Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS)
Abstract
1. Introduction
2. Results
2.1. Descriptive Analysis
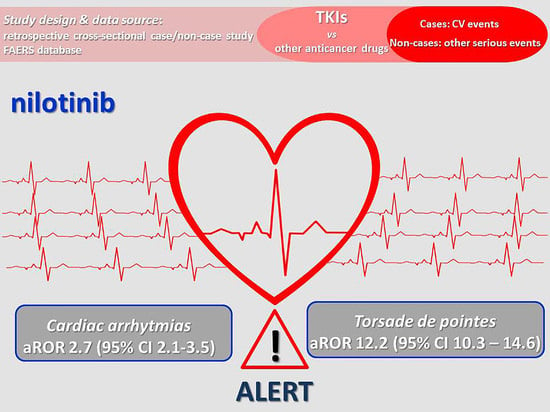
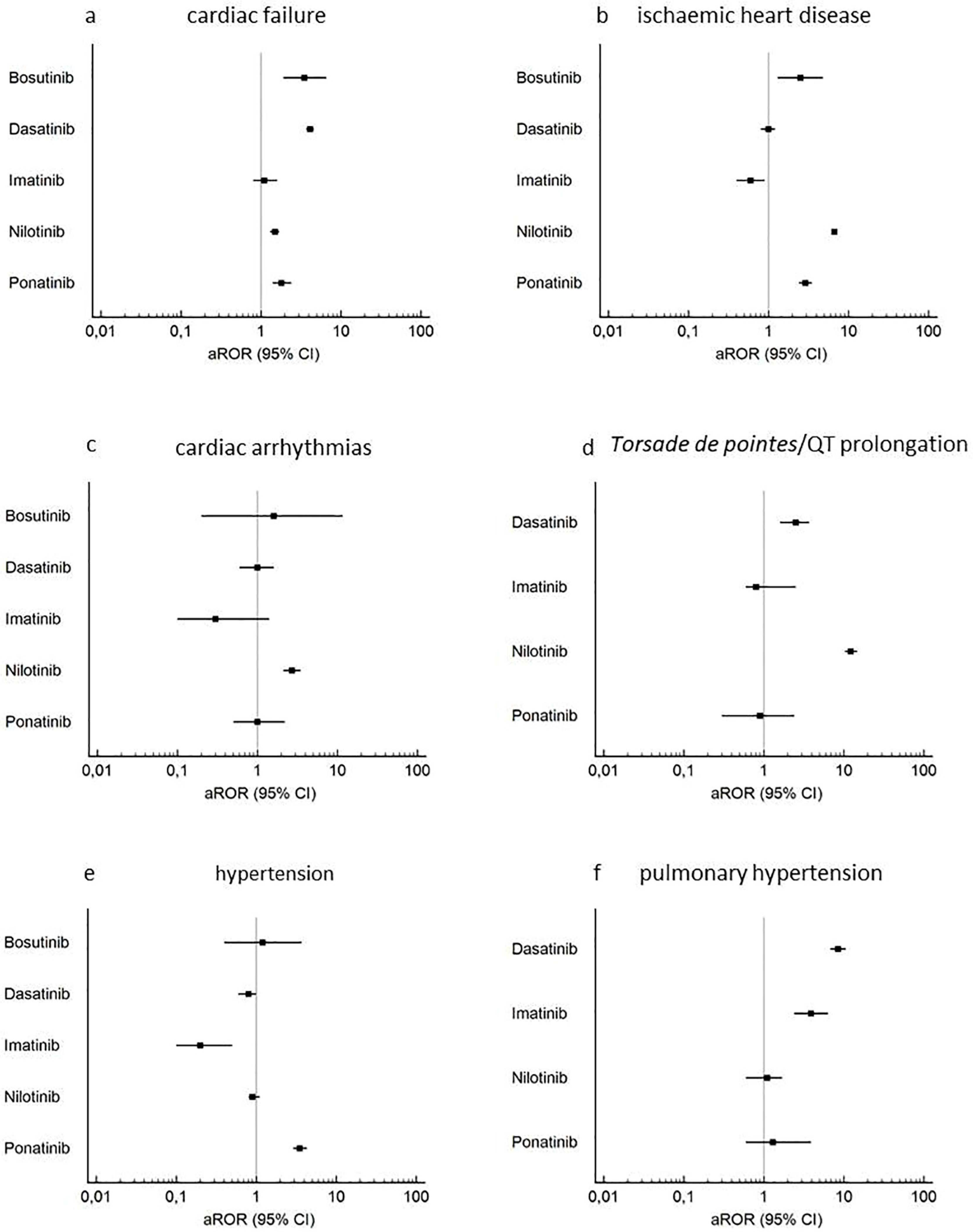
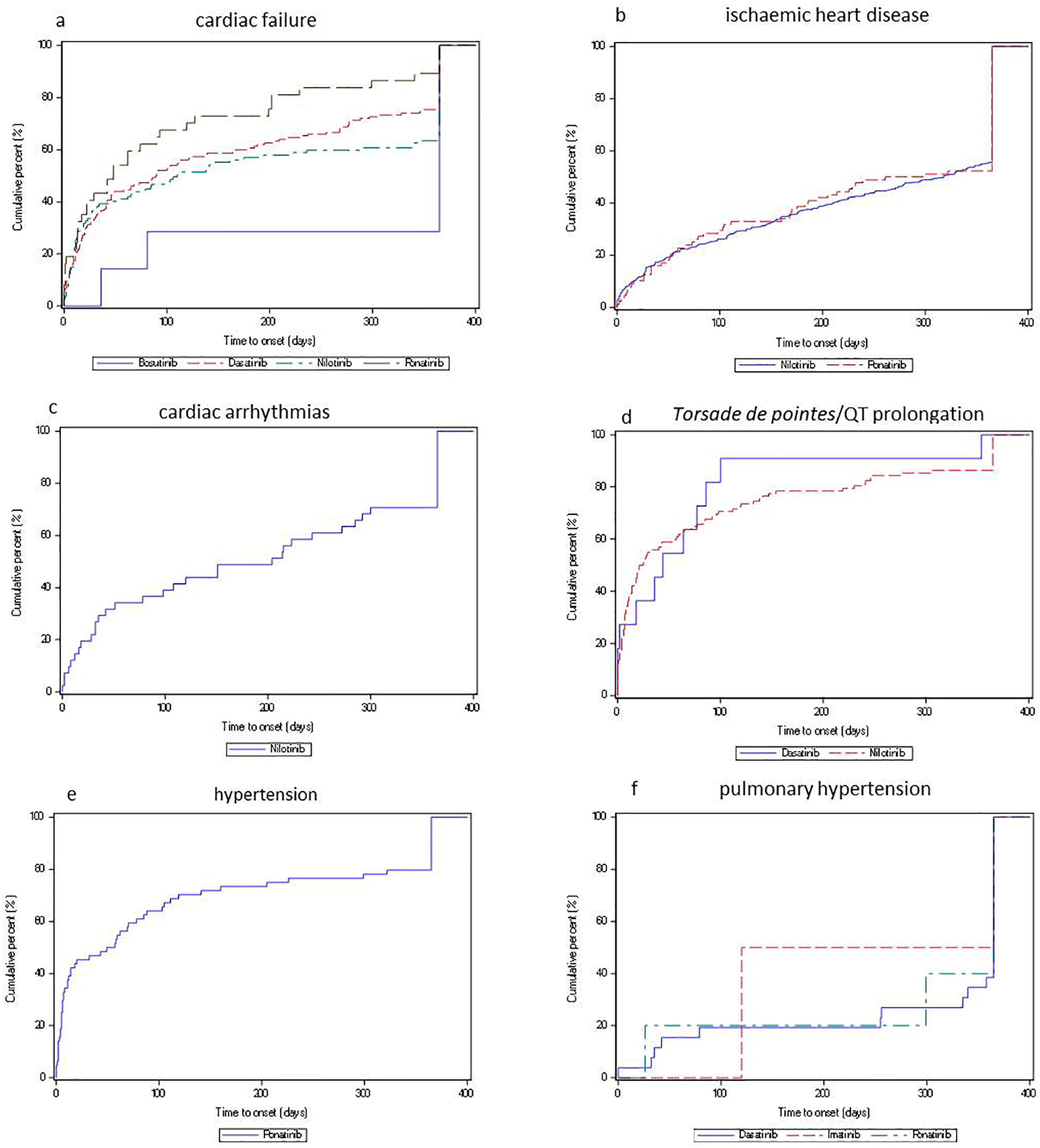
2.2. Disproportionality and Time-to-Onset Analyses
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Study Design
4.3. Extracted Variables
4.4. Data Analysis
4.4.1. Descriptive Analysis
4.4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2018, 93, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; Bjorkholm, M.; Dickman, P.W.; Hoglund, M.; Lambert, P.C.; Andersson, T.M. Life Expectancy of Patients with Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Saussele, S.; Rosti, G.; Mahon, F.X.; Janssen, J.; Hjorth-Hansen, H.; Richter, J.; Buske, C.; Committee, E.G. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv41–iv51. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Deininger, M.W.; Rosti, G.; Hochhaus, A.; Soverini, S.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Guilhot, F.; et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013, 122, 872–884. [Google Scholar] [CrossRef]
- Larson, R.A.; Hochhaus, A.; Hughes, T.P.; Clark, R.E.; Etienne, G.; Kim, D.W.; Flinn, I.W.; Kurokawa, M.; Moiraghi, B.; Yu, R.; et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia 2012, 26, 2197–2203. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H.M.; Saglio, G.; Steegmann, J.L.; Shah, N.P.; Boque, C.; Chuah, C.; Pavlovsky, C.; Mayer, J.; Cortes, J.; et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014, 123, 494–500. [Google Scholar] [CrossRef]
