The Role of Positron Emission Tomography in Clinical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Diagnostic Accuracy of 18-FDG PET Scan to Identify Malignant IPMN
3.2. Diagnostic Accuracy of International Guidelines in Identifying Malignant IPMN
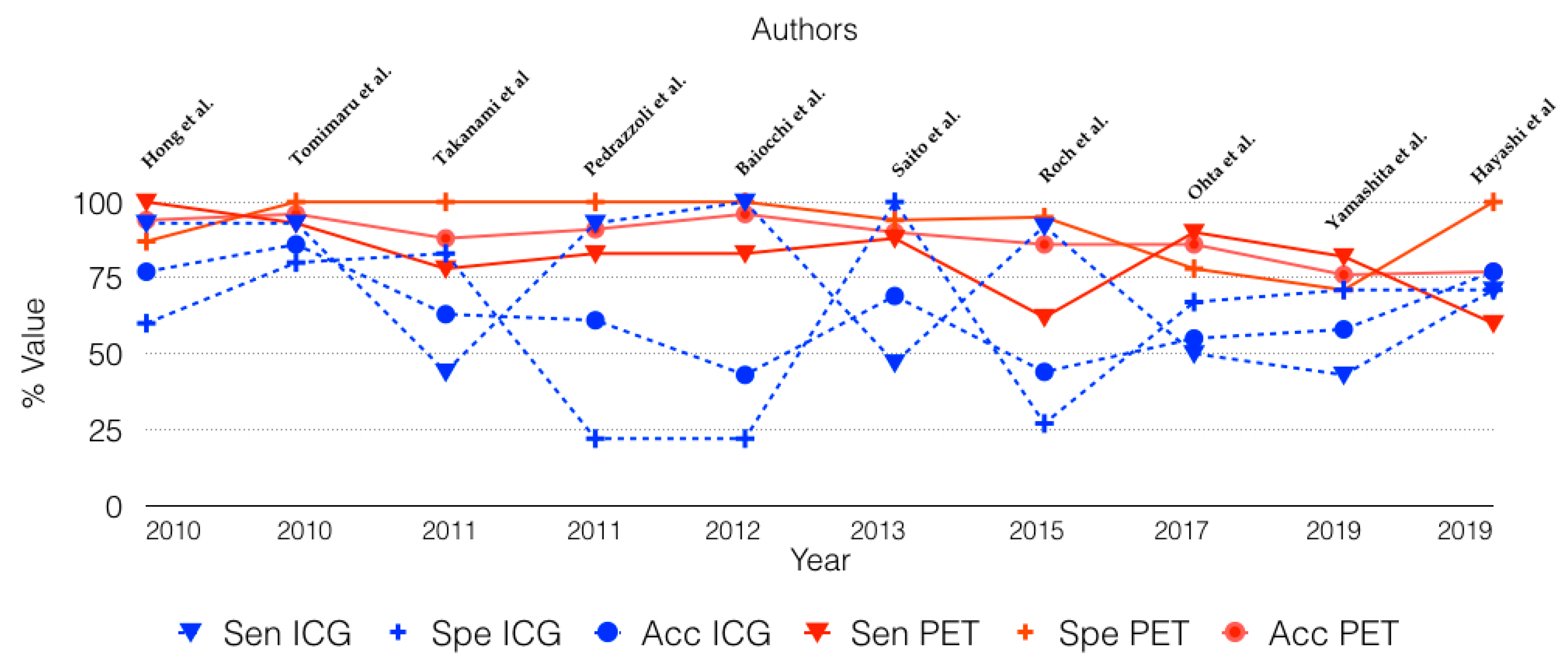
3.3. Diagnostic Accuracy Comparison of 18-FDG PET and International Consensus Guidelines in Identifying Malignant IPMN
3.4. Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Visser, B.C.; Muthusamay, V.R.; Mulvihill, S.J.; Coakley, F. Diagnostic Imaging of Cystic Pancreatic Neoplasms. Surg. Oncol. 2004, 13, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, K.G. Cystic Neoplasms of the Pancreas. Ital. J. Gastroenterol. Hepatol. 1999, 31, 183–185. [Google Scholar] [PubMed]
- Kloppel, G.; Solcia, E.; Longnecker, D.S.; Capella, C. Histological Typing of Tumours of the Exocrine Pancreas, 2nd ed.; Springer: Berlin, Germany, 1996. [Google Scholar]
- Roggin, K.K.; Chennat, J.; Oto, A.; Noffsinger, A.; Briggs, A.; Matthews, J.B. Pancreatic Cystic Neoplasm. Curr. Probl. Surg. 2010, 47, 459–510. [Google Scholar] [CrossRef] [PubMed]
- Demos, T.C.; Posniak, H.V.; Harmath, C.; Olson, M.C.; Aranha, G. Cystic Lesions of the Pancreas. Am. J. Roentgenol. 2002, 179, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S. International Association of Pancreatology. International Consensus Guidelines for Management of Intraductal Papillary Mucinous Neoplasms and Mucinous Cystic Neoplasms of the Pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.-Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International Consensus Guidelines 2012 for the Management of IPMN and MCN of the Pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of International Consensus Fukuoka Guidelines for the Management of IPMN of the Pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Pham, K.H.; Ramaswamy, M.R.; Hawkins, R.A. Advances in Positron Emission Tomography Imaging for the GI Tract. Gastrointest. Endosc. 2002, 55, S53–S63. [Google Scholar] [CrossRef]
- Kim, T.H.; Song, T.J.; Hwang, J.; Yoo, K.; Lee, W.; Lee, K.; Dong, S.; Park, C.; Park, E.; Moon, J.; et al. Predictors of Malignancy in Pure Branch Duct Type Intraductal Papillary Mucinous Neoplasm of the Pancreas: A Nationwide Multicenter Study. Pancreatology 2015, 15, 405–410. [Google Scholar] [CrossRef]
- Ohno, E.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Katano, Y.; Ohmiya, N.; Niwa, Y.; Goto, H. Intraductal Papillary Mucinous Neoplasms of the Pancreas: Differentiation of Malignant and Benign Tumors by Endoscopic Ultrasound Findings of Mural Nodules. Ann. Surg. 2009, 249, 628–634. [Google Scholar] [CrossRef]
