Cisplatin Relocalizes RNA Binding Protein HuR and Enhances the Oncolytic Activity of E4orf6 Deleted Adenovirus
Abstract
1. Introduction
2. Results
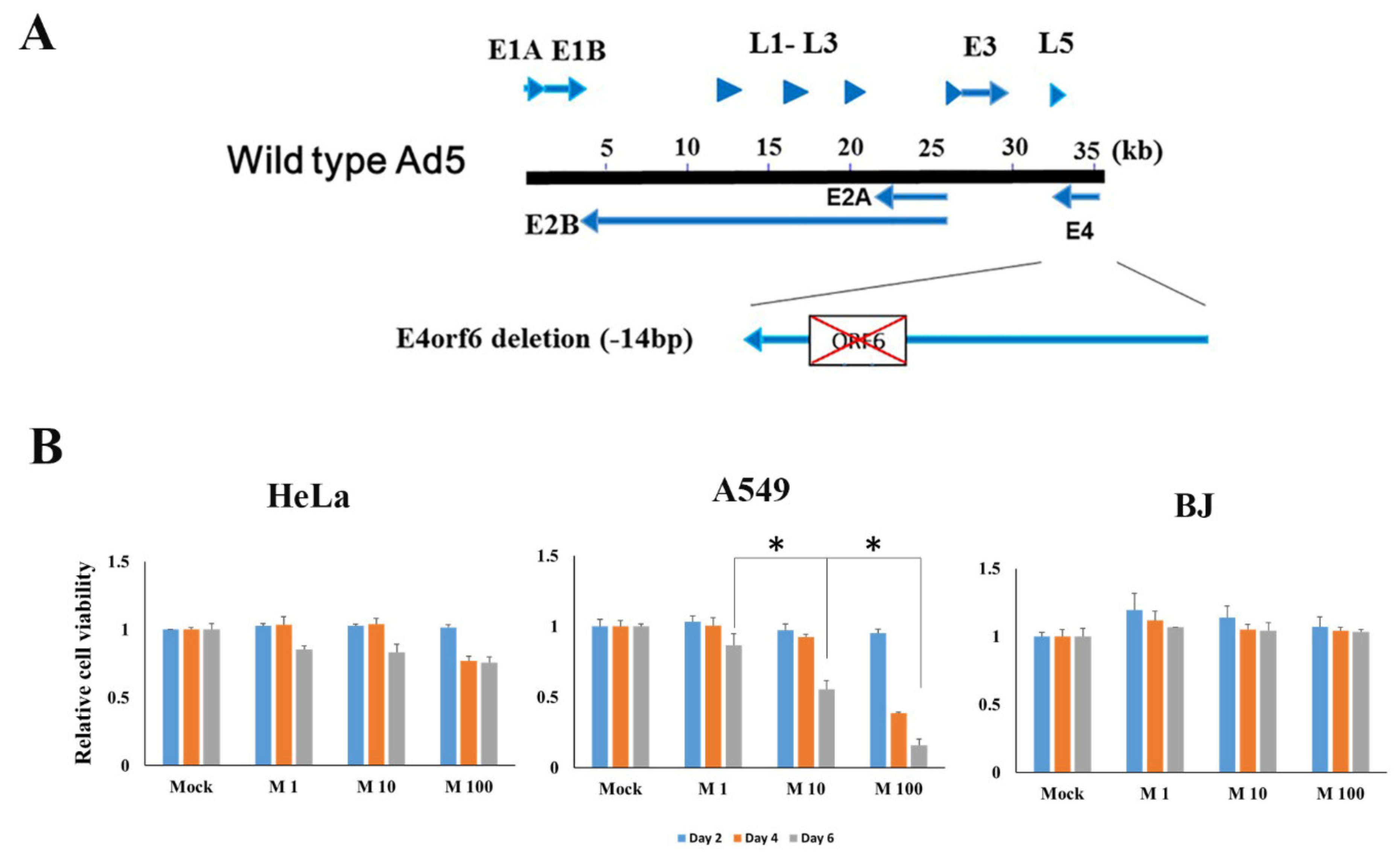
2.1. Characterization of E4orf6 Gene Deleted Mutant Adenovirus and Cancer Cell Death Efficacy
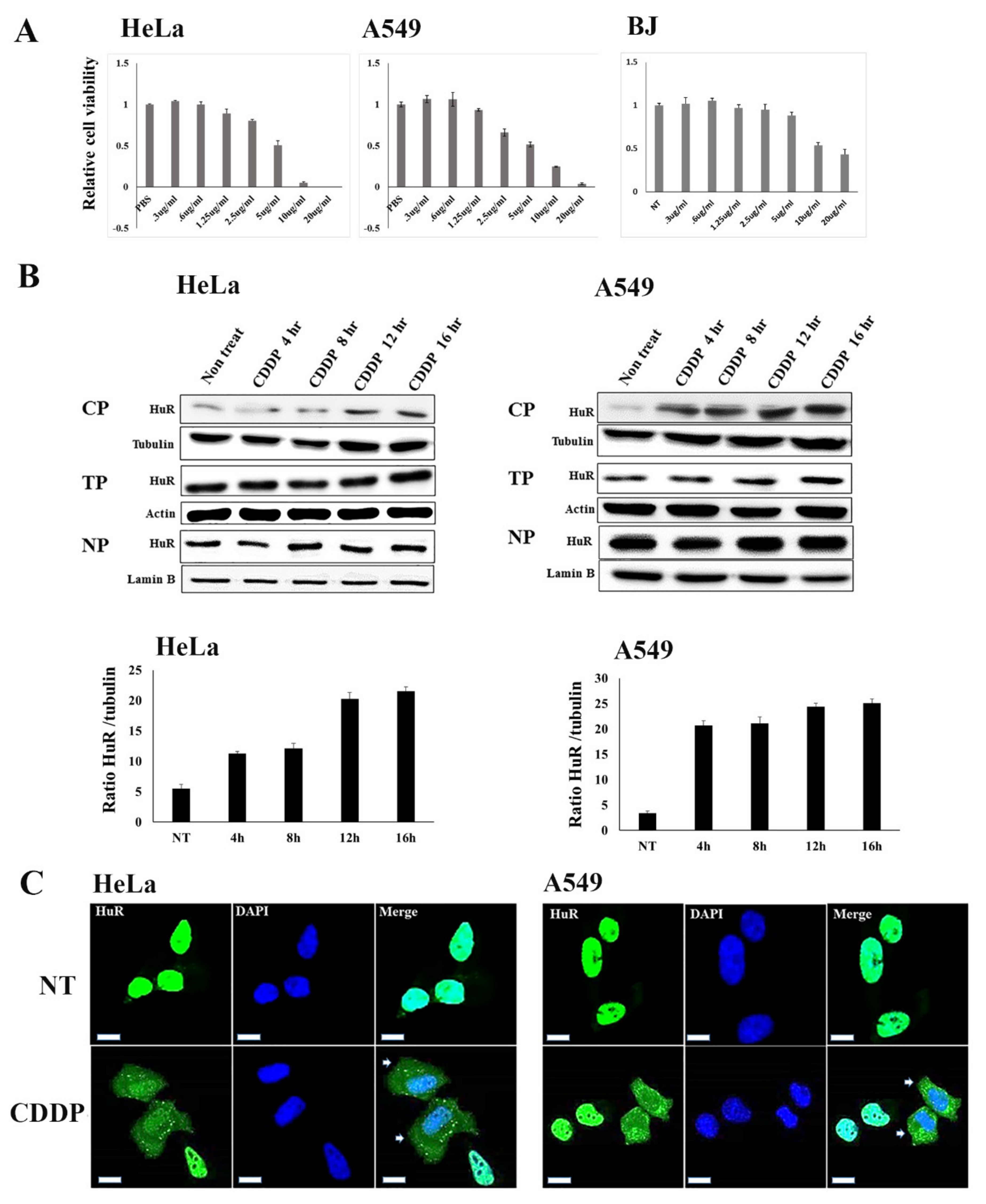
2.2. Modulatory Effect of Nucleocytoplasmic HuR Translocation by CDDP Treatment
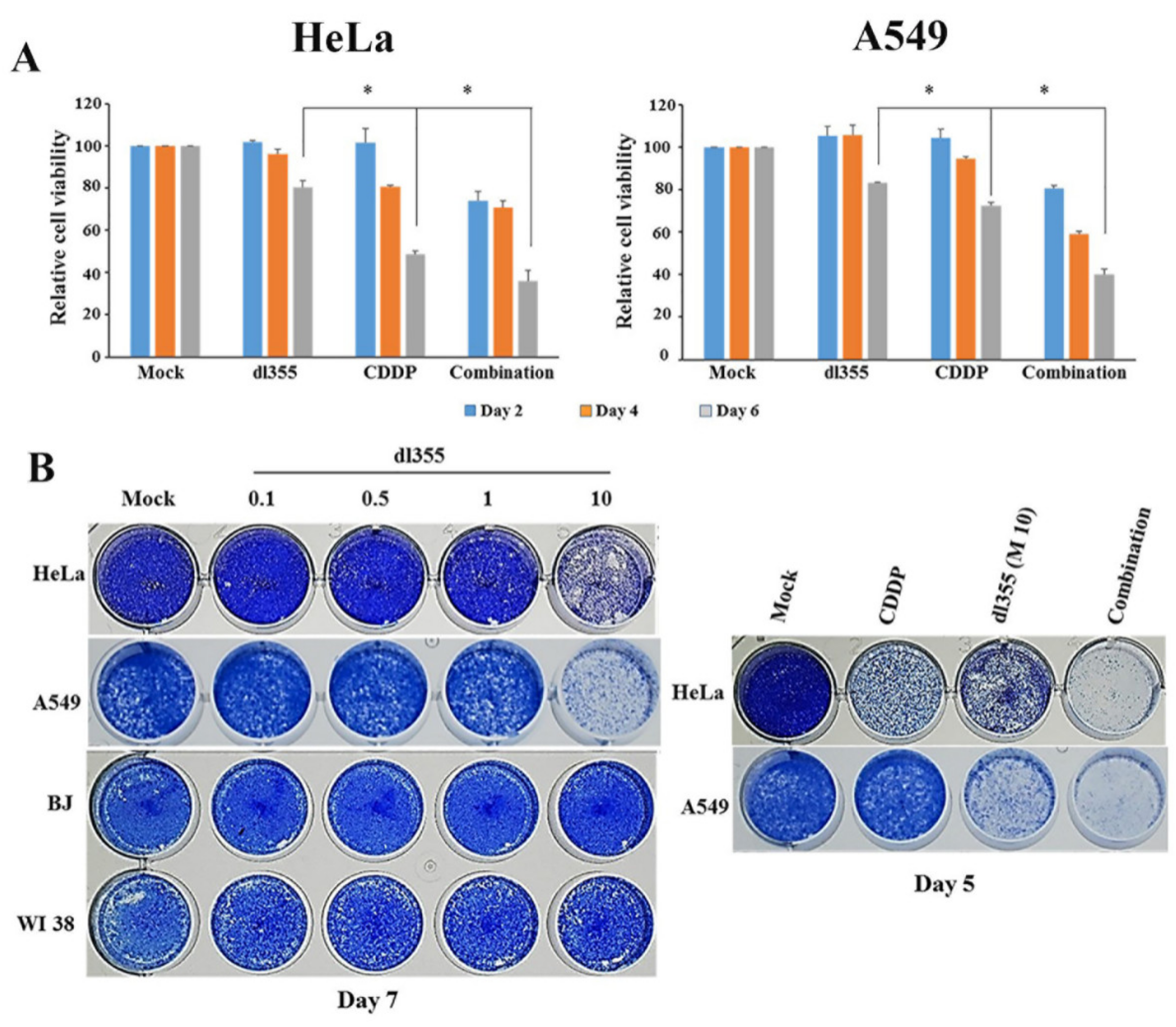
2.3. Effect of Combination Therapy on Cell Viability
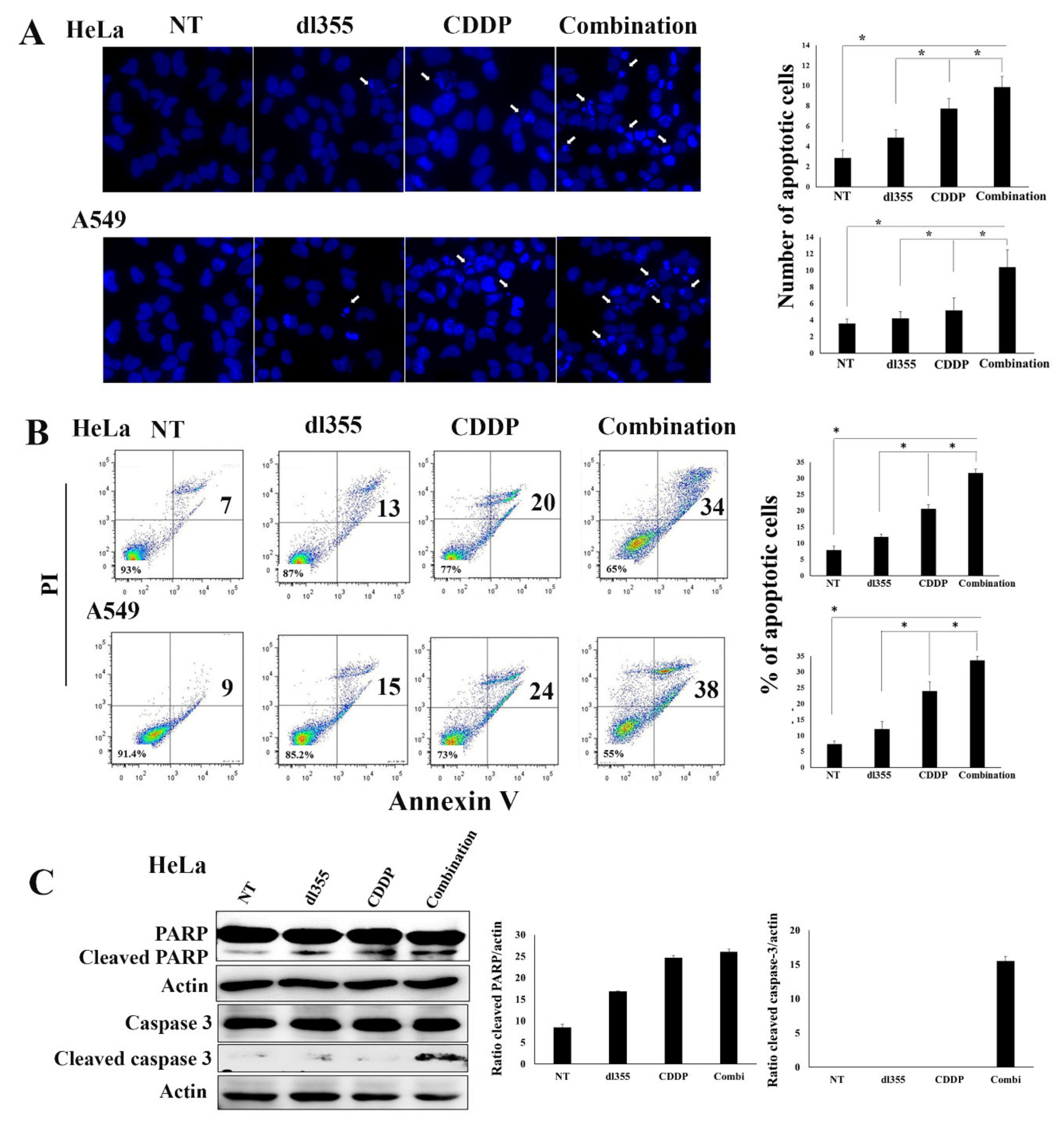
2.4. Combination Therapy Augments Apoptosis in Cancer Cells
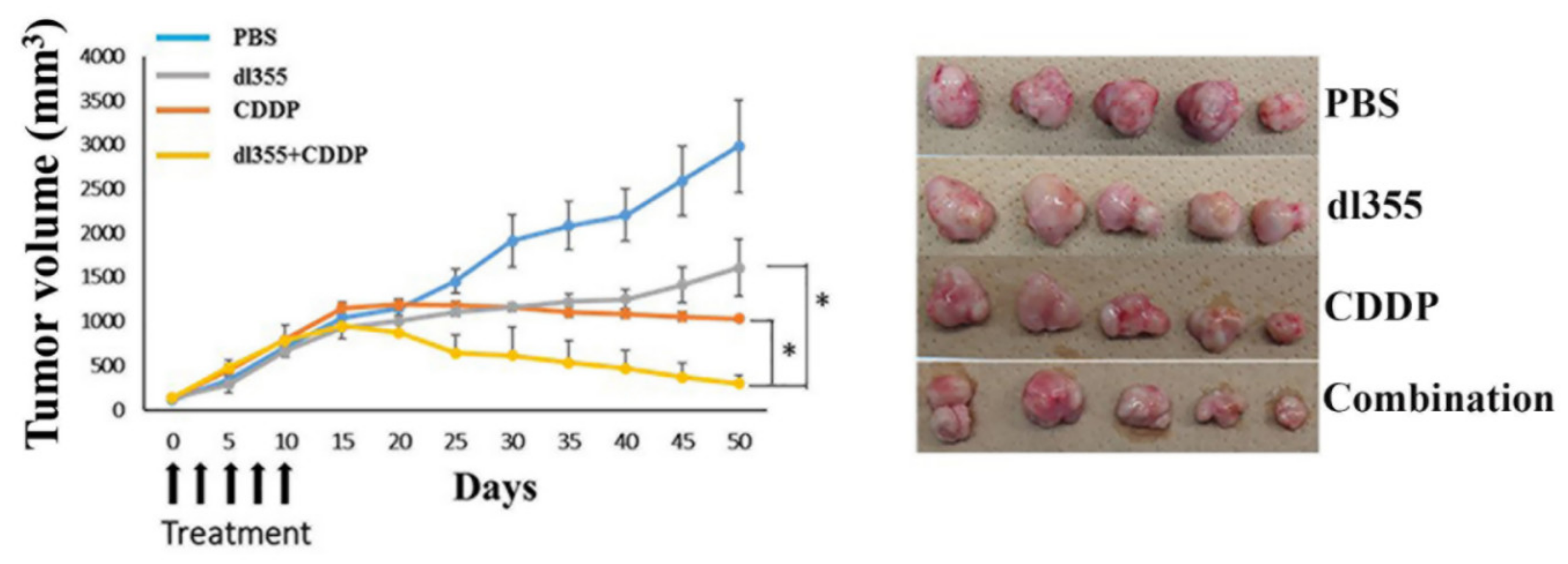
2.5. Enhanced In Vivo Antitumor Effect in Combination Therapy
2.6. CDDP - Induced In Vitro and In Vivo Virus Replication
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Culture Conditions, and Reagents
4.2. Preparation of Virus Lysates
4.3. Western Blot Analysis
4.4. Fluorescence Microscopy
4.5. Cytopathic Effect Assay
4.6. XTT Assay and Chou-Talalay Analysis
4.7. Hoechst 33342 Staining
4.8. Flow Cytometry Analysis
4.9. In Vitro Virus Proliferation Assay
4.10. In Vivo Antitumor Effect
4.11. Immunohistochemistry Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shenk, T. Adenoviridae: The viruses and their replication. In Fundamental Virology, 4th ed.; Lippincott Williams & Wilkins Ltd.: Philadelphia, PA, USA, 2001; pp. 1053–1088. [Google Scholar]
