Evaluation of Circulating miRNA Biomarkers of Testicular Germ Cell Tumors during Therapy and Follow-up―A Copenhagen Experience
Abstract
1. Introduction
2. Results
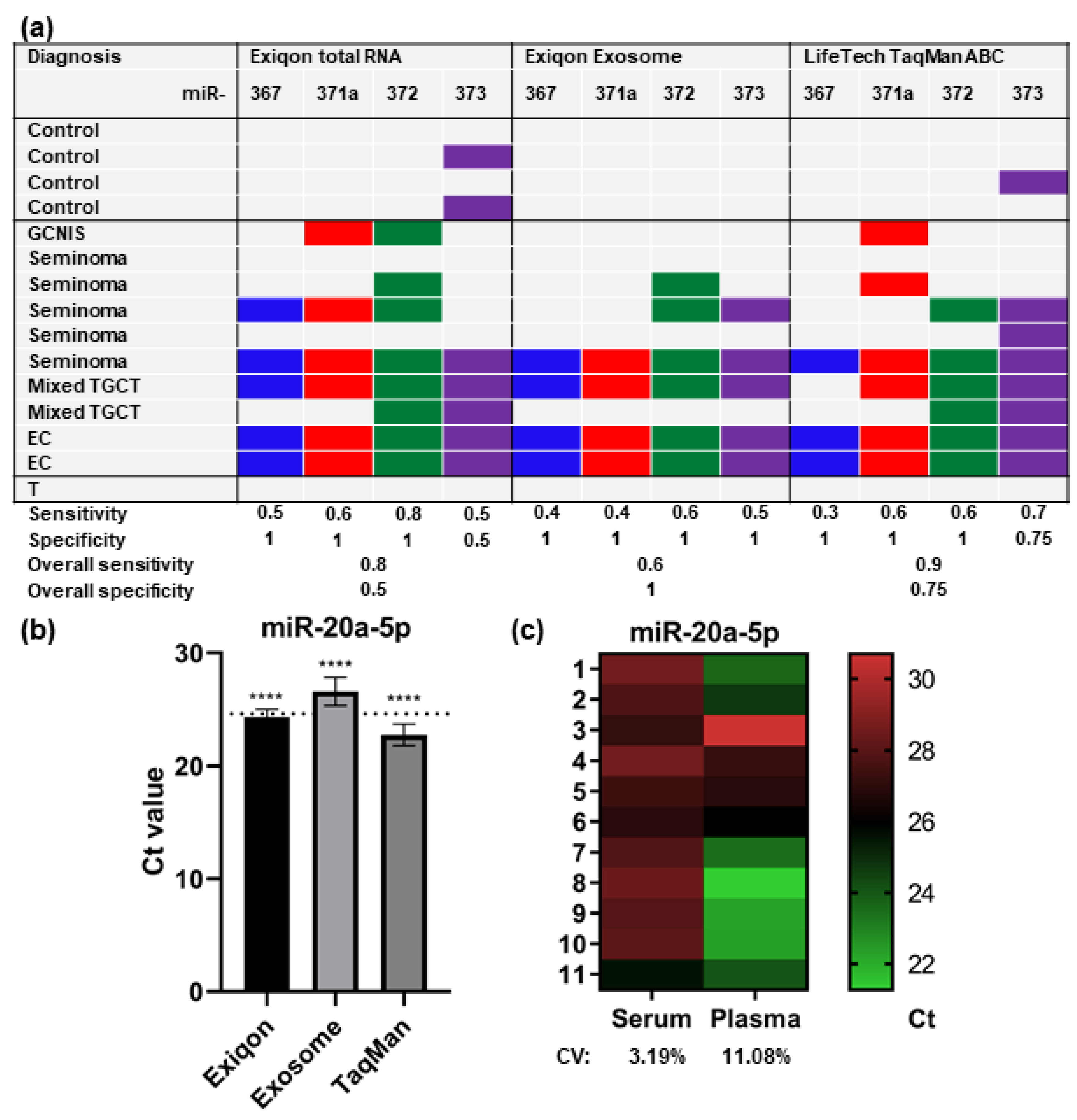
2.1. Methodological Comparison
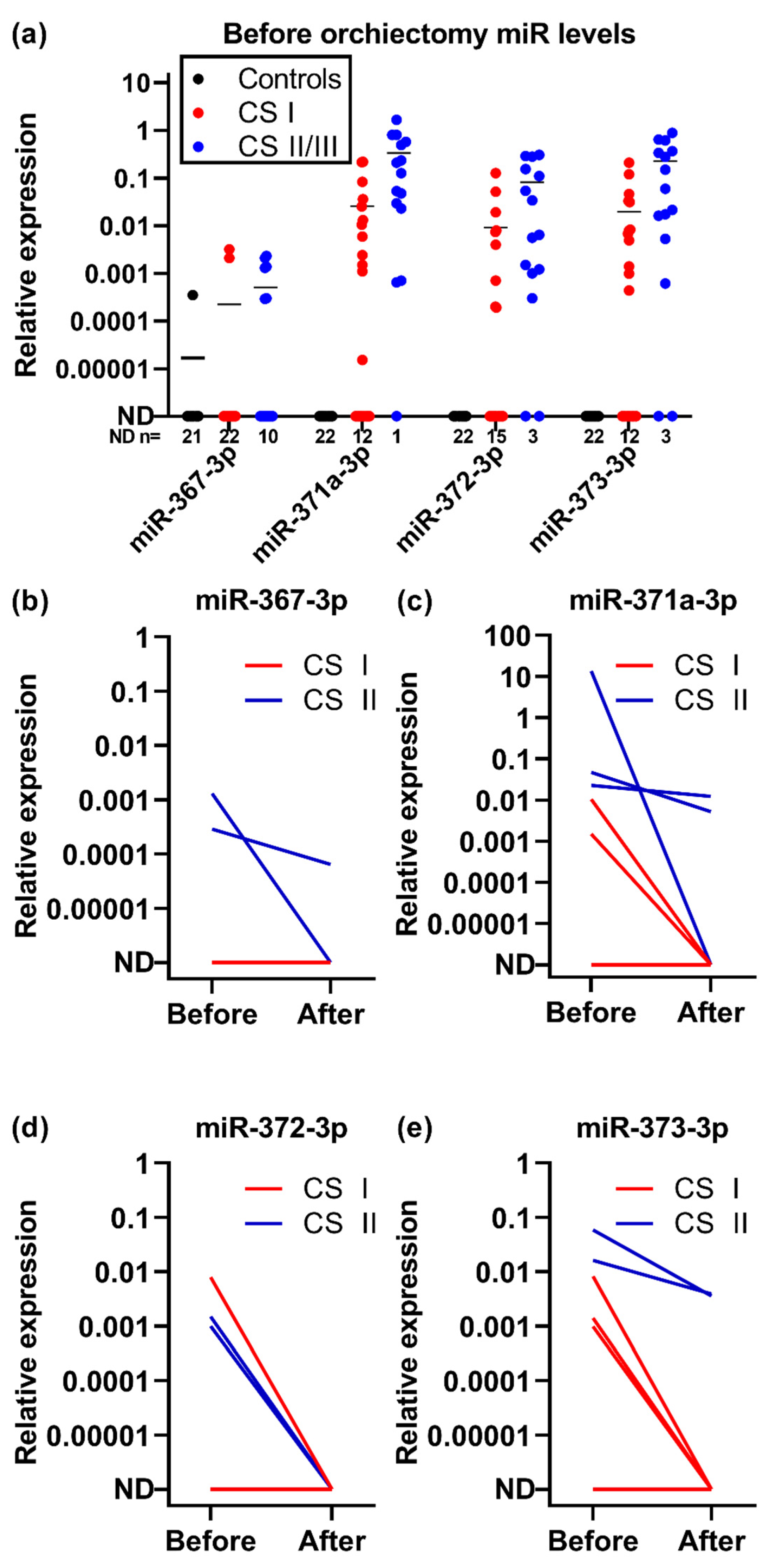
2.2. Clinical Performance
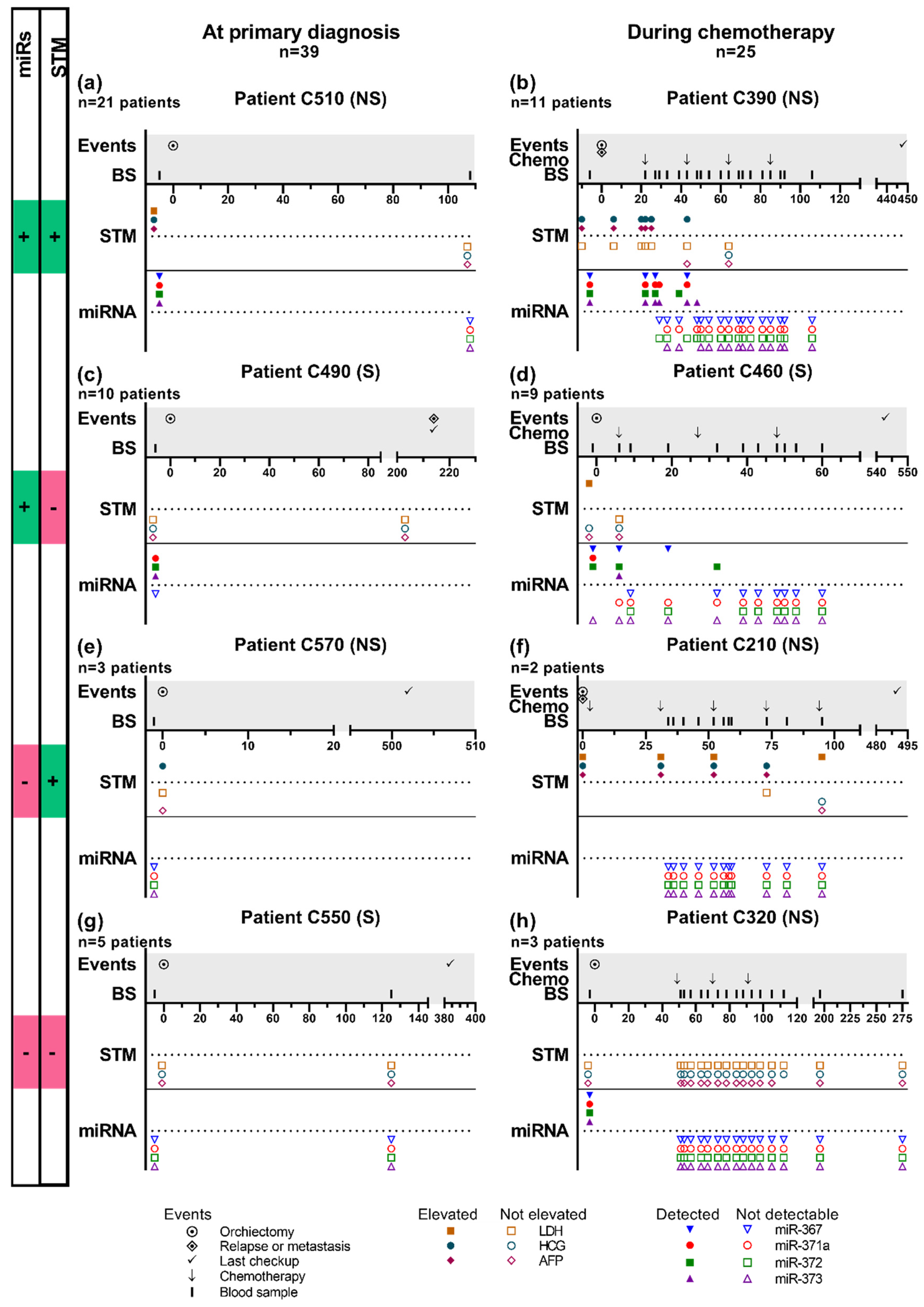
2.3. Patient Trajectories
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.1.1. Methodological Comparison
4.1.2. Clinical Study of Tumor Markers during Treatment and Follow-Up
4.2. Ethics
4.3. Laboratory Methods
4.3.1. Handling of Blood Samples
4.3.2. miRNA Measurements
4.3.3. Life Technologies
4.3.4. Exiqon Exosome Isolation and miRNA Purification
4.3.5. Exiqon miRNA Purification
4.3.6. Exiqon miRCURY LNATM Universal RT microRNA PCR
4.3.7. Measurement of Serum Tumor Markers
4.4. Data Analysis:
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gurney, J.K.; Florio, A.A.; Znaor, A.; Ferlay, J.; Laversanne, M.; Sarfati, D.; Bray, F.; McGlynn, K.A. International trends in the incidence of testicular cancer: Lessons from 35 years and 41 countries. Eur. Urol. 2019, 76, 615–623. [Google Scholar] [CrossRef]
- Ulbright, T.M.; Amin, M.B.; Balzer, B.; Berney, D.M.; Epstein, J.I.; Guo, C.; Idrees, M.T.; Looijenga, L.H.J.; Paner, G.; Rajpert-De Meyts, E.; et al. Germ cell tumours. In WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; Moch, H., Humphrey, P.A., Ulbright, T.M., Reuter, V.E., Eds.; IARC Press: Lyon, France, 2016; pp. 189–226. ISBN 978-92-832-2437-2. [Google Scholar]