- Kitano, M.; Sakamoto, H.; Komaki, T.; Kudo, M. New Techniques and Future Perspective of EUS for The Differential Diagnosis Of Pancreatic Malignancies: Contrast Harmonic Imaging: Contrast Harmonic EUS In Pancreas. Dig. Endosc. 2011, 23, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- NCSS 2019 Statistical Software; NCSS, LLC: Kaysville, UT, USA, 2019.
- Hong, H.-S.; Yun, M.; Cho, A.; Choi, J.-Y.; Kim, M.-J.; Kim, K.W.; Choi, Y.J.; Lee, J.D. The Utility of F-18 FDG PET/CT in the Evaluation of Pancreatic Intraductal Papillary Mucinous Neoplasm. Clin. Nucl. Med. 2010, 35, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Tomimaru, Y.; Takeda, Y.; Tatsumi, M.; Kim, T.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Tanemura, M.; Kitagawa, T.; Nagano, H.; et al. Utility of 2-[18F] Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography in Differential Diagnosis of Benign and Malignant Intraductal Papillary-Mucinous Neoplasm of the Pancreas. Oncol. Rep. 2010, 24, 613–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takanami, K.; Hiraide, T.; Tsuda, M.; Nakamura, Y.; Kaneta, T.; Takase, K.; Fukuda, H.; Takahashi, S. Additional Value of FDG PET/CT to Contrast-Enhanced CT in the Differentiation between Benign and Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas with Mural Nodules. Ann. Nucl. Med. 2011, 25, 501–510. [Google Scholar] [CrossRef]
- Pedrazzoli, S.; Sperti, C.; Pasquali, C.; Bissoli, S.; Chierichetti, F. Comparison of International Consensus Guidelines versus 18-FDG PET in Detecting Malignancy of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2011, 254, 971–976. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Bertagna, F.; Gheza, F.; Grazioli, L.; Calanducci, D.; Giubbini, R.; Portolani, N.; Giulini, S.M. Searching for Indicators of Malignancy in Pancreatic Intraductal Papillary Mucinous Neoplasms: The Value of 18FDG-PET Confirmed. Ann. Surg. Oncol. 2012, 19, 3574–3580. [Google Scholar] [CrossRef]
- Saito, M.; Ishihara, T.; Tada, M.; Tsuyuguchi, T.; Mikata, R.; Sakai, Y.; Tawada, K.; Sugiyama, H.; Kurosawa, J.; Otsuka, M.; et al. Use of F-18 Fluorodeoxyglucose Positron Emission Tomography with Dual-Phase Imaging to Identify Intraductal Papillary Mucinous Neoplasm. Clin. Gastroenterol. Hepatol. 2013, 11, 181–186. [Google Scholar] [CrossRef]
- Roch, A.M.; Barron, M.R.; Tann, M.; Sandrasegar, K.; Hannaford, K.N.; Ceppa, E.P.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Schmidt, C.M. Does PET with CT Have Clinical Utility in the Management of Patients with Intraductal Papillary Mucinous Neoplasm? J. Am. Coll. Surg. 2015, 221, 48–56. [Google Scholar] [CrossRef]
