Abstract
The failure of therapies directed at targets within cancer cells highlight the necessity for a paradigm change in cancer therapy. The attention of researchers has shifted towards the disruption of cancer cell interactions with the tumor microenvironment. A typical example of such a disruption is the immune checkpoint cancer therapy that disrupts interactions between the immune and the cancer cells. The interaction of cancer antigens with T cells occurs in the immunological synapses. This is characterized by several special features, i.e., the proximity of the immune cells and their target cells, strong intercellular adhesion, and secretion of signaling cytokines into the intercellular cleft. Earlier, we hypothesized that the cancer-associated fibroblasts interacting with cancer cells through a synapse-like adhesion might play an important role in cancer tumors. Studies of the interactions between cancer cells and cancer-associated fibroblasts showed that their clusterization on the membrane surface determined their strength and specificity. The hundreds of interacting pairs are involved in the binding that may indicate the formation of synapse-like structures. These interactions may be responsible for successful metastasis of cancer cells, and their identification and disruption may open new therapeutic possibilities.
1. Introduction.
1.1. The Necessity of Changing the Paradigm in Cancer Therapy
The Cancer Genome Atlas (TCGA) project revealed ~10 million mutations associated with cancer []. Nonetheless, this enormous number of mutations does not reflect the entirety of the complexity of cancer (for the definition of complexity, see Reference []). The study revealed that the heterogeneity among cancer cells was much higher than previously estimated []. Each human tumor was found to contain 4–8 heterogeneous clones. The presence of various clones and cells that differ in their genotype and/or phenotype is at the root of the underlying problem of inefficient cancer therapy, and this problem is magnified by epigenetic, metabolic, and other types of heterogeneities. Any therapy applied to a heterogeneous mixture of cancer cells will induce different responses in different cells and may be inefficient in eliminating specific clones. Changes in the intratumoral heterogeneity during tumor development predetermine failures of targeted cancer therapies directed at the individual molecular components of cancer cells [,].
However, the main problem is that cancer is a “complex system” [,] composed of interacting subunits. These interactions result in the appearance of emergent properties characteristic to the whole system [,,,], properties that cannot be predicted from the properties of the individual subunits []. In cancer, the intratumoral complexity of the true cancer cells [,,,,] should be distinguished from the complexity. This is due to their interaction with the tumor microenvironment (TME) [,].
The main tumor complexity is probably due to a large number of interactions between the true (usually epithelial) cancer cells and various cells of the TME []. Therefore, it is not surprising that the vast resources spent in the era of molecular targeted therapy have yielded only a few relatively efficacious agents. These agents include imatinib for the treatment of myeloid leukemia, trastuzumab directed at the human epidermal growth factor receptor 2 (HER2) expressed in some patients with breast cancer, and vemurafenib for melanoma expressing a mutant BRAF gene [,,]. This emphasized the necessity of changing the paradigm in cancer therapy, and consequently, the attention of researchers gradually shifted towards the disruption of cancer cell interactions with the TME.
1.2. A Brief Description of the TME and Its Importance for Cancer Progression
The American National Cancer Institute defines the TME as “The normal cells, molecules, and blood vessels that surround and feed a tumor cell.” A tumor can change its microenvironment, and the microenvironment can affect how a tumor grows and spreads. (https://www.cancer.gov/publications/dictionaries/cancer-terms/def/tumor-microenvironment). The components of the TME constitute a complex mixture of different cells and extracellular material. The cellular component includes cells of a mesenchymal origin, i.e., the fibroblasts, the cancer-associated fibroblasts (CAFs), the myofibroblasts, the mesenchymal stem cells, the adipocytes, and the endothelial cells. It also includes cells of the hematopoietic origin, namely, the lymphoid cells (the T, the B, and the NK cells) and the myeloid cells (macrophages, neutrophils, and the myeloid-derived suppressor cells) [,,,,]. The non-cellular component is represented by the extracellular matrix [,,]. Cancer and stromal components form an integrated and evolving system with multiple interactions and emergent properties [,,,,]. In their evolution, all tumors use a wide repertoire of healthy cells and adapt them to their conditions. The recruited normal cells facilitate the acquisition of the tumor-specific traits and form an ecological tumor niche that plays a significant role both in the development of the primary tumor and its metastasis [,,,,,,].
Due to the interaction of cancer and stromal cells, tumors evolve as organ-like entities. These interactions include (i) direct binary contacts between ligands and receptors exposed on the surface of cancer and stromal cells, and (ii) paracrine communication between cancer (usually epithelial) cells and various TME cells [,]. Some authors use the term “symbiotic” for tumor–stroma interactions [,]. Stromal cells modified by the malignant epithelium form a permissive microenvironment that controls the cancer progression []. The symbiosis of cancer and stromal cells includes a complimentary exchange of paracrine factors affecting the TME characteristics. The most important consequence of this exchange is the transformation of normal fibroblasts into cancer-associated fibroblasts (CAFs).
It is important to note that due to diffusion, paracrine signals can be transmitted over distances of tens of cell diameters [], forming a gradient of signals that, depending on the concentration, can induce different responses instead of a simple “yes” or “no” binary responses. The transmission of signals will presumably be efficient only between closely located cells, where it occurs in synapse-like structures. Synapses are stable adhesive domains between two neighboring cells of multicellular organisms and function in cell-to-cell communication, as well as in information processing and storage. The synapse concept was developed more than 100 years ago for neuronal cell-to-cell communication, and it was recently adapted to other cell-to-cell communication mechanisms []. Successful cancer treatment targeted at the indecipherable intracellular interactomes is impossible. The development of efficient cancer therapies should focus on a new paradigm, advanced by the immune checkpoint therapy. Generally, this paradigm focuses on interactions between cancer and the stromal cells as therapeutic targets. It was suggested [], that only direct interactions (for example, between ligands and their cognate receptors) form relatively simple binary contacts that are necessary for predictable therapeutic action. The synapse-like structures may universally mediate these interactions. An example is the successful immunotherapy of tumors based on the blocking of the immunological checkpoints (described in the paragraph below). We discuss details of the immunological synapses (ISs) as an example of the mechanism of stable intercellular interactions.
1.3. A Short Summary of the Formation of Immunological Synapses between T cells and the Activated Antigen-Presenting Cells
Activation of T cells requires three signals (Figure 1). The first signal is delivered through the interaction of T cell receptors (TCRs) with their antigen exposed on the surface of antigen-presenting cells (APCs) complexed with the proteins of the major histocompatibility complex (MHC). The second signal is antigen-independent and is delivered through the interaction between the stimulatory receptor C28 on the T cells and the protein CD80 (B7.1) or CD86 (B7.2) on the APCs []. The B7 family includes important membrane-bound ligands able to bind both co-stimulatory and co-inhibitory receptors (mentioned below). Stimulatory cytokines deliver the third signal in synapses. T cells, fully activated by all three signals, begin to proliferate and destroy carriers of antigens presented by the APCs. Apart from the co-stimulatory signals for T cells, there are also co-inhibitory signals produced by the T cells shortly after the initiation of the T cell proliferation [,,]. Inhibitory interactions prevent overactive responses to the immune stimuli, thereby preventing autoimmune reactions.
