Safety and Activity of the Combination of Ceritinib and Dasatinib in Osteosarcoma
Abstract
:1. Introduction
2. Results
2.1. Drug Screening Identifies Ceritinib and Dasatinib as Potent Inhibitors of OS Proliferation In Vitro
2.2. Dosage of Dasatinib and Ceritinib in a Therapy Protocol
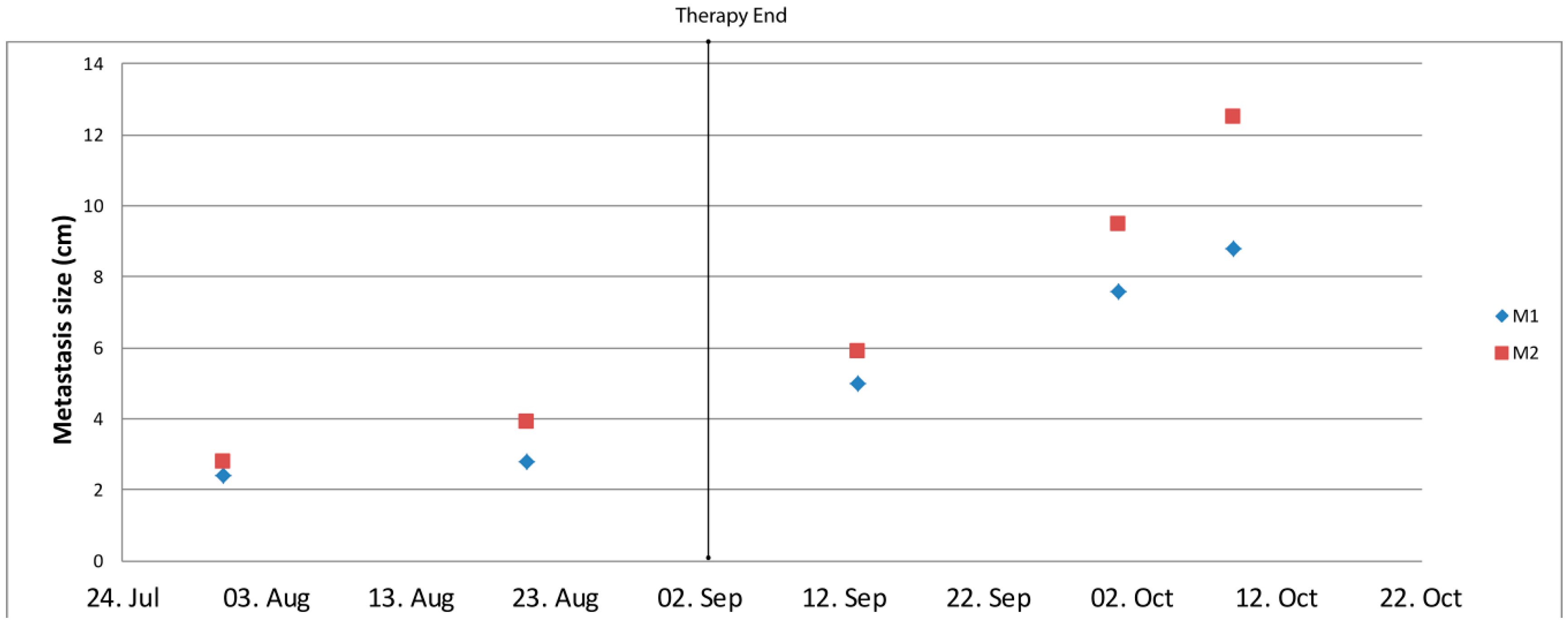
2.3. Activity of the Certinib/Dasatinib Combination
3. Discussion
4. Materials and Methods
4.1. Tumor Cells
4.2. Patient History Before Ceritinib/Dasatinib Therapy
4.3. DNA Sequencing
4.4. In Vitro Drug Screening
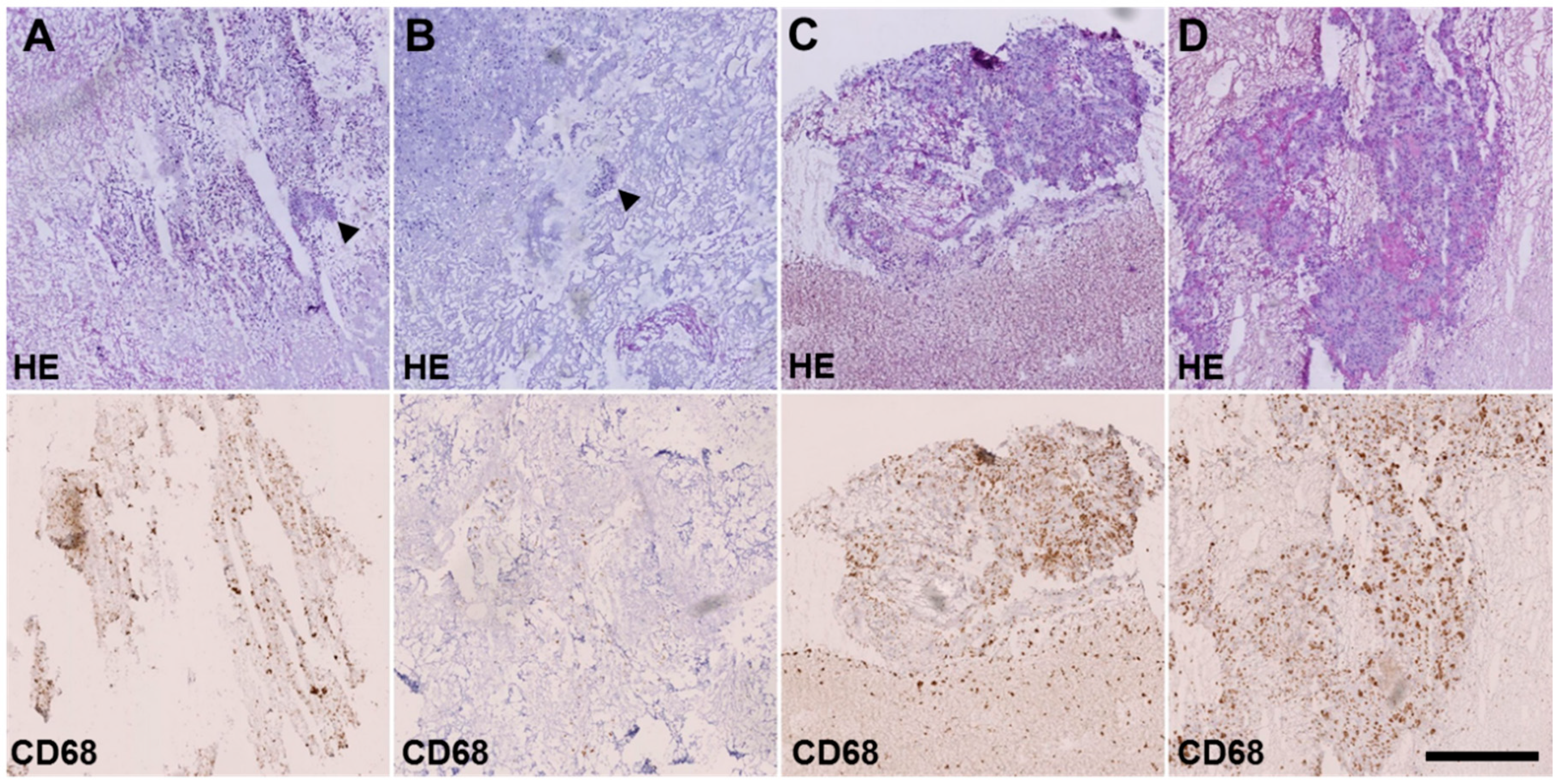
4.5. Histological Analysis
4.6. Phosphorylation Assay
4.7. Ceritinib and Dasatinib Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | AKT serine/threonine kinase 1 |
| AUC | area under the curve |
| ALK | anaplastic lymphoma kinase |
| CT | computed tomography |
| CYP | cytochrome P450 |
| EMA | European Medicines Agency |
| ERK | extracellular-signal regulated kinase |
| FAK | focal adhesion kinase |
| FDA | U.S. Food and Drug administration |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| IGF | insulin-like growth factor |
| IGF1R | insulin-like growth factor 1 receptor |
| IGFBP | insulin-like growth factor-binding protein |
| INSR | insulin receptor |
| LDH | lactate dehydrogenase |
| NSCLC | non small cell lung cancer |
| OS | osteosarcoma |
| PPP | picropodophyllin |
| Ph+ ALL | philadelphia chromosome-positive acute lymphoblastic leukemia |
| Ph+ CML | philadelphia chromosome–positive chronic myeloid leukemia |
| RECIST | Response evaluation criteria in solid tumors |
| ROS1 | ROS proto-oncogene 1 |
| Src | Proto-oncogene tyrosine-protein kinase Src |
| TKI | tyrosine kinase inhibitor |
| UPLC-MS/MS | ultra performance liquid chromatography—tandem mass spectrometer |
References
- Bielack, S.S.; Kempf-Bielack, B.; Branscheid, D.; Carrle, D.; Friedel, G.; Helmke, K.; Kevric, M.; Jundt, G.; Kuhne, T.; Maas, R.; et al. Second and subsequent recurrences of osteosarcoma: Presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J. Clin. Oncol. 2009, 27, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of osteosarcoma and current management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, D.C.; Carney, S.C.; Ahlmann, E.R.; Hendifar, A.; Chawla, S.; Fedenko, A.; Angeles, C.; Menendez, L.R. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012, 2012, 704872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overholtzer, M.; Rao, P.H.; Favis, R.; Lu, X.Y.; Elowitz, M.B.; Barany, F.; Ladanyi, M.; Gorlick, R.; Levine, A.J. The presence of p53 mutations in human osteosarcomas correlates with high levels of genomic instability. Proc. Natl. Acad. Sci. USA 2003, 100, 11547–11552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, N.; Le Deley, M.C.; Piperno-Neumann, S.; Marec-Berard, P.; Italiano, A.; Corradini, N.; Bellera, C.; Brugieres, L.; Gaspar, N. Phase-II trials in osteosarcoma recurrences: A systematic review of past experience. Eur. J. Cancer 2017, 75, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Han, X.D.; Qiu, Y.; Xiong, J.; Yu, Y.; Wang, B.; Zhu, Z.Z.; Qian, B.P.; Chen, Y.X.; Wang, S.F.; et al. Increased expression of insulin-like growth factor-1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J. Surg. Oncol. 2012, 105, 235–243. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Haase, K.; Ye, H.; Young, M.D.; Alexandrov, L.B.; Farndon, S.J.; Collord, G.; Wedge, D.C.; Martincorena, I.; et al. Recurrent mutation of IGF signalling genes and distinct patterns of genomic rearrangement in osteosarcoma. Nat. Commun. 2017, 8, 15936. [Google Scholar] [CrossRef]
- Russo, A.; Paret, C.; Alt, F.; Burhenne, J.; Fresnais, M.; Wagner, W.; Glaser, M.; Bender, H.; Huprich, S.; Harter, P.N.; et al. Ceritinib-Induced regression of an insulin-like growth factor-driven neuroepithelial brain tumor. Int. J. Mol. Sci. 2019, 20, 4267. [Google Scholar] [CrossRef] [Green Version]
- Vewinger, N.; Huprich, S.; Seidmann, L.; Russo, A.; Alt, F.; Bender, H.; Sommer, C.; Samuel, D.; Lehmann, N.; Backes, N.; et al. IGF1R Is a potential new therapeutic target for HGNET-BCOR brain tumor patients. Int. J. Mol. Sci. 2019, 20, 3027. [Google Scholar] [CrossRef] [Green Version]
- Marsilje, T.H.; Pei, W.; Chen, B.; Lu, W.; Uno, T.; Jin, Y.; Jiang, T.; Kim, S.; Li, N.; Warmuth, M.; et al. Synthesis, structure-activity relationships, and In Vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J. Med. Chem. 2013, 56, 5675–5690. [Google Scholar] [CrossRef] [PubMed]