- Halbert, D.N.; Cutt, J.R.; Shenk, T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J. Virol. 1985, 56, 250–257. [Google Scholar] [CrossRef]
- Täuber, B.; Dobner, T. Adenovirus early E4 genes in viral oncogenesis. Oncogene 2001, 20, 7847–7854. [Google Scholar] [CrossRef][Green Version]
- Javier, R.T. Adenovirus type 9 E4 open reading frame 1 encodes a transforming protein required for the production of mammary tumors in rats. J. Virol. 1994, 68, 3917–3924. [Google Scholar] [CrossRef]
- Nevels, M.; Täuber, B.; Kremmer, E.; Spruss, T.; Wolf, H.; Dobner, T. Transforming potential of the adenovirus type 5 E4orf3 protein. J. Virol. 1999, 73, 1591–1600. [Google Scholar] [CrossRef]
- Moore, M.; Horikoshi, N.; Shenk, T. Oncogenic potential of the adenovirus E4orf6 protein. Proc. Natl. Acad. Sci. USA 1996, 93, 11295–11301. [Google Scholar] [CrossRef] [PubMed]
- Nevels, M.; Rubenwolf, S.; Spruss, T.; Wolf, H.; Dobner, T. The adenovirus E4orf6 protein can promote E1A/E1B-induced focus formation by interfering with p53 tumor suppressor function. Proc. Natl. Acad. Sci. USA 1997, 94, 1206–1211. [Google Scholar] [CrossRef]