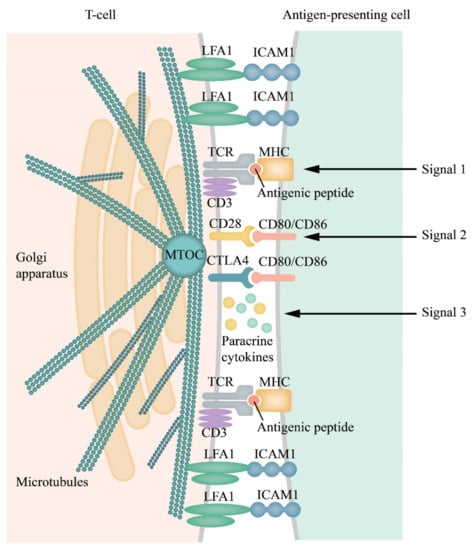
Figure 1.
Scheme of an immunological synapse (IS) and receptor/(co-receptor)–ligand interactions within the synapse cleft and distribution of receptors and adhesion molecules in separate clusters within the IS. T cell receptor (TCR)/CD3 complex interacts with an MHC peptide. Adhesion molecules, such as lymphocyte function-associated antigen 1 (LFA-1) and Inter-Cellular Adhesion Molecule 1 (ICAM-1), on the surface of both cells, are responsible for the formation and stabilization of ISs, and initiation of signal pathways generated by the TCRs []. The cytoskeleton is remodeled, the Golgi apparatus, and the microtubule-organizing center (MTOC) move to the IS formation region [,]. All these rearrangements facilitate and allow the directed secretion within the synapse [,,,]. Activation/inhibition of T cells requires three signals. The first signal is initiated by binding of the TCR complexes with antigen peptides (blue circlet) presented by MHCs of the APCs. The second signal, an antigen-independent stage, is triggered by the interaction of the co-stimulating T cell receptor CD28 with ligands B7.1 (CD80) or B7.2 (CD86), delivered by the APCs (or tumor cells). Paracrine cytokines generate the third signal. All transmembrane contacts are clustered and have been symbolized by their pairs in the figure.
The primary contact of TCRs with the antigen-loaded MHC proteins on the APCs (signal 1) induces the activation of multiple effectors including, among others, the membrane-bound integrins, the antigen-1 (LFA-1) associated with lymphocyte function and its ligand ICAM-1, signal adapters, and elements of the cytoskeleton and so on. These processes enhance interactions of the T cells with the APCs. Their contact also includes the co-stimulatory and co-inhibitory receptors (signal 2). These interactions culminate in the formation of ISs; narrow (12–15 nm) intercellular clefts where cytokines are concentrated, thereby enhancing the crosstalk between the cells (signal 3) []. ISs enable unique cell-to-cell interactions and are characterized by a number of essential features. These features include proximity of the immune cells and their target cells, strong intercellular adhesion, and secretion of signaling cytokines into the intercellular cleft [,,,]. However, data are showing that the T cells can function without forming synapses []. An essential characteristic of ISs is the mechanical forces generated due to intercellular adhesion. ISs that include the natural killer cells do not express TCRs but express activatory and inhibitory receptors that may regulate the transmission of signals and dynamic changes in the integrin-actin systems []. In general, the existing therapies targeted at blocking the co-inhibitory receptors affect the immunological synapses [].
Here we have discussed the duration of IS existence and will not discuss the mechanism and kinetics of the IS formation, which have been discussed in recent reviews [,,,,].
Cytotoxic lymphocytes (CTLs) form ISs, which only lasts a few minutes, owing to the death of target cells. This effect is probably due to the optimal CTLs function that may need fast and short-lived contact to kill as many target cells as possible. In contrast, the T lymphocytes form stable, long-lasting ISs (from 20–30 min to several hours), required for the directed and continuous secretion of cytokines []. These cytokines are located in secretory granules, and some of them undergo directed transport towards ISs. However, the transport of some cytokines, e.g., TNFs, is not directed, and the reasons for this difference remain unclear.
1.4. Clusterization of Receptors and Ligands is A Prerequisite and Signature of IS Formation
An essential feature of ISs is the formation of receptor and ligand clusters, which mediate intercellular contacts. Some authors suggest the formation of synapse-like structures for all cases of membrane signalization. For example, it is indicated in Reference [], “this in a way predicts a ‘synapse’ like entity for all membrane signaling events. Here there is no difference between a ligand/receptor pair induced higher-order lipid domain or one produced by a membrane curvature or any other biophysical means. The central purpose is to bring together enough sorted lipids and their associated protein receptors, and signaling ensues”.
In general, extracellular protein-protein interactions vary from very affine interactions with the equilibrium constant of dissociation (Kd) in the nanomolar to the picomolar range for soluble ligands. There are also extremely low-affinity interactions with the Kd within the micromolar to the millimolar range for the membrane receptor–ligand protein interactions []. Soluble ligands bind their receptors with high affinity because their concentration in the solution is usually low, and high-affinity binding ensures signal initiation. This effect is in contrast with the low affinity of the membrane-embedded proteins that often have a half-life of milliseconds in the monomeric state []. In this case, the strength of intercellular contacts depends on the clusterization of adhesion molecules comprising hundreds of receptors. This increases the avidity of the intercellular contact to a level sufficient to trigger a signaling event. Noteworthy, these adhesive events must be readily reversible. Clusterization and the associated transformations of the cytoskeleton have been shown schematically in Figure 2.
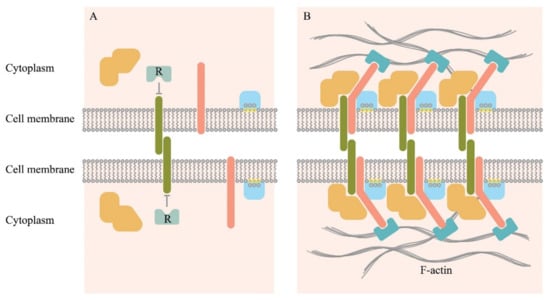
Figure 2.
Schematic representation of individual molecules freely diffusing on the membrane surface (A), and a cluster of the intercellular adhesive complexes (B). Adhesion molecules (deep green) initiate binding, which also may involve other transmembrane proteins (pink), cytoplasmic proteins that can bind to the cytosolic part of the transmembrane proteins (orange). It also involves lipid groups present on the inner surface of the plasma membrane (yellow), and proteins with lipid-binding domains (light blue). Clustering may lead to the displacement of negative regulators associated with the cytosolic part of the adhesion molecules (R). Actin microfilaments stabilize macromolecular clusters through actin-binding proteins (cyan) [].