- Ohta, K.; Tanada, M.; Sugawara, Y.; Teramoto, N.; Iguchi, H. Usefulness of Positron Emission Tomography (PET)/Contrast-Enhanced Computed Tomography (CE-CT) in Discriminating between Malignant and Benign Intraductal Papillary Mucinous Neoplasms (IPMNs). Pancreatology 2017, 17, 911–919. [Google Scholar] [CrossRef]
- Yamashita, Y.-I.; Okabe, H.; Hayashi, H.; Imai, K.; Nakagawa, S.; Nakao, Y.; Yusa, T.; Itoyama, R.; Yama, T.; Umesaki, N.; et al. Usefulness of 18-FDG PET/CT in Detecting Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Anticancer Res. 2019, 39, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Mikata, R.; Horikoshi, T.; Senoo, J.; Kusakabe, Y.; Ohyama, H.; Yasui, S.; Uchida, Y.; Uchiyama, K.; Kishimoto, T.; et al. Diffusion-Weighted Magnetic Resonance Imaging and 18-Fluorodeoxglucose Positron Emission Tomography With Computed Tomography for Evaluating Malignancy of Branch Duct and Mixed Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas 2019, 48, e43–e45. [Google Scholar] [CrossRef] [PubMed]
- Adsay, N.V. Cystic Neoplasia of the Pancreas: Pathology and Biology. J. Gastrointest. Surg. 2008, 12, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Talamini, M.A.; Pitt, H.A.; Hruban, R.H.; Boitnott, J.K.; Coleman, J.; Cameron, J.L. Spectrum of Cystic Tumors of the Pancreas. Am. J. Surg. 1992, 163, 117–123; discussion 123–124. [Google Scholar] [CrossRef]
- Yang, E.Y.; Joehl, R.J.; Talamonti, M.S. Cystic Neoplasms of the Pancreas. J. Am. Coll. Surg. 1994, 179, 747–757. [Google Scholar]
- Grützmann, R.; Niedergethmann, M.; Pilarsky, C.; Klöppel, G.; Saeger, H.D. Intraductal Papillary Mucinous Tumors of the Pancreas: Biology, Diagnosis, and Treatment. Oncologist 2010, 15, 1294–1309. [Google Scholar] [CrossRef]
- Van Dam, J. EUS in Cystic Lesions of the Pancreas. Gastrointest. Endosc. 2002, 56 (Suppl. S4), S91–S93. [Google Scholar] [CrossRef]
- Sand, J.A.; Hyoty, M.K.; Mattila, J.; Dagorn, J.C.; Nordback, I.H. Clinical Assessment Compared with Cyst Fluid Analysis in the Differential Diagnosis of Cystic Lesions in the Pancreas. Surgery 1996, 119, 275–280. [Google Scholar] [CrossRef]
- Kobari, M.; Egawa, S.; Shibuya, K.; Shimamura, H.; Sunamura, M.; Takeda, K.; Matsuno, S.; Furukawa, T. Intraductal Papillary Mucinous Tumors of the Pancreas Comprise 2 Clinical Subtypes: Differences in Clinical Characteristics and Surgical Management. Arch. Surg. 1999, 134, 1131–1136. [Google Scholar] [CrossRef]
- Sakorafas, G.H.; Sarr, M.G. Cystic Neoplasms of the Pancreas; What a Clinician Should Know. Cancer Treat. Rev. 2005, 31, 507–535. [Google Scholar] [CrossRef]