- Javier, R.; Raska, K., Jr.; Shenk, T. Requirement for the adenovirus type 9 E4 region in production of mammary tumors. Science 1992, 257, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Higashino, F.; Aoyagi, M.; Takahashi, A.; Ishino, M.; Taoka, M.; Isobe, T.; Kobayashi, M.; Totsuka, Y.; Kohgo, T.; Shindoh, M. Adenovirus E4orf6 targets pp32/LANP to control the fate of ARE-containing mRNAs by perturbing the CRM1-dependent mechanism. J. Cell Biol. 2005, 170, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, T.; Aoyagi, M.; Yasuda, M.; Kitamura, T.; Jehung, J.P.; Ishikawa, M.; Kitagawa, Y.; Totsuka, Y.; Shindoh, M.; Higashino, F. Viral-mediated stabilization of AU-rich element-containing mRNA contributes to cell transformation. Oncogene 2011, 30, 2912–2920. [Google Scholar] [CrossRef]
- Jehung, J.P.; Kitamura, T.; Matsuda, A.Y.; Kuroshima, T.; Towfik, A.; Yasuda, M.; Sano, H.; Kitagawa, Y.; Minowa, K.; Shindoh, M.; et al. Adenovirus infection induces HuRrelocalization to facilitate virus replication. BBRC 2018, 495, 1795–1800. [Google Scholar]
- Chen, C.Y.; Shyu, A.B. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995, 20, 465–470. [Google Scholar] [CrossRef]
- Jacobson, A.; Peltz, S.W. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu. Rev. Biochem. 1996, 65, 693–739. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.M.; Steitz, J.A. HuR and mRNA stability. Cell Mol. Life Sci. 2001, 58, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Hinman, M.N.; Lou, H. Diverse molecular functions of Hu proteins. Cell Mol. Life Sci. 2008, 65, 3168–3181. [Google Scholar] [CrossRef] [PubMed]