- Gilligan, T.D.; Seidenfeld, J.; Basch, E.M.; Einhorn, L.H.; Fancher, T.; Smith, D.C.; Stephenson, A.J.; Vaughn, D.J.; Cosby, R.; Hayes, D.F.; et al. American society of clinical oncology clinical practice guideline on uses of serum tumor markers in adult males with germ cell tumors. J. Clin. Oncol. 2010, 28, 3388–3404. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.-P.; Richter-Simonsen, H.; Kulejewski, M.; Ikogho, R.; Zecha, H.; Anheuser, P.; Pichlmeier, U.; Isbarn, H. Testicular germ-cell tumours: A descriptive analysis of clinical characteristics at first presentation. Urol. Int. 2018, 100, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, Y.; Beck, S.D.W.; Foster, R.S.; Bihrle, R.; Einhorn, L.H. Serum tumor markers in testicular cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Huddart, R.A.; Coleman, N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat. Rev. Urol. 2016, 13, 715–725. [Google Scholar] [CrossRef]
- Dieckmann, K.; Simonsen-Richter, H.; Kulejewski, M.; Anheuser, P.; Zecha, H.; Isbarn, H.; Pichlmeier, U. Serum tumour markers in testicular germ cell tumours: Frequencies of elevated levels and extents of marker elevation are significantly associated with clinical parameters and with response to treatment. BioMed Res. Int. 2019, 2019, 5030349. [Google Scholar] [CrossRef]
- Almstrup, K.; Lobo, J.; Mørup, N.; Belge, G.; Rajpert-De Meyts, E.; Looijenga, L.H.J.; Dieckmann, K.-P. Application of miRNAs in the diagnosis and follow-up of testicular germ cell tumours. Nat. Rev. Urol. 2020. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.; Jerónimo, C.; Henrique, R.; Looijenga, L. Human germ cell tumors are developmental cancers: Impact of epigenetics on pathobiology and clinic. Int. J. Mol. Sci. 2019, 20, 258. [Google Scholar] [CrossRef]