- Carbognin, G.; Zamboni, G.; Pinali, L.; Chiara, E.D.; Girardi, V.; Salvia, R.; Mucelli, R.P. Branch Duct IPMTs: Value of Cross-Sectional Imaging in the Assessment of Biological Behavior and Follow-Up. Abdom. Imaging 2006, 31, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Ishikawa, O.; Ikezawa, K.; Kawada, N.; Inoue, T.; Takakura, R.; Takano, Y.; Tanaka, S.; Takenaka, A. A Natural Course of Main Duct Intraductal Papillary Mucinous Neoplasm of the Pancreas with Lower Likelihood of Malignancy. Pancreas 2010, 39, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Adams, M.A.; Dorn, S.D.; Dudley-Brown, S.L.; Flamm, S.L.; Gellad, Z.F.; Gruss, C.B.; et al. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef] [PubMed]
- European Study Group on Cystic Tumours of the Pancreas. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Lekkerkerker, S.J.; Besselink, M.G.; Busch, O.R.; Verheij, J.; Engelbrecht, M.R.; Rauws, E.A.; Fockens, P.; van Hooft, J.E. Comparing 3 Guidelines on the Management of Surgically Removed Pancreatic Cysts with Regard to Pathological Outcome. Gastrointest. Endosc. 2017, 85, 1025–1031. [Google Scholar] [CrossRef]
- Imbe, K.; Nagata, N.; Hisada, Y.; Takasaki, Y.; Sekine, K.; Mishima, S.; Kawazoe, A.; Tajima, T.; Shimbo, T.; Yanase, M.; et al. Validation of the American Gastroenterological Association Guidelines on Management of Intraductal Papillary Mucinous Neoplasms: More than 5 Years of Follow-Up. Eur. Radiol. 2018, 28, 170–178. [Google Scholar] [CrossRef]
- Kalra, M.K.; Maher, M.M.; Sahani, D.V.; Digmurthy, S.; Saini, S. Current Status of Imaging in Pancreatic Diseases. J. Comput. Assist. Tomogr. 2002, 26, 661–675. [Google Scholar] [CrossRef]
- Kauhanen, S.; Rinta-Kiikka, I.; Kemppainen, J.; Grönroos, J.; Kajander, S.; Seppänen, M.; Alanen, K.; Gullichsen, R.; Nuutila, P.; Ovaska, J. Accuracy of 18F-FDG PET/CT, Multidetector CT, and MR Imaging in the Diagnosis of Pancreatic Cysts: A Prospective Single-Center Study. J. Nucl. Med. 2015, 56, 1163–1168. [Google Scholar] [CrossRef]
- Uribarri-Gonzalez, L.; Keane, M.G.; Pereira, S.P.; Iglesias-García, J.; Dominguez-Muñoz, J.E.; Lariño-Noia, J. Agreement among Magnetic Resonance Imaging/Magnetic Resonance Cholangiopancreatography (MRI-MRCP) and Endoscopic Ultrasound (EUS) in the Evaluation of Morphological Features of Branch Duct Intraductal Papillary Mucinous Neoplasm (BD-IPMN). Pancreatology 2018, 18, 170–175. [Google Scholar] [CrossRef]