- Gambacorti-Passerini, C.; Coutre, P.L.; Piazza, R. The role of bosutinib in the treatment of chronic myeloid leukemia. Future Oncol. 2020, 16, 4395–4408. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kantarjian, H.; Shah, N.P.; Bixby, D.; Mauro, M.J.; Flinn, I.; O’Hare, T.; Hu, S.; Narasimhan, N.I.; Rivera, V.M.; et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2012, 367, 2075–2088. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2013, 369, 1783–1796. [Google Scholar] [CrossRef]
- Goodrich, A.D. Ponatinib in the leukemia world: Why a reevaluation is necessary for Philadelphia chromosome-positive patients with T315I mutation. Expert Rev. Hematol. 2014, 7, 513–515. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Deininger, M. Tyrosine Kinase Inhibitor-Associated Cardiovascular Toxicity in Chronic Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Rosti, G.; Castagnetti, F.; Gugliotta, G.; Baccarani, M. Tyrosine kinase inhibitors in chronic myeloid leukaemia: Which, when, for whom? Nat. Rev. Clin. Oncol. 2017, 14, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Caldemeyer, L.; Dugan, M.; Edwards, J.; Akard, L. Long-Term Side Effects of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia. Curr. Hematol. Malig. Rep. 2016, 11, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.; Shakir, S.A. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Begaud, B.; Martin, K.; Haramburu, F.; Moore, N. Rates of spontaneous reporting of adverse drug reactions in France. JAMA 2002, 288, 1588. [Google Scholar] [CrossRef] [PubMed]
- Kerkela, R.; Grazette, L.; Yacobi, R.; Iliescu, C.; Patten, R.; Beahm, C.; Walters, B.; Shevtsov, S.; Pesant, S.; Clubb, F.J.; et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 2006, 12, 908–916. [Google Scholar] [CrossRef]