A relatively well-studied example is the clusterization of cadherins during the formation of the cadherin-mediated intercellular contacts []. The emergent intercellular adhesion is initiated by the binding of cadherin ectodomains on cell surfaces. Due to diffusion, the formed cadherin trans-dimers gather into small clusters at the sites of cell adhesion. With the participation of intracellular transformations of the cytoskeleton bound to the inner parts of the cadherins, the clusters are stabilized, and they expand. As a result, cell adhesion is enhanced strongly. Monomers and small inactive nanoclusters can coexist on the cell membrane. Small nanoclusters usually slowly diffuse or can be fixed through the actin cytoskeleton. The size of the nanoclusters in the ligand-free state may be probably below the functional threshold, and therefore, may be unable to stably bind their ligands and transmit a signal. On binding a ligand, the already existing small nanocluster can include accessory monomers.
Activation of the nanoclusters through binding ligands leads to an enlargement of nanoclusters, making them functional. Nanoclusterization is a general organization principle for many membrane receptors. It is rarely completed, and nanoclusters often coexist with randomly distributed non-clustered components. This coexistence may play a functional role or a regulatory role. Nanoclusters may function as complexes assembled in advance and capable of fast activation on binding a ligand []. A receptor cluster in the T cell synapses initiates the recruitment of hundreds of molecules to the membrane, interacts with the actin cytoskeleton¸ and plays a significant role in signal transmission. The formation of signal clusters leads to functional results that are difficult to predict from individual components []. This complex system interacts having emergent properties []. Transmission of intercellular adhesion signals in other cellular systems is similar to processes in the T cell immunological synapses. One of the recent examples is the ephrin type-A receptor 2 (EphA2)/EphrinA1 system that regulates cell adhesion, motility, and angiogenesis. The binding of EphA2 to EphrinA1 results in the formation of clusters that undergo actin-directed transport on the cell membrane []. These may display features similar to features found in a T cell immunological synapse. Clusterization provides stability for signaling by enhancing ligand-receptor functional local concentration and reducing the possible effect of the protein-degrading enzymes on the interaction result. Clusterization also results in higher specificity and provides an additional level of cell control [,].
A fundamental property of synapse is the proximity of the interacting cells. Such proximity was reported in an X-ray structural analysis of a CD200R and CD200 protein complex. CD200 (earlier known as OX2) is a widespread cellular surface protein that interacts with the receptor CD200R, expressed in the myeloid cells and some lymphoid cells. The authors calculated a distance of ~12 nm between the interacting cells, which corresponds to the spatial parameters of an immunological synapse. Since CD200 is also expressed in the non-lymphoid cells, synapse-like interactions may be widely used [,].
In summary, one of the essential features of the synapse-like intercellular contacts is the presence of receptor clusters on one of the interacting cells and ligand clusters on the other. These clusters are associated with the remodeling of the intracellular cytoskeletons. This allows the polarization of the cell secretory mechanism in immunological synapses, which provides another feature of synapse-directed secretion []. The existence of such membrane ligand-receptor pair clusters on the interacting cells should imply the existence of synapse-like structures [,,].
1.5. Remodeling of Cytoskeletons in Intercellular Interactions
Intercellular interactions induce a radical remodeling of the cytoskeleton (Figure 2). As a result, the Golgi apparatus moves to the IS, thereby allowing directed secretion within the synapse (Figure 1). The location of the centrosome is also drastically changed upon recognition of the target cell. The centrosome moves from the back-end of the cell to its front edge where a synapse forms [,,,,]. The involvement of the cytoskeleton in cluster formation has been shown schematically in Figure 2. This process is rather well-studied for the E-cadherin-mediated intercellular interactions. It involves the p120 catenin that, together with the beta- and the alfa-catenins, binds the cytoplasmic domain of cadherin. Alfa-catenin directly binds F-actin. This process stabilizes the clusterization of cadherin [,,].
Adhesion induces remodeling of the cytoskeleton and affects the cell polarity, as discussed above. It is also related to some cellular processes, including differentiation and proliferation. Disorders of cell polarity are associated with disorders of development. Therefore, many tumors show the loss of E-cadherin-mediated intercellular adhesion []. These complex processes have a genetic basis and an epigenetic basis that is mostly unclear. In recent years, there have been attempts to decipher it, and some representative results have been presented below. An extensive siRNA screening revealed tens of genes that were probably involved in the regulation of adhesion (see the review []), through involvement in β-catenin and β1-integrin pathways, regulation of the actin cytoskeleton, and EGFR signaling. Noteworthy, among the genes mutated in lung carcinoma, a significant proportion of genes participate in the regulation of the cytoskeleton state, including the genes IQGAP3, EPB41, CDC42, PARD6G, PTK2B, and KALRN. The proportion of these genes has been found to increase in metastases. This suggested the involvement of these genes in the process of metastasis []. The transcription factor GATA4, crucially important in the early liver development, has been shown to be involved in the pathophysiology of hepatoblastoma, an embryonic tumor of childhood. Suppression of the GATA4 gene (using RNA interference) disturbed the migration of the human hepatoblastoma cells, HUH6. Moreover, the expression of genes involved in the cytoskeleton organization, intercellular adhesion, and dynamics of the extracellular matrix was found to be changed. One hundred and six differentially expressed genes (34 up-regulated and 72 down-regulated) were identified [].
Furthermore, the relationships between several proteins involved in intercellular adhesion have been identified, and relentless efforts continue to determine the full range of such proteins, especially those regulating these processes []. In particular, 27 genes have been identified in which mutations disrupt intercellular adhesion during collective migration. For example, p73, which is important for folliculogenesis of the ovaries, functions as a vital regulator of a gene network involved in cell-to-cell adhesion and migration []. The nuclear retinoic acid receptors (retinoic acid receptor alpha, RARα, retinoic acid receptor beta, RARβ, and retinoic acid receptor gamma, RARγ) are ligand-dependent transcription factors regulating the expression of genes related to cell differentiation and proliferation. A whole-genome analysis has been performed for the RAR-regulated genes in the mouse embryonic fibroblasts (MEFs) with a comparison of the wild type MEFs with MEFs having all three RARs knocked out []. The absence of RARs was found to be associated with cell adhesion, and the knock-out MEFs were unable to adhere and to spread on substrates and displayed a disrupted network of actin filaments.
Although a relationship between metabolism and cell adhesion has been reported, the exact molecular details of their interaction remain to be understood. Minsky et al. [] showed that PGC-1α, a major transcription co-activator of metabolic gene expression, takes part in inhibiting the expression of cell adhesion genes. Using cell lines, primary cells, and mice, the authors demonstrated that both endogenous and exogenous PGC-1α inhibited the expression of different cell adhesion molecules. In addition, PGC-1α modulates the adhesion of primary fibroblasts and the hepatic stellate cells to the proteins of the extracellular matrix. These results outlined the relationship between central pathways controlling metabolic regulation and cell adhesion and identified PGC-1α as one of the connecting links between these major cell networks []. The examples mentioned above show that although the problem is of fundamental interest, the data available now are too scarce. They do not allow deduction of the molecular genetic mechanisms of remodeling cytoskeletons and the formation of the ligand-receptor clusters in the process of cell-cell adhesion.