- Dieckmann, K.-P.; Radtke, A.; Geczi, L.; Matthies, C.; Anheuser, P.; Eckardt, U.; Sommer, J.; Zengerling, F.; Trenti, E.; Pichler, R.; et al. Serum levels of MicroRNA-371a-3p (M371 Test) as a new biomarker of testicular germ cell tumors: Results of a prospective multicentric study. J. Clin. Oncol. 2019, 37, 1412–1423. [Google Scholar] [CrossRef]
- Mego, M.; Agthoven, T.; Gronesova, P.; Chovanec, M.; Miskovska, V.; Mardiak, J.; Looijenga, L.H.J. Clinical utility of plasma miR-371a-3p in germ cell tumors. J. Cell. Mol. Med. 2018, 23, 1128–1136. [Google Scholar]
- Rosas Plaza, X.; van Agthoven, T.; Meijer, C.; van Vugt, M.A.T.M.; de Jong, S.; Gietema, J.A.; Looijenga, L.H.J. miR-371a-3p, miR-373-3p and miR-367-3p as serum biomarkers in metastatic testicular germ cell cancers before, during and after chemotherapy. Cells 2019, 8, 1221. [Google Scholar] [CrossRef]
- van Agthoven, T.; Looijenga, L.H.J. Accurate primary germ cell cancer diagnosis using serum based microRNA detection (ampTSmiR test). Oncotarget 2017, 8, 58037. [Google Scholar] [CrossRef] [PubMed]
- Leão, R.; van Agthoven, T.; Figueiredo, A.; Jewett, M.A.S.; Fadaak, K.; Sweet, J.; Ahmad, A.E.; Anson-Cartwright, L.; Chung, P.; Hansen, A.; et al. Serum miRNA predicts viable disease after chemotherapy in patients with testicular nonseminoma germ cell tumor. J. Urol. 2018, 200, 126–135. [Google Scholar] [CrossRef]