- Patras de Campaigno, E.; Bondon-Guitton, E.; Laurent, G.; Montastruc, F.; Montastruc, J.L.; Lapeyre-Mestre, M.; Despas, F. Identification of cellular targets involved in cardiac failure caused by PKI in oncology: An approach combining pharmacovigilance and pharmacodynamics. Br. J. Clin. Pharmacol. 2017, 83, 1544–1555. [Google Scholar] [CrossRef]
- Woosley, R.L.; Heise, C.W.; Romero, K.A. QTdrugs List, Accession Date, AZCERT, Inc. 1822 Innovation Park Dr., Oro Valley, AZ 85755. Available online: www.Crediblemeds.org (accessed on 29 March 2020).
- Weisberg, E.; Manley, P.W.; Breitenstein, W.; Bruggen, J.; Cowan-Jacob, S.W.; Ray, A.; Huntly, B.; Fabbro, D.; Fendrich, G.; Hall-Meyers, E.; et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005, 7, 129–141. [Google Scholar] [CrossRef]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016, 30, 1044–1054. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, V.; Hernandez-Boluda, J.C. Tyrosine Kinase Inhibitors Available for Chronic Myeloid Leukemia: Efficacy and Safety. Front. Oncol. 2019, 9, 603. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Shah, N.P.; Cortes, J.E.; Baccarani, M.; Agarwal, M.B.; Undurraga, M.S.; Wang, J.; Ipina, J.J.; Kim, D.W.; Ogura, M.; et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood 2012, 119, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Talpaz, M.; Shah, N.P.; Kantarjian, H.; Donato, N.; Nicoll, J.; Paquette, R.; Cortes, J.; O’Brien, S.; Nicaise, C.; Bleickardt, E.; et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2006, 354, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Drug Safety Communication: Sprycel (Dasatinib) and Risk of Pulmonary Arterial Hypertension. 2011. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-sprycel-dasatinib-and-risk-pulmonary-arterial-hypertension (accessed on 29 March 2020).