- Lewandrowski, K.; Lee, J.; Southern, J.; Centeno, B.; Warshaw, A. Cyst Fluid Analysis in the Differential Diagnosis of Pancreatic Cysts: A New Approach to the Preoperative Assessment of Pancreatic Cystic Lesions. Am. J. Roentgenol. 1995, 164, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Pasquali, C.; Guolo, P.; Polverosi, R.; Liessi, G.; Pedrazzoli, S. Serum Tumor Markers and Cyst Fluid Analysis Are Useful for the Diagnosis of Pancreatic Cystic Tumors. Cancer 1996, 78, 237–243. [Google Scholar] [CrossRef]
- Hernandez, L.V.; Mishra, G.; Forsmark, C.; Draganov, P.V.; Petersen, J.M.; Hochwald, S.N.; Vogel, S.B.; Bhutani, M.S. Role of Endoscopic Ultrasound (EUS) and EUS-Guided Fine Needle Aspiration in the Diagnosis and Treatment of Cystic Lesions of the Pancreas. Pancreas 2002, 25, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Pelaez-Luna, M.; Chari, S.T.; Smyrk, T.C.; Takahashi, N.; Clain, J.E.; Levy, M.J.; Pearson, R.K.; Petersen, B.T.; Topazian, M.D.; Vege, S.S.; et al. Do Consensus Indications for Resection in Branch Duct Intraductal Papillary Mucinous Neoplasm Predict Malignancy? A Study of 147 Patients. Am. J. Gastroenterol. 2007, 102, 1759–1764. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Salvia, R.; Crippa, S.; Warshaw, A.L.; Bassi, C.; Falconi, M.; Thayer, S.P.; Lauwers, G.Y.; Capelli, P.; Mino-Kenudson, M.; et al. Branch-Duct Intraductal Papillary Mucinous Neoplasms: Observations in 145 Patients Who Underwent Resection. Gastroenterology 2007, 133, 72–79; quiz 309–310. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.S.; Weinberg, B.; Dawson, D.W.; Reber, H.; Hines, O.J.; Tomlinson, J.S.; Chaudhari, V.; Raman, S.; Farrell, J.J. Evaluation of the Guidelines for Management of Pancreatic Branch-Duct Intraductal Papillary Mucinous Neoplasm. Clin. Gastroenterol. Hepatol. 2008, 6, 815–819; quiz 719. [Google Scholar] [CrossRef]
- Srinivasan, N.; Teo, J.-Y.; Chin, Y.-K.; Hennedige, T.; Tan, D.M.; Low, A.S.; Thng, C.H.; Goh, B.K.P. Systematic Review of the Clinical Utility and Validity of the Sendai and Fukuoka Consensus Guidelines for the Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas. HPB 2018, 20, 497–504. [Google Scholar] [CrossRef]
- Sperti, C.; Pasquali, C.; Chierichetti, F.; Liessi, G.; Ferlin, G.; Pedrazzoli, S. Value of 18-Fluorodeoxyglucose Positron Emission Tomography in the Management of Patients with Cystic Tumors of the Pancreas. Ann. Surg. 2001, 234, 675–680. [Google Scholar] [CrossRef]
- Sperti, C.; Pasquali, C.; Decet, G.; Chierichetti, F.; Liessi, G.; Pedrazzoli, S. F-18-Fluorodeoxyglucose Positron Emission Tomography in Differentiating Malignant from Benign Pancreatic Cysts: A Prospective Study. J. Gastrointest. Surg. 2005, 9, 22–28; discussion 28–29. [Google Scholar] [CrossRef]