- López de Silanes, I.; Lal, A.; Gorospe, M. HuR Post-transcriptional paths to malignancy. RNA Biol. 2005, 2, 11–13. [Google Scholar] [CrossRef]
- López de Silanes, I.; Fan, J.; Yang, X.; Zonderman, A.B.; Potapova, O.; Pizer, E.S.; Gorospe, M. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene 2003, 22, 7146–7154. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.P.; Rugman, F.; Dorman, E.B.; Stoney, P.J.; Wilson, J.A.; McCormick, M.; Veevers, A.; Stell, P.M. Cisplatinum and bleomycin for advanced or recurrent squamous cell carcinoma of the head and neck: A randomized factorial phase III controlled trial. Cancer Chemother. Pharmacol. 1985, 15, 283–289. [Google Scholar] [CrossRef]
- Williams, S.D.; Birch, R.; Einhorn, L.H.; Irwin, L.; Greco, F.A.; Loehrer, P.J. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N. Engl. J. Med. 1987, 316, 1435–1440. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, B.; Liu, J.; Cai, R.; Wang, Y.; Qian, C. Synergistic induction of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying TRAIL. Mol. Cell Biochem. 2007, 304, 315–323. [Google Scholar] [CrossRef]
- Wu, Y.M.; Zhang, K.J.; Yue, X.T.; Wang, Y.Q.; Yang, Y.; Li, G.C.; Li, N.; Wang, Y.G. Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacol. Sin. 2009, 30, 467–477. [Google Scholar] [CrossRef]