1.6. Circulating Cancer Cells Form Clusters through Tomo- and Heterotypic Intercellular Adhesions That Are Responsible for Metastasis and Possess the Stemness Property
Carcinoma cells can metastasize, still maintaining cell–cell contacts [,,]. One reason for this may be that the epithelial cancer cells use stromal cells during invasion [,] (see more detail below). Solid tumors secrete a large number of highly heterogeneous circulating tumor cells (CTCs) into the bloodstream [,,,,,,]. Still, only a small proportion of the CTCs (0.2% reported by Tripathi et al. []) can survive and ultimately result in metastatic changes. Efficient metastasis (>90% []) has been attributed to the CTCs clusters, sometimes referred to as the circulating tumor microemboli [], defined as groups of two or more aggregated CTCs. According to the estimates, tumor cells detach from the primary tumor at 3.2 × 106 cells per gram of tumor per day, but more than half of the detached tumor cells die. Approximately only one cell per 106–107 leukocytes remain []. The molecular mechanisms responsible for the formation and spread of clusters, and the pathways supporting their survival and metastatic potential remain mostly unknown [].
Most data on CTC clusters participation in metastasis describe homotypic clusters [,]. It is evident that adhesion and cytoskeleton processes actively participate in such kind of clusterization. Furthermore, changes in the cell adhesion properties are required to establish and maintain the trait of cancer cell stemness []. Persistent and adhesion-dependent survival signals in the CTC clusters can support the survival stimuli, thereby facilitating active metastases. While individual CTCs may experience problems with survival, such as oxidative stresses and immune effects, leading to apoptosis, the CTCs in clusters remain protected []. In particular, the CD44-dependent aggregation in blood circulation confers traits to the CTC clusters that are similar to those of cancer stem cells, which leads to a more efficient metastasis in the secondary organs [,].
However, CTCs can also contain other components, such as leukocytes, endothelial cells, platelets, and cancer-associated fibroblasts (CAFs) that provide a microenvironment favorable for survival []. The role of CAFs in metastasis has been widely studied [,,]. The interactions between CAFs and cancer cells were reported to produce a reciprocal and convergent set of signaling activities that promote cancer invasion and metastasis []. Santi et al. suggested that cancer and stromal cells of invasive tumors may have been in direct contact and may have established complex crosstalk during tumor development []. CAFs induce the formation of metastasizing clusters of tumor cells, with the participation of an intercellular adhesion []. According to the authors, CAFs may drive the formation of tumor cell clusters composed of two distinct cancer cell populations, one in a highly epithelial state and another in a hybrid epithelial/mesenchymal state and confer invasive and metastatic traits upon tumor cells. The stromal cell-derived factor 1 (SDF-1) and transforming growth factor-β (TGF-β) mediate the tumor cell cluster formation, invasion, and metastasis via Src activation. The authors also detected in cancer cells, CAFs induced cell–cell adhesion molecules (E-cad, CAM5, or CAM), causing the formation of tumor cell clusters. One can suggest that these same molecules take part in the adhesion between CAFs and tumor cells, providing a tight contact (synapse?) for efficient SDF-1 and TGF-β crosstalk. Following the above data, CAF, as has been shown [], can promote aggressive metastatic phenotypes of non-invasive bladder cancer cells through an EMT induced by the secretion of IL-6. A critical study [] showed that CAFs induced invasion through a heterophilic adhesion to both the participating N-cadherin on the membranes of CAFs and the E cadherin on the membranes of the cancer cells. The weakening of this adhesion blocked the ability of the CAFs to direct the collective migration of cells and cancer cell invasion. Nectins and afadin (organizers of cell contacts) were recruited simultaneously to interfaces between the CAFs and the cancer cells. These data suggest that active heterophilic adhesion between CAFs and cancer cells may lead to a cooperative tumor invasion. Contacts between the CAFs and the cancer cells may be formed due to interactions of the Eph-receptors and the corresponding ephrine ligands []. It suggests that these direct contacts may form synapse-like structures that may enhance the paracrine communications. One of these communication strategies may be the directed secretion of soluble growth factors and chemokines [].
A remarkable example of direct contacts between the stromal (the fibroblasts and the mesothelial cells) and the cancer cells can be seen in spheroids of the ovarian carcinoma ascites [,,,]. When in the abdominal cavity, tumor cells combine with the free-floating myofibroblast cells forming multicellular heterotypic spheroids. This enables the tumor cells to avoid anoikis and acquire a more invasive phenotype. Macrophages have also been demonstrated to play an active role in the formation of spheroids []. The multicellular spheroids attach to the mesothelial cells using various cell adhesion molecules. Adhesion molecules, including integrins and cadherins, mediate adhesion between cells and cell interaction with the extracellular matrix and play a role in the formation and metastasis of ovarian cancer []. However, the mechanisms of CAFs–cancer cell interactions during collective migration are still far from being investigated. In particular, the question of whether the signaling clusters are formed between the two entities remains untouched.
1.7. Why are CAFs “Chosen” for Cancer Cell Partners and Direct Contacts
Cancer-associated fibroblasts (CAFs) are ideal stromal partners for the collective invasion of cancer cells [,]. The CAFs were shown to be one of the predominant cell types in the stroma [,,,,,]. They are a heterogeneous cell “family” or a “group” demonstrating mesenchymal-like properties. CAFs are often close to or in direct contact with the tumor cells [,,,]. However, only a few studies have provided experimental data supporting the direct interaction of CAFs and cancer cells and its functional consequences. It has been hypothesized that the transformation of normal fibroblasts into CAFs occurs due to the continuous signals from the malignant cells [,,,]. In response, CAF populations produce paracrine signals, which affect cancer progression. The most evident and important consequence of such an interaction is the involvement of CAFs in the stimulation of EMT of cancer cells, as well as in their invasion and metastasis [,,,,,,], as a special case of collective cell migration typical for multicellular organisms []. Gaggioli et al. [] showed that in collectively invading co-cultures of the squamous cell carcinoma (SCC) cells and the stromal fibroblasts, the leading cells were always the fibroblasts. The special tests demonstrated that the invasion by the SCC cells requires either proximity to or direct contact with the CAFs. One more argument for this can be found in the review by Yamaguchi et al. [].
To study the input of the direct intercellular contacts and the paracrine signal factors in the metastasis of the non-small cell lung carcinoma (NSCLC) cells, Choe et al. [] used two variants of co-culturing. These included direct co-culturing of an NSCLC cell line with the primary CAF cultures from patients with a resected NSCLC, and an indirect co-culturing with a permeable membrane. In these experiments, the CAFs induced an EMT more actively in direct co-culturing, indicating that physical contacts between the NSCLC cells and the CAFs can control the metastatic potential of the NSCLC cells. It does not exclude the possible role of the paracrine interaction. This is enhanced by the physical cell interactions similar to that in immunological synapses. A review by Santi et al. [] contains data showing that the CAFs adjacent to the cancerous regions can increase the invasiveness of the cancer cells under cell–cell interactions assisted by various pro-invasive molecules, such as cytokines, chemokines, and inflammatory mediators.