- van Erp, A.E.M.; Hillebrandt-Roeffen, M.H.S.; van Houdt, L.; Fleuren, E.D.G.; van der Graaf, W.T.A.; Versleijen-Jonkers, Y.M.H. Targeting anaplastic lymphoma kinase (ALK) in rhabdomyosarcoma (RMS) with the second-generation ALK inhibitor ceritinib. Target. Oncol. 2017, 12, 815–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, H.Y.; Yun, H.J.; Lee, J.S.; Lee, H.J.; Cho, J.; Jang, H.J.; Park, S.H.; Liu, D.; Oh, S.H.; Lee, J.J.; et al. Targeting the insulin-like growth factor receptor and Src signaling network for the treatment of non-small cell lung cancer. Mol. Cancer 2015, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Laschi, M.; Bernardini, G.; Geminiani, M.; Ghezzi, L.; Amato, L.; Braconi, D.; Millucci, L.; Frediani, B.; Spreafico, A.; Franchi, A.; et al. Establishment of four new human primary cell cultures from chemo-naive italian osteosarcoma patients. J. Cell. Physiol. 2015, 230, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Shor, A.C.; Keschman, E.A.; Lee, F.Y.; Muro-Cacho, C.; Letson, G.D.; Trent, J.C.; Pledger, W.J.; Jove, R. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res. 2007, 67, 2800–2808. [Google Scholar] [CrossRef] [Green Version]
- Friedman, A.A.; Letai, A.; Fisher, D.E.; Flaherty, K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 2015, 15, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, M.J.; Cooke, A.; Rosenfeld-Franklin, M.; Buck, E.; Foreman, K.; Landfair, D.; O’Connor, M.; Pirritt, C.; Sun, Y.; Yao, Y.; et al. Discovery of OSI-906: A selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med. Chem. 2009, 1, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Gable, K.L.; Maddux, B.A.; Penaranda, C.; Zavodovskaya, M.; Campbell, M.J.; Lobo, M.; Robinson, L.; Schow, S.; Kerner, J.A.; Goldfine, I.D.; et al. Diarylureas are small-molecule inhibitors of insulin-like growth factor I receptor signaling and breast cancer cell growth. Mol. Cancer Ther. 2006, 5, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girnita, A.; Girnita, L.; del Prete, F.; Bartolazzi, A.; Larsson, O.; Axelson, M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004, 64, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Poondru, S.; Chaves, J.; Yuen, G.; Parker, B.; Conklin, E.; Singh, M.; Nagata, M.; Gill, S. Mass balance, pharmacokinetics, and metabolism of linsitinib in cancer patients. Cancer Chemother. Pharmacol. 