-Enzyme Inhibitors; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 415–437. [Google Scholar]
- Moraes, T.F.; Reithmeier, R.A.F. Membrane transport metabolons. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 2687–2706. [Google Scholar] [CrossRef]
- Becker, H.M.; Klier, M.; Deitmer, J.W. Carbonic anhydrases and their interplay with acid/base-coupled membrane transporters. In Sub-cellular biochemistry; Frost, S.C., McKenna, R., Eds.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7358-5. [Google Scholar]
- Nachliel, E.; Gutman, M.; Kiryati, S.; Dencher, N.A.; Gutman, M. Protonation dynamics of the extracellular and cytoplasmic surface of bacteriorhodopsin in the purple membrane. Proc. Natl. Acad. Sci. USA 1996, 93, 10747–10752. [Google Scholar] [CrossRef]
- Marantz, Y.; Nachliel, E.; Aagaard, A.; Brzezinski, P.; Gutman, M. The proton collecting function of the inner surface of cytochrome c oxidase from Rhodobacter sphaeroides. Proc. Natl. Acad. Sci. USA 1998, 95, 8590–8595. [Google Scholar] [CrossRef]
- Ädelroth, P.; Brzezinski, P. Surface-mediated proton-transfer reactions in membrane-bound proteins. Biochim. Biophys. Acta-Bioenerg. 2004, 1655, 102–115. [Google Scholar] [CrossRef]
- Fisher, S.Z.; Maupin, C.M.; Budayova-Spano, M.; Govindasamy, L.; Tu, C.; Agbandje-McKenna, M.; Silverman, D.N.; Voth, G.A.; McKenna, R. Atomic crystal and molecular dynamics simulation structures of human carbonic anhydrase II: Insights into the proton transfer mechanism. Biochemistry 2007, 46, 2930–2937. [Google Scholar] [CrossRef]
- Shinobu, A.; Agmon, N. Mapping proton wires in proteins: Carbonic anhydrase and GFP chromophore biosynthesis. J. Phys. Chem. A 2009, 113, 7253–7266. [Google Scholar] [CrossRef]
- Innocenti, A.; Pastorekova, S.; Pastorek, J.; Scozzafava, A.; De Simone, G.; Supuran, C.T. The proteoglycan region of the tumor-associated carbonic anhydrase isoform IX acts as anintrinsic buffer optimizing CO2 hydration at acidic pH values characteristic of solid tumors. Bioorg. Med. Chem. Lett. 2009, 19, 5825–5828. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.F.; Quon, A.; Dyck, J.R.B.; Casey, J.R. Carbonic anhydrase II promotes cardiomyocyte hypertrophy. Can. J. Physiol. Pharmacol. 2012, 90, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Svichar, N.; Chesler, M. Surface carbonic anhydrase activity on astrocytes and neurons facilitates lactate transport. Glia 2003, 41, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Svichar, N.; Waheed, A.; Sly, W.S.; Hennings, J.C.; Hubner, C.A.; Chesler, M. Carbonic Anhydrases CA4 and CA14 Both Enhance AE3-Mediated Cl−-HCO3− Exchange in Hippocampal Neurons. J. Neurosci. 2009, 29, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Robey, I.; Gatenby, R.A. Causes and consequences of increased glucose metabolism of cancers. J. Nucl. Med. 2008, 49, 24–43. [Google Scholar] [CrossRef]
- Doherty, J.R.; Yang, C.; Scott, K.E.N.; Cameron, M.D.; Fallahi, M.; Li, W.; Hall, M.A.; Amelio, A.L.; Mishra, J.K.; Li, F.; et al. Blocking lactate export by inhibiting the myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 2014, 74, 908–920. [Google Scholar] [CrossRef]
- Parks, S.K.; Cormerais, Y.; Marchiq, I.; Pouyssegur, J. Hypoxia optimises tumour growth by controlling nutrient import and acidic metabolite export. Mol. Aspects Med. 2016, 47–48, 3–14. [Google Scholar] [CrossRef]
- Ullah, M.S.; Davies, A.J.; Halestrap, A.P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem. 2006, 281, 9030–9037. [Google Scholar] [CrossRef]
- Meijer, T.W.H.; Schuurbiers, O.C.J.; Kaanders, J.H.A.M.; Looijen-Salamon, M.G.; de Geus-Oei, L.F.; Verhagen, A.F.T.M.; Lok, J.; van der Heijden, H.F.M.; Rademakers, S.E.; Span, P.N.; et al. Differences in metabolism between adeno- and squamous cell non-small cell lung carcinomas: Spatial distribution and prognostic value of GLUT1 and MCT4. Lung Cancer 2012, 76, 316–323. [Google Scholar] [CrossRef]