The malicious role of the direct CAF contacts with the cancer cells makes the disruption of these contacts an important target for cancer therapy. Yamaguchi et al. [] attempted to find the inhibitors of direct interactions between the CAFs and the cancer cells. They found that the Src inhibitor, Dasatinib, efficiently blocked the physical bonds between the CAFs and the scirrhous gastric cancer (SGC) cells with a minimal cytotoxic effect. Dasatinib was also effective against the peritoneal dissemination of SGC cells in a mouse model. According to histological analysis, mice treated with Dasatinib were found to contain fewer metastasizing tumors associated with the stromal fibroblasts than in the controls. It implies that the direct interaction between the CAFs and the SGC cells can be a target for anti-metastasis therapy []. However, the authors recommended that the results be treated with caution as a decrease in CAF levels led to a faster progression of pancreatic cancer. Despite the inconsistency of these results, they emphasized the requirement for safety tests for inhibitors of the CAF–cancer cell interactions in anti-cancer therapy. In contrast, using the CAF–cancer contacts instead of the CAFs as therapeutic targets is a safer approach, as this strategy will not affect the CAF levels.
2. Conclusions
The Power of Clusters in Signal Transmission, and Their Vulnerability to a Directed Disruption
Any intercellular recognition between two membranes may include hundreds, possibly thousands, of receptors that may enhance the avidity of intercellular contacts to a level sufficient to trigger a signal event [,,].
Synapse-like structures can be identified by several features:
- The proximity of the interacting cells.
- The presence of receptor clusters and corresponding ligands on the interacting cells.
- The presence of strong interactions that allow cancer cells to migrate together with the stromal cells within circulating clusters.
- A remodeled cytoskeleton in the interacting cells.
- Characteristic changes in the transcription regulation [] and possible epigenetic changes.
The detection of synapse-like structures that emerge during the interaction of cancer and the stromal cells, mostly with the CAFs, will open a new dimension in cancer treatment. This may supplement the immune checkpoint therapy, which is also targeted at disrupting synapses between the cancer cells and cells of the immune system. The formation of the clusters suggests that several different incoming signals could already be integrated at the plasma membrane level via direct allosteric interactions between the protomers that form the cluster []. It should lead to the emergence of new unpredictable features different from those expected from the properties of the interacting monomeric ligand-receptor pairs. The elucidation of these properties can open new therapeutic horizons. The proximity of adhesion molecules in clusters in itself opens up new possibilities for therapeutic agents directed at nearby receptor-ligand pairs in the clusters. For example, the application of bivalent ligands composed of two functional pharmacophores linked by a spacer. This is considered in pharmacology as one of the most promising strategies for the treatment of homo or heterodimeric receptors (see, for example, [,]. Such kind of therapy may be a new way of tumor destruction.
The above pertains to cancerous tumors and their metastasis, and there is no doubt that these processes involve many, if not all, cells of the stromal environment of cancer. Studies will be needed on the selection of the most “malicious” partners of the cancer cells that protect them from a therapeutic action and facilitate their proliferation and metastasis. Among these partners are CAFs that, as suggested in this review, may interact with the cancer cells forming synapse-like structures. It justifies the title of a paper []: “Cancer-associated-fibroblasts and tumor cells: a diabolic liaison driving cancer progression”. Disrupting these detrimental connections is a challenging but still achievable and promising task.
Author Contributions
Author Contributions: E.D.S.—conceptualization and original draft preparation. I.V.A.—visualization, I.V.A., I.P.C., and S.V.K.—design and editing manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
RFBR funded the reported study according to the research project № 17-00-00194 (17-00-00190) and the Russian Science Foundation project № 19-15-00317.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ledford, H. End of cancer-genome project prompts rethink. Nature 2015, 517, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov, E.D. Systems biology and personalized medicine: To be or not to be? Ross. Fiziol. Zhurnal Im. IM Sechenova 2014, 100, 505–541. [Google Scholar]
- Sverdlov, E.D. Genetic surgery—A right strategy to attack cancer. Curr. Gene Ther. 2011, 11, 501–531. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, M. The microcosmos of intratumor heterogeneity: The space-time of cancer evolution. Oncogene 2019, 39, 2031–2039. [Google Scholar] [CrossRef]
- Mallick, P. Physical Sciences and Engineering Advances in Life Sciences and Oncology; Springer International Publishing: New York, NY, USA, 2015; pp. 5–29. [Google Scholar]
- Noble, D. A theory of biological relativity: No privileged level of causation. Interface Focus 2012, 2, 55–64. [Google Scholar] [CrossRef]
- Noble, D. A biological relativity view of the relationships between genomes and phenotypes. Prog. Biophys. Mol. Biol. 2013, 111, 59–65. [Google Scholar] [CrossRef]
- Rickles, D.; Hawe, P.; Shiell, A. A simple guide to chaos and complexity. J. Epidemiol. Community Health 2007, 61, 933–937. [Google Scholar] [CrossRef]
- Suki, B.; Bates, J.H.; Frey, U. Complexity and emergent phenomena. Compr. Physiol. 2011, 1, 995–1029. [Google Scholar]
- Gershenson, C. Facing Complexity: Prediction vs. Adaptation. In Complexity Perspectives on Language, Communication and Society; Massip-Bonet, À., Bastardas-Boada, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–14. Available online: http://arxiv.org/ftp/arxiv/papers/1112/1112.3843.pdf (accessed on 2 February 2020).