2016, 77, 829–837. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.W.; Mehra, R.; Tan, D.S.; Felip, E.; Chow, L.Q.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist-Nii, Y.; Eksborg, S.; Axelson, M.; Harmenberg, J.; Ekman, S.; Bergqvist, M.; Beck, O. Determination of picropodophyllin and its isomer podophyllotoxin in human serum samples with electrospray ionization of hexylamine adducts by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Zwaan, C.M.; Rizzari, C.; Mechinaud, F.; Lancaster, D.L.; Lehrnbecher, T.; van der Velden, V.H.; Beverloo, B.B.; den Boer, M.L.; Pieters, R.; Reinhardt, D.; et al. Dasatinib in children and adolescents with relapsed or refractory leukemia: Results of the CA180-018 phase I dose-escalation study of the innovative therapies for children with cancer consortium. J. Clin. Oncol. 2013, 31, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Kovac, M.; Blattmann, C.; Ribi, S.; Smida, J.; Mueller, N.S.; Engert, F.; Castro-Giner, F.; Weischenfeldt, J.; Kovacova, M.; Krieg, A.; et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat. Commun. 2015, 6, 8940. [Google Scholar] [CrossRef]
- Italiano, A.; Penel, N.; Toulmonde, M.; Bompas, E.; Piperno-Neumann, S.; Pulido, M.; Entz-Werle, N.; Le Cesne, A.; Chevreau, C.M.; Duffaud, F.; et al. LBA67Cabozantinib in patients with advanced osteosarcomas and Ewing sarcomas: A French Sarcoma Group (FSG)/US National Cancer Institute phase II collaborative study. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Geoerger, B.; Schulte, J.; Zwaan, C.M.; Casanova, M.; Fischer, M.; Moreno, L.; Trahair, T.; Jimenez, I.; Kang, H.J.; Pappo, A.S.; et al. Phase I study of ceritinib in pediatric patients (Pts) with malignancies harboring a genetic alteration in ALK (ALK+): Safety, pharmacokinetic (PK), and efficacy results. J. Clin. Oncol. 2015, 33, 10005. [Google Scholar] [CrossRef]