- Nagamachi, S.; Nishii, R.; Wakamatsu, H.; Mizutani, Y.; Kiyohara, S.; Fujita, S.; Futami, S.; Sakae, T.; Furukoji, E.; Tamura, S.; et al. The Usefulness of 18F-FDG PET/MRI Fusion Image in Diagnosing Pancreatic Tumor: Comparison with 18F-FDG PET/CT. Ann. Nucl. Med. 2013, 27, 554–563. [Google Scholar] [CrossRef]
- Chen, B.-B.; Tien, Y.-W.; Chang, M.-C.; Cheng, M.-F.; Chang, Y.-T.; Wu, C.-H.; Chen, X.-J.; Kuo, T.-C.; Yang, S.-H.; Shih, I.-L.; et al. PET/MRI in Pancreatic and Periampullary Cancer: Correlating Diffusion-Weighted Imaging, MR Spectroscopy and Glucose Metabolic Activity with Clinical Stage and Prognosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
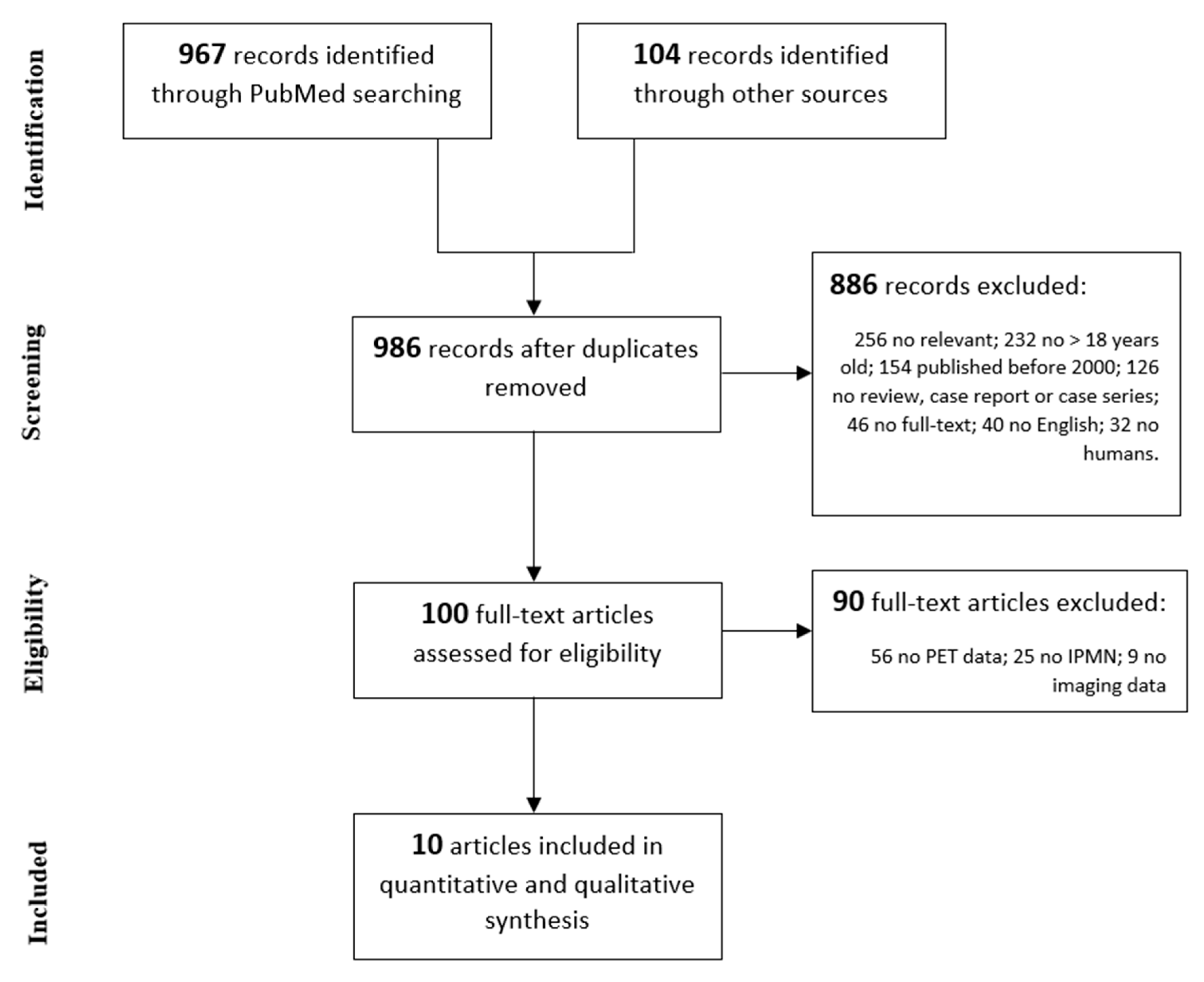
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Articles published from 01/01/2000 to 10/31/2019 | Case report or small case series (<5 patients) |
| Written in English | No PET data available |
| Study in humans > 18 years old | No radiological imaging data available |
| No histopathological proven IPMN |
| Author | Year | Patients/Histo | Benign (n) | Malignant (n) | Sensitivity (%) | Specificity (%) | Accuracy (%) | SUV Cut-Off |
|---|---|---|---|---|---|---|---|---|
| Hong et al. [15] | 2010 | 31/31 | 15 | 16 | 100 | 87 | 94 | 2.5 |
| Tomimaru et al. [16] | 2010 | 72/29 | 15 | 14 | 93 | 100 | 96 | 2.5 |
| Takanami et al. [17] | 2011 | 59/16 | 7 | 9 | 78 | 100 | 88 | 2.3 |
| Pedrazzoli et al. [18] | 2011 | 145/69 | 33 | 36 | 83 | 100 | 91 | 2.5 |
| Baiocchi et al. [19] | 2012 | 44/44 | 32 | 12 | 83 | 100 | 96 | 2.5 |
| Saito et al. [20] | 2013 | 48/48 | 16 | 32 | 88 | 94 | 90 | 2.0 |
| Roch et al. [21] | 2015 | 50/50 | 37 | 13 | 62 | 95 | 86 | 3.0 |
| Ohta et al. [22] | 2017 | 29/29 | 9 | 20 | 90 | 78 | 86 | † |
| Yamashita et al. [23] | 2019 | 79/38 | 18 | 20 | 82 | 71 | 76 | 1.3 |
| Hayashi et al. [24] | 2019 | 65/65 | 28 | 37 | 60 | 100 | 77 | 2.5 |
| Total | 622/419 | 210 | 209 | 80 | 95 | 87 |
| Author | Year | Tot/Histo | Benign (n°) | Malignant (n°) | Sensitivity % | Specificity (%) | Accuracy (%) | Diagnostic Criteria |
|---|---|---|---|---|---|---|---|---|
| Hong et al. [15] | 2010 | 31/31 | 15 | 16 | 93 | 60 | 77 | † |
| Tomimaru et al. [16] | 2010 | 72/29 | 15 | 14 | 93 | 80 | 86 | Mural nodule |
| Takanami et al. [17] | 2011 | 59/16 | 7 | 9 | 44 | 83 | 63 | MPD >5 mm |
| Pedrazzoli et al. [18] | 2011 | 145/80 | 36 | 44 | 93 | 22 | 61 | SCG |
| Baiocchi et al. [19] | 2012 | 44/42 | 30 | 12 | 100 | 22 | 43 | SCG |
| Saito et al. [20] | 2013 | 32/32 | 13 | 19 | 47 | 100 | 69 | MPD ≥ 7 mm |