- Sverdlov, E. Transcribed Junk Remains Junk If It Does Not Acquire A Selected Function in Evolution. Bioessays 2017, 39, 1700164. [Google Scholar] [CrossRef]
- Sverdlov, E.D. Multidimensional Complexity of Cancer. Simple Solutions Are Needed. Biochemistry (Mosc) 2016, 81, 731–738. [Google Scholar] [CrossRef]
- Frank, S.A. Evolution in health and medicine Sackler colloquium: Somatic evolutionary genomics: Mutations during development cause highly variable genetic mosaicism with risk of cancer and neurodegeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Prahallad, A.; Bernards, R. Opportunities and challenges provided by crosstalk between signalling pathways in cancer. Oncogene 2015, 35, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Merlo, L.M.; Pepper, J.W.; Reid, B.J.; Maley, C.C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 2006, 6, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tannock, I.F.; Hickman, J.A. Limits to Personalized Cancer Medicine. N. Engl. J. Med. 2016, 375, 1289–1294. [Google Scholar] [CrossRef]
- Prasad, V. Perspective: The precision-oncology illusion. Nature 2016, 537, S63. [Google Scholar] [CrossRef]
- Gillies, R.J.; Verduzco, D.; Gatenby, R.A. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer 2012, 12, 487–493. [Google Scholar] [CrossRef]
- Bhome, R.; Bullock, M.D.; Al Saihati, H.A.; Goh, R.W.; Primrose, J.N.; Sayan, A.E.; Mirnezami, A.H. A top-down view of the tumor microenvironment: Structure, cells and signaling. Front. Cell Dev. Biol. 2015, 3, 33. [Google Scholar] [CrossRef]
- Bhome, R.; Al Saihati, H.A.; Goh, R.W.; Bullock, M.D.; Primrose, J.N.; Thomas, G.J.; Sayan, A.E.; Mirnezami, A.H. Translational aspects in targeting the stromal tumour microenvironment: From bench to bedside. New Horiz. Transl. Med. 2016, 3, 9–21. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Kalluri, R. A peek into cancer-associated fibroblasts: Origins, functions and translational impact. Dis. Model. Mech. 2018, 11, dmm029447. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Moscat, J.; Diaz-Meco, M. Metabolism shapes the tumor microenvironment. Curr. Opin. Cell Biol. 2017, 48, 47–53. [Google Scholar] [CrossRef]
- Orimo, A.; Weinberg, R.A. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle 2006, 5, 1597–1601. [Google Scholar] [CrossRef]
- Sverdlov, E. Missed Druggable Cancer Hallmark: Cancer-Stroma Symbiotic Crosstalk as Paradigm and Hypothesis for Cancer Therapy. Bioessays 2018, 40, 1800079. [Google Scholar] [CrossRef]
- Gonda, T.A.; Varro, A.; Wang, T.C.; Tycko, B. Molecular biology of cancer-associated fibroblasts: Can these cells be targeted in anti-cancer therapy? Semin. Cell Dev. Biol. 2010, 21, 2–10. [Google Scholar] [CrossRef]
- De Palma, M.; Hanahan, D. The biology of personalized cancer medicine: Facing individual complexities underlying hallmark capabilities. Mol. Oncol. 2012, 6, 111–127. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: Challenges and opportunities. BMC Med. 2015, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Gandellini, P.; Andriani, F.; Merlino, G.; D’Aiuto, F.; Roz, L.; Callari, M. Complexity in the tumour microenvironment: Cancer associated fibroblast gene expression patterns identify both common and unique features of tumour-stroma crosstalk across cancer types. Semin. Cancer Biol. 2015, 35, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Walter, S.; Walzl, A.; Kramer, N.; Unger, C.; Scherzer, M.; Unterleuthner, D.; Hengstschlager, M.; Krupitza, G.; Dolznig, H. Increased complexity in carcinomas: Analyzing and modeling the interaction of human cancer cells with their microenvironment. Semin. Cancer Biol. 2015, 35, 107–124. [Google Scholar] [CrossRef]
- Zi, F.; He, J.; He, D.; Li, Y.; Yang, L.; Cai, Z. Fibroblast activation protein alpha in tumor microenvironment: Recent progression and implications (review). Mol. Med. Rep. 2015, 11, 3203–3211. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Dazzi, F. Classification and biology of tumour associated stromal cells. Immunol. Lett. 2015, 168, 175–182. [Google Scholar] [CrossRef]
- Perrimon, N.; Pitsouli, C.; Shilo, B.Z. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb. Perspect. Biol. 2012, 4, a005975. [Google Scholar] [CrossRef]
- Bizzarri, M.; Cucina, A. Tumor and the microenvironment: A chance to reframe the paradigm of carcinogenesis? BioMed Res. Int. 2014, 2014, 934038. [Google Scholar] [CrossRef]
- Guo, F.; Wang, Y.; Liu, J.; Mok, S.C.; Xue, F.; Zhang, W. CXCL12/CXCR4: A symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 2016, 35, 816–826. [Google Scholar] [CrossRef]
- Sluka, P.; Davis, I.D. Cell mates: Paracrine and stromal targets for prostate cancer therapy. Nat. Rev. Urol. 2013, 10, 441–451. [Google Scholar] [CrossRef]
- Baluska, F.M.S. Synaptic view of eukaryotic cell. Int. J. Gen. Syst. 2014, 43, 740–756. [Google Scholar] [CrossRef]
- Sadelain, M.; Riviere, I.; Brentjens, R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 2003, 3, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Rumio, C.; Corti, A. Tumor cell-associated immune checkpoint molecules—Drivers of malignancy and stemness. Biochim. Biophys. Acta 2017, 1868, 571–583. [Google Scholar] [CrossRef]
- Meissner, J.M.; Sikorski, A.F.; Nawara, T.; Grzesiak, J.; Marycz, K.; Boguslawska, D.M.; Michalczyk, I.; Lecomte, M.C.; Machnicka, B. alphaII-spectrin in T cells is involved in the regulation of cell-cell contact leading to immunological synapse formation? PLoS ONE 2017, 12, e0189545. [Google Scholar] [CrossRef]
- Angus, K.L.; Griffiths, G.M. Cell polarisation and the immunological synapse. Curr. Opin. Cell Biol. 2013, 25, 85–91. [Google Scholar] [CrossRef]
- Ritter, A.T.; Angus, K.L.; Griffiths, G.M. The role of the cytoskeleton at the immunological synapse. Immunol. Rev. 2013, 256, 107–117. [Google Scholar] [CrossRef]
- Dustin, M.L. The immunological synapse. Cancer Immunol. Res. 2014, 2, 1023–1033. [Google Scholar] [CrossRef]
- Bertrand, F.; Muller, S.; Roh, K.H.; Laurent, C.; Dupre, L.; Valitutti, S. An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proc. Natl. Acad. Sci. USA 2013, 110, 6073–6078. [Google Scholar] [CrossRef]
- Hafner, A.E.; Rieger, H. Spatial Cytoskeleton Organization Supports Targeted Intracellular Transport. Biophys. J. 2018, 114, 1420–1432. [Google Scholar] [CrossRef]
- Xie, J.; Tato, C.M.; Davis, M.M. How the immune system talks to itself: The varied role of synapses. Immunol. Rev. 2013, 251, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Sanchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 2004, 4, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L.; Baldari, C.T. The Immune Synapse: Past, Present, and Future. Methods Mol. Biol. 2017, 1584, 1–5. [Google Scholar] [PubMed]