- Shah, N.P.; Kantarjian, H.M.; Kim, D.W.; Rea, D.; Dorlhiac-Llacer, P.E.; Milone, J.H.; Vela-Ojeda, J.; Silver, R.T.; Khoury, H.J.; Charbonnier, A.; et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and-intolerant chronic-phase chronic myeloid leukemia. J. Clin. Oncol. 2008, 26, 3204–3212. [Google Scholar] [CrossRef]
- Huvos, A.G.; Rosen, G.; Marcove, R.C. Primary osteogenic sarcoma: Pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch. Pathol. Lab. Med. 1977, 101, 14–18. [Google Scholar]
- Salzer-Kuntschik, M.; Delling, G.; Beron, G.; Sigmund, R. Morphological grades of regression in osteosarcoma after polychemotherapy—Study COSS 80. J. Cancer Res. Clin. Oncol. 1983, 106 (Suppl. 21–24). [Google Scholar] [CrossRef]
- Schuetze, S.M.; Wathen, J.K.; Lucas, D.R.; Choy, E.; Samuels, B.L.; Staddon, A.P.; Ganjoo, K.N.; von Mehren, M.; Chow, W.A.; Loeb, D.M.; et al. SARC009: Phase 2 study of dasatinib in patients with previously treated, high-grade, advanced sarcoma. Cancer 2016, 122, 868–874. [Google Scholar] [CrossRef]
- Pappo, A.S.; Vassal, G.; Crowley, J.J.; Bolejack, V.; Hogendoorn, P.C.; Chugh, R.; Ladanyi, M.; Grippo, J.F.; Dall, G.; Staddon, A.P.; et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: Results of a sarcoma alliance for research through collaboration study. Cancer 2014, 120, 2448–2456. [Google Scholar] [CrossRef] [Green Version]
- Guenther, L.M.; Rowe, R.G.; Acharya, P.T.; Swenson, D.W.; Meyer, S.C.; Clinton, C.M.; Guo, D.; Sridharan, M.; London, W.B.; Grier, H.E.; et al. Response evaluation criteria in solid tumors (RECIST) following neoadjuvant chemotherapy in osteosarcoma. Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef]
- Lo, P.C.; Dahlberg, S.E.; Nishino, M.; Johnson, B.E.; Sequist, L.V.; Jackman, D.M.; Janne, P.A.; Oxnard, G.R. Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer 2015, 121, 2570–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiken, R.; Axelson, M.; Harmenberg, J.; Klockare, M.; Larsson, O.; Wassberg, C. Phase I clinical trial of AXL1717 for treatment of relapsed malignant astrocytomas: Analysis of dose and response. Oncotarget 2017, 8, 81501–81510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekman, S.; Frodin, J.E.; Harmenberg, J.; Bergman, A.; Hedlund, A.; Dahg, P.; Alvfors, C.; Stahl, B.; Bergstrom, S.; Bergqvist, M. Clinical phase I study with an insulin-like growth factor-1 receptor inhibitor: Experiences in patients with squamous non-small cell lung carcinoma. Acta Oncol. 