- Choi, J.H.; Lim, I.; Noh, W.C.; Kim, H.-A.; Seong, M.-K.; Jang, S.; Seol, H.; Moon, H.; Byun, B.H.; Kim, B.I.; et al. Prediction of tumor differentiation using sequential PET/CT and MRI in patients with breast cancer. Ann. Nucl. Med. 2018, 32, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Smallbone, K.; Maini, P.K.; Rose, F.; Averill, J.; Nagle, R.B.; Worrall, L.; Gillies, R.J. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br. J. Cancer 2007, 97, 646–653. [Google Scholar] [CrossRef]
- Waterman, E.A.; Cross, N.A.; Lippitt, J.M.; Cross, S.S.; Rehman, I.; Holen, I.; Hamdy, F.C.; Eaton, C.L. The antibody MAB8051 directed against osteoprotegerin detects carbonic anhydrase II: Implications for association studies with human cancers. Int. J. Cancer 2007, 121, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Parkkila, S.; Lasota, J.; Fletcher, J.A.; Ou, W.B.; Kivelä, A.J.; Nuorva, K.; Parkkila, A.K.; Ollikainen, J.; Sly, W.S.; Waheed, A.; et al. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod. Pathol. 2010, 23, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Huang, W.; Yao, Y.; Wang, Y.; Li, Z.; Shao, B.; Zhong, J.; Tang, M.; Liang, S.; Zhao, X.; et al. CA II, a potential biomarker by proteomic analysis, exerts significant inhibitory effect on the growth of colorectal cancer cells. Int. J. Oncol. 2013, 43, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mokhtari, R.B.; Pan, J.; Cutz, E.; Yeger, H. Carbonic anhydrase II mediates malignant behavior of pulmonary neuroendocrine tumors. Am. J. Respir. Cell Mol. Biol. 2015, 52, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.M. Carbonic anhydrase IX and acid transport in cancer. Br. J. Cancer 2019, 75, 199–219. [Google Scholar] [CrossRef]
- Klein, M.; Seeger, P.; Schuricht, B.; Alper, S.L.; Schwab, A. Polarization of Na(+)/H(+) and Cl(-)/HCO (3)(-) exchangers in migrating renal epithelial cells. J. Gen. Physiol. 2000, 115, 599–608. [Google Scholar] [CrossRef]
- Stock, C.; Gassner, B.; Hauck, C.R.; Arnold, H.; Mally, S.; Eble, J.A.; Dieterich, P.; Schwab, A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J. Physiol. 2005, 567, 225–238. [Google Scholar] [CrossRef]
- Stock, C.; Cardone, R.A.; Busco, G.; Krähling, H.; Schwab, A.; Reshkin, S.J. Protons extruded by NHE1: Digestive or glue? Eur. J. Cell Biol. 2008, 87, 591–599. [Google Scholar] [CrossRef]
- Busco, G.; Cardone, R.A.; Greco, M.R.; Bellizzi, A.; Colella, M.; Antelmi, E.; Mancini, M.T.; Dell’Aquila, M.E.; Casavola, V.; Paradiso, A.; et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 2010, 24, 3903–3915. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Huang, D.C.; Orlowski, J.; Furthmayr, H.; Barber, D.L. Direct binding of the Na-H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol. Cell 2000, 6, 1425–1436. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflugers Arch. Eur. J. Physiol. 2008, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Bentzon, J.F.; Dam, V.S.; Aalkjaer, C. Na+, HCO3−-cotransporter NBCn1 increases pHi gradients, filopodia, and migration of smooth muscle cells and promotes arterial remodelling. Cardiovasc. Res. 2016, 111, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.M.; Castorino, J.J.; Philp, N.J. Interaction of monocarboxylate transporter 4 with beta1-integrin and its role in cell migration. Am. J. Physiol. Cell Physiol. 2009, 296, C414–C421. [Google Scholar] [CrossRef]
- Stock, C.; Pedersen, S.F. Roles of pH and the Na+/H+ exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective? Semin. Cancer Biol. 2017, 43, 5–16. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Svastova, E.; Pastorekova, S. Carbonic anhydrase IX. Cell Adh. Migr. 2013, 7, 226–231. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell. Biochem. 2014, 75, 349–359. [Google Scholar]