- Giles, F.J.; O’Dwyer, M.; Swords, R. Class effects of tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia 2009, 23, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Pariente, A.; Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salame, G.; Fourrier-Reglat, A.; Haramburu, F.; Begaud, B.; Moore, N.; Association Française des Centres Régionaux de Pharmacovigilance (CRPV). Effect of competition bias in safety signal generation: Analysis of a research database of spontaneous reports in France. Drug Saf. 2012, 35, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.; Salvo, F.; Ahmed, I.; Robinson, P.; Moore, N.; Begaud, B.; Tubert-Bitter, P.; Pariente, A. A Method for the Minimization of Competition Bias in Signal Detection from Spontaneous Reporting Databases. Drug Saf. 2016, 39, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kim, D.W.; Pinilla-Ibarz, J.; le Coutre, P.D.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: Final 5-year results of the phase 2 PACE trial. Blood 2018, 132, 393–404. [Google Scholar] [CrossRef]
- Heiblig, M.; Rea, D.; Chretien, M.L.; Charbonnier, A.; Rousselot, P.; Coiteux, V.; Escoffre-Barbe, M.; Dubruille, V.; Huguet, F.; Cayssials, E.; et al. Ponatinib evaluation and safety in real-life chronic myelogenous leukemia patients failing more than two tyrosine kinase inhibitors: The PEARL observational study. Exp. Hematol. 2018, 67, 41–48. [Google Scholar] [CrossRef]
- Luciano, L.; Annunziata, M.; Attolico, I.; Di Raimondo, F.; Maggi, A.; Malato, A.; Martino, B.; Palmieri, F.; Pane, F.; Sgherza, N.; et al. The multi-tyrosine kinase inhibitor ponatinib for chronic myeloid leukemia: Real-world data. Eur. J. Haematol. 2020. [Google Scholar] [CrossRef]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A novel multi-tyrosine kinase inhibitor against human malignancies. Oncotargets Ther. 2019, 12, 635–645. [Google Scholar] [CrossRef]
- Brown, E.G. Methods and pitfalls in searching drug safety databases utilising the Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf. 2003, 26, 145–158. [Google Scholar] [CrossRef]
- Sakaeda, T.; Tamon, A.; Kadoyama, K.; Okuno, Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 2013, 10, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Geniaux, H.; Assaf, D.; Miremont-Salame, G.; Raspaud, B.; Gouverneur, A.; Robinson, P.; Pariente, A.; Salvo, F. Performance of the standardised MedDRA(R) queries for case retrieval in the French spontaneous reporting database. Drug Saf. 2014, 37, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Poluzzi, E.; Raschi, E.; Piccinni, C.; De Ponti, F. Data Mining Techniques in Pharmacovigilance: Analysis of the Publicly Accessible FDA Adverse Event Reporting System (AERS). In Data Mining Applications in Engineering and Medicine; IntechOpen: London, UK, 2012. [Google Scholar]
| Characteristics | TKIs n = 3930 | Other Anticancer Drugs n = 60,302 | Total n = 64,232 |
|---|---|---|---|
| Patient’s age, years, median (Q1-Q3) | 65 (55–73) | 63 (52–71) | 63 (52–72) |
| Patient’s age | |||
| ≤25 26–50 51–75 ≥ 76 Unknown | 41 (1.0) 436 (11.1) 1729 (44.0) 490 (12.5) 1234 (31.4) | 2878 (4.8) 7009 (11.6) 27,051 (44.9) 6615 (11.0) 16,749 (27.8) | 2919 (4.5) 7445 (11.6) 28,780 (44.8) 7105 (11.1) 17,983 (28) |
| Patient’s sex | |||
| Female Male Unknown | 1423 (36.2) 2079 (52.9) 428 (10.9) | 27,205 (45.1) 24,951 (41.4) 8146 (13,5) | 28,628 (44.6) 27,030 (42.1) 8574 (13,35) |
| Type of reporter | |||
| Health professional Nonhealth professional Unknown | 2877 (73.2) 951 (24.2) 102 (2.6) | 44,575 (73.9) 12,908 (21.4) 2819 (4.7) | 47,452 (73.9) 13,859 (21.6) 2921 (4.5) |