2011, 50, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiou, V.L.; Burotto, M. Pseudoprogression and immune-related response in solid tumors. J. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef] [Green Version]
- Dumars, C.; Ngyuen, J.M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.X.; Yao, Y. Metastatic osteosarcoma to the liver and the kidney: A case report and review of the literature. BMJ Case Rep. 2009, 2009. [Google Scholar] [CrossRef]
- Duan, Z.; Choy, E.; Harmon, D.; Yang, C.; Ryu, K.; Schwab, J.; Mankin, H.; Hornicek, F.J. Insulin-Like growth factor-I receptor tyrosine kinase inhibitor cyclolignan picropodophyllin inhibits proliferation and induces apoptosis in multidrug resistant osteosarcoma cell lines. Mol. Cancer Ther. 2009, 8, 2122–2130. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Yu, Y.; Zhu, Y.J.; Davis, S.; Walker, R.; Meltzer, P.S.; Helman, L.J.; Cao, L. Downregulation of IGFBP2 is associated with resistance to IGF1R therapy in rhabdomyosarcoma. Oncogene 2014, 33, 5697–5705. [Google Scholar] [CrossRef] [Green Version]
- Waraky, A.; Akopyan, K.; Parrow, V.; Stromberg, T.; Axelson, M.; Abrahmsen, L.; Lindqvist, A.; Larsson, O.; Aleem, E. Picropodophyllin causes mitotic arrest and catastrophe by depolymerizing microtubules via insulin-like growth factor-1 receptor-independent mechanism. Oncotarget 2014, 5, 8379–8392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, R.; Sasaki, T.; Minami, Y.; Hibino, Y.; Okumura, S.; Sado, M.; Miyokawa, N.; Hayashi, S.; Kitada, M.; Ohsaki, Y. Activation of Src signaling mediates acquired resistance to ALK inhibition in lung cancer. Int. J. Oncol. 2017, 51, 1533–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.; Fiandalo, M.V.; Pop, E.; Stocking, J.J.; Azabdaftari, G.; Li, J.; Wei, H.; Ma, D.; Qu, J.; Mohler, J.L.; et al. Proteomic analysis of charcoal-stripped fetal bovine serum reveals changes in the insulin-like growth factor signaling pathway. J. Proteome Res. 2018, 17, 2963–2977. [Google Scholar] [CrossRef]

| Sample no. | Gender | Age | Primary Tumor or Metastasis | Localization |