- Matsuda, A.Y.; Mikawa, Y.; Habiba, U.; Kitamura, T.; Yasuda, M.; Alam, M.T.; Kitagawa, Y.; Minowa, K.; Shindoh, M.; Higashino, F. Oncolytic potential of an E4-deficient adenovirus that can recognize the stabilization of AU-rich element-containing mRNA in cancer cells. Oncol. Rep. 2019, 41, 954–960. [Google Scholar]
- Yu, D.C.; Chen, Y.; Dilley, J.; Li, Y.; Embry, M.; Zhang, H.; Nguyen, N.; Amin, P. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001, 61, 517–525. [Google Scholar]
- Heise, C.; Sampson-Johannes, A.; Williams, A.; Mccormick, F.; Von Hoff, D.D.; Kirn, D.H. Onyx-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 1997, 3, 639–645. [Google Scholar] [CrossRef]
- Zeng, M.; Cerniglia, G.J.; Eck, S.L.; Stevens, C.W. High-efficiency stable gene transfer of adenovirus into mammalian cells using ionizing radiation. Hum. Gene Ther. 1997, 10, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Levinson, A.D. Cancer therapy reform. Science 2010, 328, 137. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, H.; Häcker, S.; Wittmann, S.; Maurer, M.; Borkhardt, A.; Toloczko, A.; Debatin, K.M.; Fulda, S.; Jeremias, I. Cytotoxic drug-induced, p53-mediated upregulation of caspase-8 in tumor cells. Oncogene 2008, 27, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.C.; Wang, Y.; Meng, J.H.; Hill, R.; Sweeney, K.; Kirn, D.; Lemoine, N.R.; Hallden, G. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008, 15, 40–50. [Google Scholar] [CrossRef][Green Version]
- Takakura, M.; Nakamura, M.; Kyo, S.; Hashimoto, M.; Mori, N.; Ikoma, T.; Mizumoto, Y.; Fujiwara, T.; Urata, Y.; Inoue, M. Intraperitoneal administration of telomerase-specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther. 2010, 17, 11–19. [Google Scholar] [CrossRef]
- Kotb, A.; Subramanya, S.; Xiaoling, Y.; Ashish, L.; Hyeon, K.; Yuki, K.; Stefanie, G.; Kevin, G.B.; Davida, K.; Rafael, C.; et al. Ubiquitin-mediated proteolysis of HuR by heat shock. EMBO J. 2009, 28, 1271–1282. [Google Scholar]
- Habiba, U.; Kuroshima, T.; Matsuda, A.Y.; Matsuda, T.; Chowdhury, A.F.M.A.; Jehung, J.P.; Hossain, E.; Sano, H.; Kitagawa, Y.; Shindoh, M.; et al. HuR translocation to the cytoplasm of cancer cells in actin-independent manner. Experimental Cell Reh. 2018, 369, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Gallouzi, E.; Steitz, J.A. Delineation of mRNA Export Pathways by the Use of Cell-Permeable Peptides. Science 2001, 294, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.L.; Post, D.E.; Khuri, F.R.; Erwin, G.; Meir, V. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clin. Cancer Res. 2004, 10, 5299–5312. [Google Scholar] [CrossRef]