| Reporting country | |||
| United Sates Japan Germany France Other countries Unknown | 1263 (32.1) 418 (10.6) 293 (7.5) 275 (7) 1505 (38.3) 176 (4.5) | 21,841 (36.2) 5788 (9.6) 4415 (7.3) 4519 (7.5) 22,625 (37.5) 1114 (1.84) | 23,104 (36.0) 6206 (9.7) 4708 (7.3) 4794 (7.5) 24,130 (37.6) 1290 (2) |
| Drug role in event occurrence | |||
| Primary suspect drug Secondary suspect drug Concomitant Interacting | 3269 (83.2) 611 (15.5) 40 (1.0) 10 (0.2) | 29,869 (49.5) 19,588 (32.5) 10,745 (17.2) 100 (0.2) | 33,138 (51.6) 20,199 (31.4) 10,785 (16.8) 110 (0.2) |
| Outcome of event | |||
| Death Life-threatening Hospitalization-initial or prolonged Disability Required intervention Congenital anomaly Other serious events | 396 (10.1) 171 (4.3) 1375 (35.0) 63 (1.6) 2 (0) 1 (0) 1922 (48.9) | 7920 (13.1) 3105 (5.1) 22,434 (37.2) 1056 (1.7) 150 (0.2) 14 (0) 25,623 (42.4) | 8316 (12.9) 3276 (5.1) 23,809 (37.1) 1119 (1.7) 152 (0.2) 15 (0) 27,545 (42.9) |
| Cardiovascular toxicity, SMQs | |||
| Cardiac arrhythmias Cardiac failure Cardiomyopathy Embolic and thrombotic events Hypertension Ischemic heart disease Pulmonary hypertension Torsade de Pointes/QT prolongation | 126 (3.2) 669 (17.0) 78 (1.2) 1049 (26.7) 306 (7.8) 1306 (33.2) 127 (3.2) 269 (6.8) | 1982 (3.2) 8402 (13.9) 3238 (5.4) 27,911 (46.3) 8236 (13.7) 8833 (14.6) 854 (1.4) 846 (1.4) | 2108 (3.3) 9071 (14.1) 3316 (5.2) 28,960 (45.1) 8542 (13.3) 10,139 (15.8) 981 (1.5) 1115 (1.7) |
| Characteristics | Cases n = 3930 | Non-Cases n = 20,443 | Total * n = 24,373 |
|---|---|---|---|
| Type of TKIs | |||
| Bosutinib Dasatinib Imatinib Nilotinib Ponatinib | 38 (1.0) 835 (21.2) 173 (4.4) 2319 (59) 565 (14.4) | 220 (1.1) 6382 (31.2) 2291 (11.2) 9014 (44.1) 2536 (12.4) | 258 (1.0) 7217 (29.6) 2464 (10.1) 11,333 (46.5) 3101 (12.7) |
| Reported indication | |||
| Chronic myeloid leukemia Acute myeloid leukemia Acute lymphocytic leukemia Myeloid leukemia Gastrointestinal stromal tumour Hypertension Other indication Unknown | 2678 (68.1) 16 (0.4) 159 (4.0) 22 (0.6) 52 (1.3) 19 (0.5) 341 (8.7) 643 (16.4) | 12,450 (60.9) 150 (0.7) 1448 (7.1) 136 (0.7) 471 (2.3) 23 (0.1) 2376 (11.6) 3389 (16.6) | 15,128 (62.1) 166 (0.7) 1607 (6.6) 158 (0.6) 523 (2.1) 42 (0.2) 2717 (11.1) 4032 (16.5) |
| Reporting year | |||
| 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 Unknown | 120 (3.1) 106 (2.7) 163 (4.1) 209 (5.3) 284 (7.2) 422 (10.8) 546 (13.9) 415 (10.6) 481 (12.2) 501 (12.8) 679 (17.3) 4 (0.1) | 488 (2.4) 720 (3.5) 922 (4.5) 1157 (5.7) 1470 (7.2) 2042 (10.0) 2421 (11.9) 2023 (9.9) 2853 (14) 2803 (13.7) 3530 (17.3) 14 (0) | 608 (2.5) 826 (3.4) 1085 (4.4) 1366 (5.6) 1754 (7.2) 2464 (10.1) 2967 (12.2) 2438 (10.0) 3334 (13.7) 3304 (13.6) 4209 (17.3) 18 (0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirmi, S.; El Abd, A.; Letinier, L.; Navarra, M.; Salvo, F. Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS). Cancers 2020, 12, 826. https://doi.org/10.3390/cancers12040826
Cirmi S, El Abd A, Letinier L, Navarra M, Salvo F. Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS). Cancers. 2020; 12(4):826. https://doi.org/10.3390/cancers12040826
Chicago/Turabian StyleCirmi, Santa, Asmae El Abd, Louis Letinier, Michele Navarra, and Francesco Salvo. 2020. "Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS)" Cancers 12, no. 4: 826. https://doi.org/10.3390/cancers12040826
APA StyleCirmi, S., El Abd, A., Letinier, L., Navarra, M., & Salvo, F. (2020). Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS). Cancers, 12(4), 826. https://doi.org/10.3390/cancers12040826