- Dustin, M.L.; Choudhuri, K. Signaling and Polarized Communication across the T Cell Immunological Synapse. Annu. Rev. Cell Dev. Biol. 2016, 32, 303–325. [Google Scholar] [CrossRef]
- Terry, S.; Savagner, P.; Ortiz-Cuaran, S.; Mahjoubi, L.; Saintigny, P.; Thiery, J.P.; Chouaib, S. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 2017, 11, 824–846. [Google Scholar] [CrossRef]
- Pettmann, J.; Santos, A.M.; Dushek, O.; Davis, S.J. Membrane Ultrastructure and T Cell Activation. Front. Immunol. 2018, 9, 2152. [Google Scholar] [CrossRef]
- Fooksman, D.R.; Vardhana, S.; Vasiliver-Shamis, G.; Liese, J.; Blair, D.A.; Waite, J.; Sacristan, C.; Victora, G.D.; Zanin-Zhorov, A.; Dustin, M.L. Functional anatomy of T cell activation and synapse formation. Annu. Rev. Immunol. 2010, 28, 79–105. [Google Scholar] [CrossRef]
- Mayya, V.; Judokusumo, E.; Abu Shah, E.; Peel, C.G.; Neiswanger, W.; Depoil, D.; Blair, D.A.; Wiggins, C.H.; Kam, L.C.; Dustin, M.L. Durable Interactions of T Cells with T Cell Receptor Stimuli in the Absence of a Stable Immunological Synapse. Cell Rep. 2018, 22, 340–349. [Google Scholar] [CrossRef]
- Dustin, M.L. A dynamic view of the immunological synapse. Semin. Immunol. 2005, 17, 400–410. [Google Scholar] [CrossRef]
- Calvo, V.; Izquierdo, M. Imaging Polarized Secretory Traffic at the Immune Synapse in Livin T Lymphocytes. Front. Immunol. 2018, 9, 684. [Google Scholar] [CrossRef]
- Shi, Y. To forge a solid immune recognition. Protein Cell 2012, 3, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.J. Signal initiation in biological systems: The properties and detection of transient extracellular protein interactions. Mol. Biosyst. 2009, 5, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Cebecauer, M.; Spitaler, M.; Serge, A.; Magee, A.I. Signalling complexes and clusters: Functional advantages and methodological hurdles. J. Cell Sci. 2010, 123, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Mege, R.M.; Ishiyama, N. Integration of Cadherin Adhesion and Cytoskeleton at Adherens Junctions. Cold Spring Harb. Perspect. Biol. 2017, 9, a028738. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Parajo, M.F.; Cambi, A.; Torreno-Pina, J.A.; Thompson, N.; Jacobson, K. Nanoclustering as a dominant feature of plasma membrane organization. J. Cell Sci. 2014, 127, 4995–5005. [Google Scholar] [CrossRef]
- Hartman, N.C.; Groves, J.T. Signaling clusters in the cell membrane. Curr. Opin. Cell Biol. 2011, 23, 370–376. [Google Scholar] [CrossRef]
- Yap, A.S.; Gomez, G.A.; Parton, R.G. Adherens Junctions Revisualized: Organizing Cadherins as Nanoassemblies. Dev. Cell 2015, 35, 12–20. [Google Scholar] [CrossRef]
- Nussinov, R.; Jang, H. Dynamic multiprotein assemblies shape the spatial structure of cell signaling. Prog. Biophys. Mol. Biol. 2014, 116, 158–164. [Google Scholar] [CrossRef]
- Nussinov, R.; Jang, H.; Tsai, C.J. Oligomerization and nanocluster organization render specificity. Biol. Rev. Camb. Philos. Soc. 2015, 90, 587–598. [Google Scholar] [CrossRef]
- Hatherley, D.; Lea, S.M.; Johnson, S.; Barclay, A.N. Structures of CD200/CD200 receptor family and implications for topology, regulation, and evolution. Structure 2013, 21, 820–832. [Google Scholar] [CrossRef]
- Wright, G.J.; Puklavec, M.J.; Willis, A.C.; Hoek, R.M.; Sedgwick, J.D.; Brown, M.H.; Barclay, A.N. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 2000, 13, 233–242. [Google Scholar] [CrossRef]
- Serge, A. The Molecular Architecture of Cell Adhesion: Dynamic Remodeling Revealed by Videonanoscopy. Front. Cell Dev. Biol. 2016, 4, 413. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.D.; Yap, A.S. Towards a Dynamic Understanding of Cadherin-Based Mechanobiology. Trends Cell Biol. 2015, 25, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, A.; Gottardi, C.J.; Yap, A.S. Cadherins and cancer: How does cadherin dysfunction promote tumor progression? Oncogene 2008, 27, 6920–6929. [Google Scholar] [CrossRef] [PubMed]
- McKayed, K.K.; Simpson, J.C. Actin in action: Imaging approaches to study cytoskeleton structure and function. Cells 2013, 2, 715–731. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, X.; Li, F.; Xiao, D.; Hou, Y.; Zhu, S.; Liu, D.; Ye, X.; Ye, M.; Yang, J.; et al. Frequent alterations in cytoskeleton remodelling genes in primary and metastatic lung adenocarcinomas. Nat. Commun. 2015, 6, 10131. [Google Scholar] [CrossRef]
- Soini, T.; Eloranta, K.; Pihlajoki, M.; Kyronlahti, A.; Akinrinade, O.; Andersson, N.; Lohi, J.; Pakarinen, M.P.; Wilson, D.B.; Heikinheimo, M. Transcription factor GATA4 associates with mesenchymal-like gene expression in human hepatoblastoma cells. Tumour. Biol. 2018, 40, 1010428318785498. [Google Scholar] [CrossRef]
- Gallegos, L.L.; Ng, M.R.; Sowa, M.E.; Selfors, L.M.; White, A.; Zervantonakis, I.K.; Singh, P.; Dhakal, S.; Harper, J.W.; Brugge, J.S. A protein interaction map for cell-cell adhesion regulators identifies DUSP23 as a novel phosphatase for beta-catenin. Sci. Rep. 2016, 6, 1–15. [Google Scholar]
- Santos Guasch, G.L.; Beeler, J.S.; Marshall, C.B.; Shaver, T.M.; Sheng, Q.; Johnson, K.N.; Boyd, K.L.; Venters, B.J.; Cook, R.S.; Pietenpol, J.A. p73 Is Required for Ovarian Follicle Development and Regulates a Gene Network Involved in Cell-to-Cell Adhesion. iScience 2018, 8, 236–249. [Google Scholar] [CrossRef]
- Al Tanoury, Z.; Piskunov, A.; Andriamoratsiresy, D.; Gaouar, S.; Lutzing, R.; Ye, T.; Jost, B.; Keime, C.; Rochette-Egly, C. Genes involved in cell adhesion and signaling: A new repertoire of retinoic acid receptor target genes in mouse embryonic fibroblasts. J. Cell Sci. 2014, 127, 521–533. [Google Scholar] [CrossRef]
- Minsky, N.; Roeder, R.G. Inhibition of Adhesion Molecule Gene Expression and Cell Adhesion by the Metabolic Regulator PGC-1alpha. PLoS ONE 2016, 11, e0165598. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Mayor, R. Tuning Collective Cell Migration by Cell–Cell Junction Regulation. Cold Spring Harb. Perspect. Biol. 2017, 9, a029199. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Gao, D.; Redfern, A.; Thompson, E.W. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer. 2019, 19, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Harney, A.S.; Arwert, E.N.; Entenberg, D.; Wang, Y.; Guo, P.; Qian, B.Z.; Oktay, M.H.; Pollard, J.W.; Jones, J.G.; Condeelis, J.S. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015, 5, 932–943. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112. [Google Scholar] [CrossRef]
- Hong, Y.; Fang, F.; Zhang, Q. Circulating tumor cell clusters: What we know and what we expect (Review). Int. J. Oncol. 2016, 49, 2206–2216. [Google Scholar] [CrossRef]
- Yu, M. Metastasis Stemming from Circulating Tumor Cell Clusters. Trends Cell Biol. 2019, 29, 275–276. [Google Scholar] [CrossRef]