- Kruyt, F.A.; Curiel, D.T. Toward a new generation of conditionally replicating adenoviruses: Pairing tumor selectivity with maximal oncolysis. Hum. Gene Ther. 2002, 13, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Post, D.E.; Khuri, F.R.; Simons, J.W.; Van Meir, E.G. Replicative oncolytic adenoviruses in multimodal cancer regimens. Hum. Gene Ther. 2003, 14, 933–946. [Google Scholar] [CrossRef]
- Libertini, S.; Iacuzzo, I.; Ferraro, A.; Vitale, M.; Bifulco, M.; Fusco, A.; Portella, G. Lovastatin enhances the replication of the oncolytic adenovirus dl1520 and its antineoplastic activity against anaplastic thyroid carcinoma cells. Endocrinology 2007, 148, 5186–5194. [Google Scholar] [CrossRef]
- Pan, J.J.; Zhang, S.W.; Chen, C.B.; Xiao, S.W.; Sun, Y.; Liu, C.Q.; Su, X.; Li, D.M.; Xu, G.; Xu, B.; et al. Effect of recombinant adenovirus-p53 combined with radiotherapy on long-term prognosis of advanced nasopharyngeal carcinoma. J. Clin. Oncol. 2009, 27, 799–804. [Google Scholar] [CrossRef]
- Jabar, A.H.; Errington-Mais, F.; Vile, R.G.; Selby, P.J.; Melcher, A.A.; Griffin, S. Progress in clinical oncolytic virus-based therapy for hepatocellular carcinoma. J. Gen. Virol. 2015, 96, 1533–1550. [Google Scholar] [CrossRef]