|---|---|---|---|---|
| 301 | m | 13 | Primary tumor | Distal femur left |
| 313 1 | m | 18 | Metastasis | Lung |
| 331 1 | m | 18 | Metastasis | Lung |
| 351 | f | 19 | Metastasis | Lung |
| 386 2 | f | 16 | Metastasis | Lung |
| 403 2 | f | 17 | Metastasis | Lung |
| 414 | m | 15 | Primary tumor | Proximal tibia left |
| 416 | m | 15 | Primary tumor | Proximal tibia left |
| Analysis | Sample | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Vital tumor area/total area [%] | 0.02 | 0.4 | 3.6 | 20.8 |
| Huvos grading * | III | III | II | II |
| Salzer-Kuntschik grading ** | II | II | III | IV |
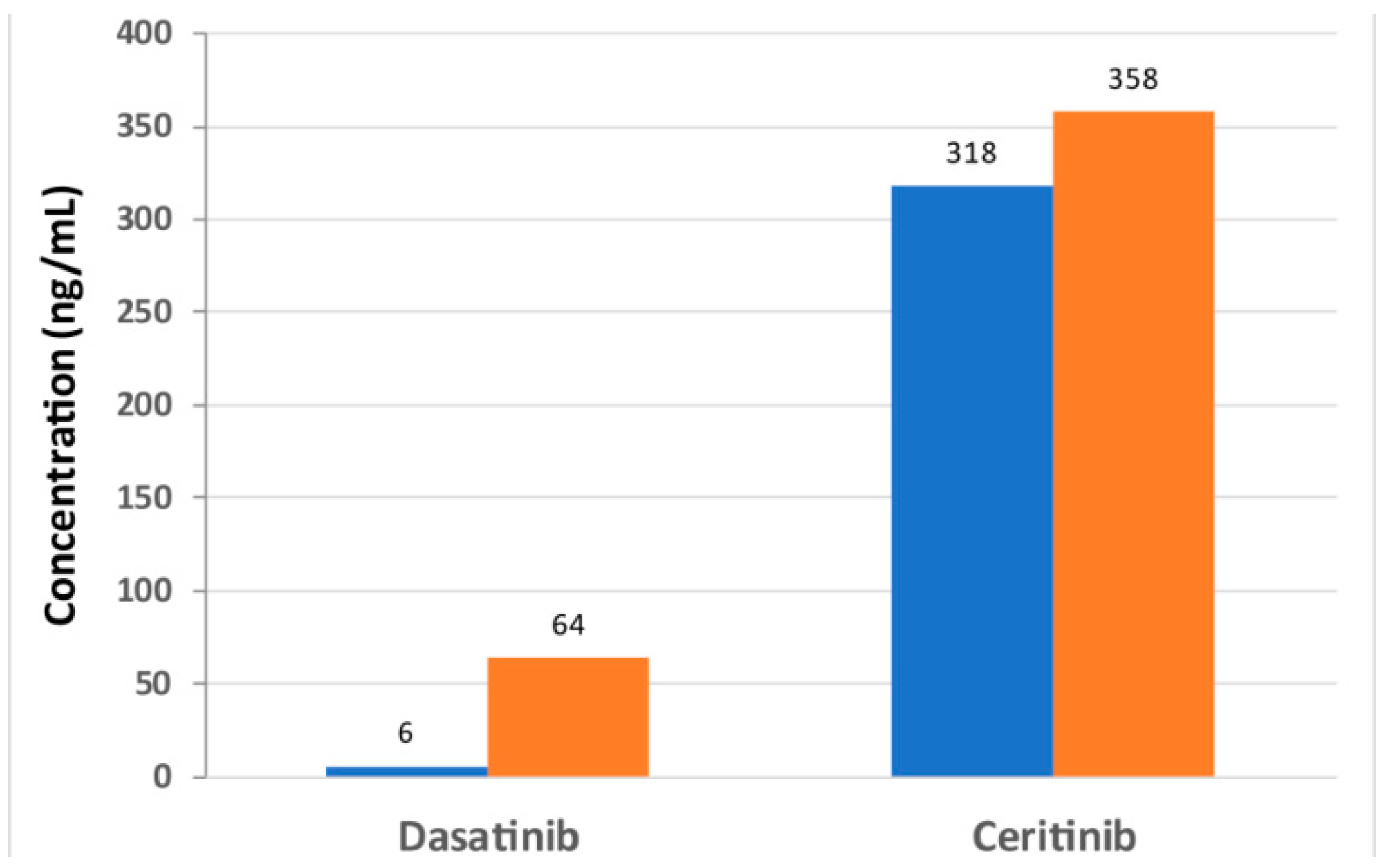
| Dasatinib concentration [ng/g] | 52.9 | 12.5 | 47 | 0 |
| Ceritinib concentration [ng/g] | 1703 | 1021 | 743 | 687 |
| % CD68 cells | 26.27% | 10.15% | 55.46% | 31.45% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, O.; Paret, C.; Russo, A.; Burhenne, J.; Fresnais, M.; Steimel, K.; Seidmann, L.; Wagner, D.-C.; Vewinger, N.; Lehmann, N.; et al. Safety and Activity of the Combination of Ceritinib and Dasatinib in Osteosarcoma. Cancers 2020, 12, 793. https://doi.org/10.3390/cancers12040793
Beck O, Paret C, Russo A, Burhenne J, Fresnais M, Steimel K, Seidmann L, Wagner D-C, Vewinger N, Lehmann N, et al. Safety and Activity of the Combination of Ceritinib and Dasatinib in Osteosarcoma. Cancers. 2020; 12(4):793. https://doi.org/10.3390/cancers12040793
Chicago/Turabian StyleBeck, Olaf, Claudia Paret, Alexandra Russo, Jürgen Burhenne, Margaux Fresnais, Kevin Steimel, Larissa Seidmann, Daniel-Christoph Wagner, Nadine Vewinger, Nadine Lehmann, and et al. 2020. "Safety and Activity of the Combination of Ceritinib and Dasatinib in Osteosarcoma" Cancers 12, no. 4: 793. https://doi.org/10.3390/cancers12040793
APA StyleBeck, O., Paret, C., Russo, A., Burhenne, J., Fresnais, M., Steimel, K., Seidmann, L., Wagner, D.-C., Vewinger, N., Lehmann, N., Sprang, M., Backes, N., Roth, L., Neu, M. A., Wingerter, A., Henninger, N., El Malki, K., Otto, H., Alt, F., ... Faber, J. (2020). Safety and Activity of the Combination of Ceritinib and Dasatinib in Osteosarcoma. Cancers, 12(4), 793. https://doi.org/10.3390/cancers12040793