- Giuliano, M.; Shaikh, A.; Lo, H.C.; Arpino, G.; De Placido, S.; Zhang, X.H.; Cristofanilli, M.; Schiff, R.; Trivedi, M.V. Perspective on Circulating Tumor Cell Clusters: Why It Takes a Village to Metastasize. Cancer Res. 2018, 78, 845–852. [Google Scholar] [CrossRef]
- Wang, W.C.; Zhang, X.F.; Peng, J.; Li, X.F.; Wang, A.L.; Bie, Y.Q.; Shi, L.H.; Lin, M.B.; Zhang, X.F. Survival Mechanisms and Influence Factors of Circulating Tumor Cells. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.C.; Martin, S.S. Insights on CTC Biology and Clinical Impact Emerging from Advances in Capture Technology. Cells 2019, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Agnoletto, C.; Corra, F.; Minotti, L.; Baldassari, F.; Crudele, F.; Cook, W.J.J.; Di Leva, G.; d’Adamo, A.P.; Gasparini, P.; Volinia, S. Heterogeneity in Circulating Tumor Cells: The Relevance of the Stem-Cell Subset. Cancers 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Jolly, M.K.; Woodward, W.A.; Levine, H.; Deem, M.W. Analysis of Hierarchical Organization in Gene Expression Networks Reveals Underlying Principles of Collective Tumor Cell Dissemination and Metastatic Aggressiveness of Inflammatory Breast Cancer. Front. Oncol. 2018, 8, 244. [Google Scholar] [CrossRef]
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B.; et al. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov. 2019, 9, 96–113. [Google Scholar] [CrossRef]
- Rodrigues, P.; Vanharanta, S. Circulating Tumor Cells: Come Together, Right Now, Over Metastasis. Cancer Discov. 2019, 9, 22–24. [Google Scholar] [CrossRef]
- Basu, S.; Cheriyamundath, S.; Ben-Ze’ev, A. Cell-cell adhesion: Linking Wnt/beta-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Santi, A.; Kugeratski, F.G.; Zanivan, S. Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics 2018, 18, 1700167. [Google Scholar] [CrossRef]
- Marsh, T.; Pietras, K.; McAllister, S.S. Fibroblasts as architects of cancer pathogenesis. Biochim. Biophys. Acta 2013, 1832, 1070–1078. [Google Scholar] [CrossRef]
- Kwa, M.Q.; Herum, K.M.; Brakebusch, C. Cancer-associated fibroblasts: How do they contribute to metastasis? Clin. Exp. Metastasis 2019, 36, 71–86. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ito, Y.; Mezawa, Y.; Sulidan, K.; Daigo, Y.; Hiraga, T.; Mogushi, K.; Wali, N.; Suzuki, H.; Itoh, T.; et al. Stromal fibroblasts induce metastatic tumor cell clusters via epithelial-mesenchymal plasticity. Life Sci. Alliance 2019, 2, e201900425. [Google Scholar] [CrossRef] [PubMed]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Labernadie, A.; Kato, T.; Brugues, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; Gonzalez-Tarrago, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Cancer cells exploit the Eph-ephrin system to promote invasion and metastasis: Tales of unwitting partners. Sci. Signal. 2011, 4, pe28. [Google Scholar] [CrossRef]
- Mattes, B.; Scholpp, S. Emerging role of contact-mediated cell communication in tissue development and diseases. Histochem. Cell Biol. 2018, 150, 431–442. [Google Scholar] [CrossRef]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef]
- Lupia, M.; Cavallaro, U. Ovarian cancer stem cells: Still an elusive entity? Mol. Cancer 2017, 16, 64. [Google Scholar] [CrossRef]
- Ghoneum, A.; Afify, H.; Salih, Z.; Kelly, M.; Said, N. Role of tumor microenvironment in the pathobiology of ovarian cancer: Insights and therapeutic opportunities. Cancer Med. 2018, 7, 5047–5056. [Google Scholar] [CrossRef]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; Karaminejadranjbar, M.; Hu, Z.; et al. An evolving story of the metastatic voyage of ovarian cancer cells: Cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 2019, 38, 2885–2898. [Google Scholar] [CrossRef]
- Azimian-Zavareh, V.; Hossein, G.; Ebrahimi, M.; Dehghani-Ghobadi, Z. Wnt11 alters integrin and cadherin expression by ovarian cancer spheroids and inhibits tumorigenesis and metastasis. Exp. Cell Res. 2018, 369, 90–104. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef] [PubMed]
- De Wever, O.; Van Bockstal, M.; Mareel, M.; Hendrix, A.; Bracke, M. Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Semin. Cancer Biol. 2014, 25, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Heneberg, P. Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Crit. Rev. Oncol. Hematol. 2016, 97, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Augsten, M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front. Oncol. 2014, 4, 62. [Google Scholar] [CrossRef]
- Tao, L.; Huang, G.; Song, H.; Chen, Y.; Chen, L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol. Lett. 2017, 14, 2611–2620. [Google Scholar] [CrossRef]
- Semba, S.; Kodama, Y.; Ohnuma, K.; Mizuuchi, E.; Masuda, R.; Yashiro, M.; Hirakawa, K.; Yokozaki, H. Direct cancer-stromal interaction increases fibroblast proliferation and enhances invasive properties of scirrhous-type gastric carcinoma cells. Br. J. Cancer 2009, 101, 1365–1373. [Google Scholar] [CrossRef]
- Choe, C.; Shin, Y.S.; Kim, S.H.; Jeon, M.J.; Choi, S.J.; Lee, J.; Kim, J. Tumor-stromal interactions with direct cell contacts enhance motility of non-small cell lung cancer cells through the hedgehog signaling pathway. Anticancer. Res. 2013, 33, 3715–3723. [Google Scholar]
- He, X.J.; Tao, H.Q.; Hu, Z.M.; Ma, Y.Y.; Xu, J.; Wang, H.J.; Xia, Y.J.; Li, L.; Fei, B.Y.; Li, Y.Q.; et al. Expression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin beta1. Cancer Sci. 2014, 105, 1402–1410. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Sakai, R. Direct Interaction between Carcinoma Cells and Cancer Associated Fibroblasts for the Regulation of Cancer Invasion. Cancers 2015, 7, 2054–2062. [Google Scholar] [CrossRef]
- Theveneau, E.; Linker, C. Leaders in collective migration: Are front cells really endowed with a particular set of skills? F1000Research 2017, 6, 1899. [Google Scholar] [CrossRef] [PubMed]
- Jaqaman, K.; Grinstein, S. Regulation from within: The cytoskeleton in transmembrane signaling. Trends Cell Biol. 2012, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L. Receptor-Receptor Interactions as a Widespread Phenomenon: Novel Targets for Drug Development? Front. Endocrinol. (Lausanne) 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Berque-Bestel, I.; Lezoualc’h, F.; Jockers, R. Bivalent ligands as specific pharmacological tools for G protein-coupled receptor dimers. Curr. Drug Discov. Technol. 2008, 5, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Cirri, P.; Chiarugi, P. Cancer-associated-fibroblasts and tumour cells: A diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012, 31, 195–208. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).