- Wong, H.H.; Lemoine, N.R.; Wang, Y. Oncolytic Viruses for Cancer Therapy: Overcoming the Obstacles. Viruses 2010, 2, 78–106. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habiba, U.; Hossain, E.; Yanagawa-Matsuda, A.; Chowdhury, A.F.M.A.; Tsuda, M.; Zaman, A.-u.-; Tanaka, S.; Higashino, F. Cisplatin Relocalizes RNA Binding Protein HuR and Enhances the Oncolytic Activity of E4orf6 Deleted Adenovirus. Cancers 2020, 12, 809. https://doi.org/10.3390/cancers12040809
Habiba U, Hossain E, Yanagawa-Matsuda A, Chowdhury AFMA, Tsuda M, Zaman A-u-, Tanaka S, Higashino F. Cisplatin Relocalizes RNA Binding Protein HuR and Enhances the Oncolytic Activity of E4orf6 Deleted Adenovirus. Cancers. 2020; 12(4):809. https://doi.org/10.3390/cancers12040809
Chicago/Turabian StyleHabiba, Umma, Elora Hossain, Aya Yanagawa-Matsuda, Abu Faem Mohammad Almas Chowdhury, Masumi Tsuda, Asad-uz- Zaman, Shinya Tanaka, and Fumihiro Higashino. 2020. "Cisplatin Relocalizes RNA Binding Protein HuR and Enhances the Oncolytic Activity of E4orf6 Deleted Adenovirus" Cancers 12, no. 4: 809. https://doi.org/10.3390/cancers12040809
APA StyleHabiba, U., Hossain, E., Yanagawa-Matsuda, A., Chowdhury, A. F. M. A., Tsuda, M., Zaman, A.-u.-, Tanaka, S., & Higashino, F. (2020). Cisplatin Relocalizes RNA Binding Protein HuR and Enhances the Oncolytic Activity of E4orf6 Deleted Adenovirus. Cancers, 12(4), 809. https://doi.org/10.3390/cancers12040809