- Gupta, D.; Chen, P.P. Glaucoma. Am. Fam. Physician 2016, 93, 668–674. [Google Scholar] [PubMed]
- Aggarwal, M.; Kondeti, B.; McKenna, R. Anticonvulsant/antiepileptic carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ruusuvuori, E.; Kaila, K. Carbonic anhydrases and brain pH in the control of neuronal excitability. Subcell. Biochem. 2014, 75, 271–290. [Google Scholar] [PubMed]
- Swenson, E.R. Carbonic anhydrase inhibitors and high altitude illnesses. Subcell. Biochem. 2014, 75, 361–386. [Google Scholar]
- Davis, C.; Hackett, P. Advances in the Prevention and Treatment of High Altitude Illness. Emerg. Med. Clin. North. Am. 2017, 35, 241–260. [Google Scholar] [CrossRef]
- Singh, S.; Lomelino, C.L.; Mboge, M.Y.; Frost, S.C.; McKenna, R. Cancer drug development of carbonic anhydrase inhibitors beyond the active site. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Avkiran, M.; Marber, M.S. Na(+)/H(+) exchange inhibitors for cardioprotective therapy: Progress, problems and prospects. J. Am. Coll. Cardiol. 2002, 39, 747–753. [Google Scholar] [CrossRef]
- Avkiran, M.; Cook, A.R.; Cuello, F. Targeting Na+/H+ exchanger regulation for cardiac protection: A RSKy approach? Curr. Opin. Pharmacol. 2008, 8, 133–140. [Google Scholar] [CrossRef]
- Mentzer, R.M.; Bartels, C.; Bolli, R.; Boyce, S.; Buckberg, G.D.; Chaitman, B.; Haverich, A.; Knight, J.; Menasché, P.; Myers, M.L.; et al. Sodium-Hydrogen Exchange Inhibition by Cariporide to Reduce the Risk of Ischemic Cardiac Events in Patients Undergoing Coronary Artery Bypass Grafting: Results of the EXPEDITION Study. Ann. Thorac. Surg. 2008, 85, 1261–1270. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Brown, K.D.; Bozdag, M.; Chen, Z.; Carta, F.; Mathias, J.V.; Frost, S.C.; McKenna, R.; Supuran, C.T.; Wolff, A.; et al. Selective inhibition of carbonic anhydrase IX over carbonic anhydrase XII in breast cancer cells using benzene sulfonamides: Disconnect between activity and growth inhibition. PLoS ONE 2018, 13, e0207417. [Google Scholar] [CrossRef]
| Transporter | Interacts with CA Isoform | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA I | CA II | CA III | CA IV | CA IX | CA XIV | ||||||||
| AE1 (SLC4A1) | ⊠ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | [39,56,57,58,61,70,72,74,75,76] | |||||
| AE2 (SLC4A2) | ☑ | ☑ | ☑ | ☑ | ☑ | [39,57,61,70] | |||||||
| AE3 (SLC4A3) | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | [61,70,72,77] | ||||||
| DRA (SLC26A3) | ☑ | ⊠ | [70] | ||||||||||
| SLC26A6 | ☑ | ☑ | [78] | ||||||||||
| SLC26A7 | ☑ | [72] | |||||||||||
| NBCe1 (SLC4A4) | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | [39,62,63,64,65,66,71,73,79,80] | ||||
| NBCn1 (SLC4A7) | ☑ | ☑ | [64] | ||||||||||
| NHE1 (SLC9A1) | ☑ | ☑ | ☑ | ☑ | ☑ | [81,67,79,82,83] | |||||||
| NHE3 (SLC9A3) | ☑ | ☑ | [68] | ||||||||||
| SNAT1 (SLC38A3) | ☑ | ☑ | ☑ | ☑ | [84,85] | ||||||||
| MCT1 (SLC16A1) | ⊠ | ☑ | ☑ | ⊠ | ☑ | ☑ * | ☑ | ☑ | [86,87,88,89,90,91,92,93,94,95,96] | ||||
| MCT2 (SLC16A7) | ⊠ | ⊠ | ☑ | ☑ * | [94,95,97,98] | ||||||||
| MCT4 (SLC16A3) | ☑ | ☑ | ⊠ | ☑ | ☑ * | ☑ | ☑ | [88,90,92,94,95,98,99,100,101] | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, H.M.; Deitmer, J.W. Transport Metabolons and Acid/Base Balance in Tumor Cells. Cancers 2020, 12, 899. https://doi.org/10.3390/cancers12040899
Becker HM, Deitmer JW. Transport Metabolons and Acid/Base Balance in Tumor Cells. Cancers. 2020; 12(4):899. https://doi.org/10.3390/cancers12040899
Chicago/Turabian StyleBecker, Holger M., and Joachim W. Deitmer. 2020. "Transport Metabolons and Acid/Base Balance in Tumor Cells" Cancers 12, no. 4: 899. https://doi.org/10.3390/cancers12040899
APA StyleBecker, H. M., & Deitmer, J. W. (2020). Transport Metabolons and Acid/Base Balance in Tumor Cells. Cancers, 12(4), 899. https://doi.org/10.3390/cancers12040899