| Roch et al. [21] | 2015 | 50/50 | 37 | 13 | 92 | 27 | 44 | FCG |
| Ohta et al. [22] | 2017 | 29/29 | 9 | 20 | 50 | 67 | 55 | FCG |
| Yamashita et al. [23] | 2019 | 79/38 | 18 | 20 | 43 | 71 | 58 | MPD ≥ 10 mm |
| Hayashi et al. [24] | 2019 | 65/65 | 28 | 37 | 71 | 85 | 77 | FCG |
| Total | 622/412 | 208 | 204 | 67 | 58 | 63 |
| IPMN Type | ICG Guidelines (2017) [8] | EU Guidelines (2018) [37] | ACG Guidelines (2018) [35] | AGA Guidelines (2015) [36] |
|---|---|---|---|---|
| BD–IPMN | High-risk stigmata: Enhancing mural nodule ≥5 mm MPD ≥10 mm Jaundice | Absolute indications: Solid mass Enhancing mural nodule ≥5 mm MPD ≥10 mm Jaundice | High-risk features: Mural nodule/solid component MPD ≥5 mm MPD caliber change and atrophy Cyst size ≥3 cm Cyst growth ≥3 mm/year Jaundice Acute pancreatitis Elevated serum CA 19-9 New-onset DM | High-risk features: Solid component Dilated MPD Cyst size ≥3 cm |
| Worrisome features: Growth ≥5 mm/2 years Cyst size ≥3 cm Enhancing mural nodule ≥5 mm Enhancing thickened cyst wall MPD 5–9 mm MPD diameter change Elevated serum CA 19-9 Acute pancreatitis | Relative indications: Cyst growth ≥5 mm/year Cyst size ≥4 cm Enhancing mural nodule ≥5 mm MPD 5–9.9 mm Elevated serum CA 19-9 New-onset DM Acute pancreatitis | |||
| MD/MT-IPMN | ≥1 High-risk stigmata | All | Not mentioned | Not mentioned |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafini, S.; Sperti, C.; Brazzale, A.R.; Cecchin, D.; Zucchetta, P.; Pierobon, E.S.; Ponzoni, A.; Valmasoni, M.; Moletta, L. The Role of Positron Emission Tomography in Clinical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancers 2020, 12, 807. https://doi.org/10.3390/cancers12040807
Serafini S, Sperti C, Brazzale AR, Cecchin D, Zucchetta P, Pierobon ES, Ponzoni A, Valmasoni M, Moletta L. The Role of Positron Emission Tomography in Clinical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancers. 2020; 12(4):807. https://doi.org/10.3390/cancers12040807
Chicago/Turabian StyleSerafini, Simone, Cosimo Sperti, Alessandra Rosalba Brazzale, Diego Cecchin, Pietro Zucchetta, Elisa Sefora Pierobon, Alberto Ponzoni, Michele Valmasoni, and Lucia Moletta. 2020. "The Role of Positron Emission Tomography in Clinical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas" Cancers 12, no. 4: 807. https://doi.org/10.3390/cancers12040807
APA StyleSerafini, S., Sperti, C., Brazzale, A. R., Cecchin, D., Zucchetta, P., Pierobon, E. S., Ponzoni, A., Valmasoni, M., & Moletta, L. (2020). The Role of Positron Emission Tomography in Clinical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancers, 12(4), 807. https://doi.org/10.3390/cancers12040807