- Dieckmann, K.-P.; Spiekermann, M.; Balks, T.; Ikogho, R.; Anheuser, P.; Wosniok, W.; Loening, T.; Bullerdiek, J.; Belge, G. MicroRNA miR-371a-3p—A novel serum biomarker of testicular germ cell tumors: Evidence for specificity from measurements in testicular vein blood and in neoplastic hydrocele fluid. Urol. Int. 2016, 97, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.-P.; Spiekermann, M.; Balks, T.; Flor, I.; Löning, T.; Bullerdiek, J.; Belge, G. MicroRNAs miR-371-3 in serum as diagnostic tools in the management of testicular germ cell tumours. Br. J. Cancer 2012, 107, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.-P.; Radtke, A.; Spiekermann, M.; Balks, T.; Matthies, C.; Becker, P.; Ruf, C.; Oing, C.; Oechsle, K.; Bokemeyer, C.; et al. Serum levels of MicroRNA miR-371a-3p: A sensitive and specific new biomarker for germ cell tumours. Eur. Urol. 2017, 71, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Hennig, F.; Ikogho, R.; Hammel, J.; Anheuser, P.; Wülfing, C.; Belge, G.; Dieckmann, K.-P. The novel biomarker of germ cell tumours, Micro-RNA-371a-3p, has a very rapid decay in patients with clinical stage 1. Urol. Int. 2018, 100, 470–475. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Lauritsen, J.; Gundgaard, M.G.; Agerbæk, M.; Holm, N.V.; Christensen, I.J.; von der Maase, H.; Daugaard, G. A nationwide cohort study of stage I seminoma patients followed on a surveillance program. Eur. Urol. 2014, 66, 1172–1178. [Google Scholar] [CrossRef]
- Daugaard, G.; Gundgaard, M.G.; Mortensen, M.S.; Agerbæk, M.; Holm, N.V.; Rørth, M.; von der Maase, H.; Christensen, I.J.; Lauritsen, J. Surveillance for stage I nonseminoma testicular cancer: Outcomes and long-term follow-up in a population-based cohort. J. Clin. Oncol. 2014, 32, 3817–3823. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; van den Berg, A.; Dorssers, L.C.J.; Belge, G.; Dieckmann, K.; Roest, H.P.; van der Laan, L.J.W.; Gietema, J.; Hamilton, R.J.; et al. Identification and validation model for informative liquid biopsy-based microRNA biomarkers: Insights from germ cell tumor in vitro, in vivo and patient-derived data. Cells 2019, 8, 1637. [Google Scholar] [CrossRef]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kääb, S.; Wakili, R.; et al. Stability of circulating blood-based microRNAs-Pre-Analytic methodological considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef] [PubMed]
- Terbuch, A.; Adiprasito, J.; Stiegelbauer, V.; Seles, M.; Klec, C.; Pichler, G.; Resel, M.; Posch, F.; Lembeck, A.; Stöger, H.; et al. MiR-371a-3p serum levels are increased in recurrence of testicular germ cell tumor patients. Int. J. Mol. Sci. 2018, 19, 3130. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, M.; Belge, G.; Winter, N.; Ikogho, R.; Balks, T.; Bullerdiek, J.; Dieckmann, K.-P. MicroRNA miR-371a-3p in serum of patients with germ cell tumours: Evaluations for establishing a serum biomarker. Andrology 2015, 3, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.M.; Rijlaarsdam, M.A.; Eini, R.; Dorssers, L.C.J.; Biermann, K.; Murray, M.J.; Nicholson, J.C.; Coleman, N.; Dieckmann, K.-P.; Belge, G.; et al. Targeted serum miRNA (TSmiR) test for diagnosis and follow-up of (testicular) germ cell cancer patients: A proof of principle. Mol. Oncol. 2013, 7, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
| Clinical Cohort | Number of Cases (%) |
|---|---|
| Controls | 22 (100%) |
| TGCT patients | 52 (100%) |
| GCNIS | 1 (1.9%) |
| S | 23 (44.2%) |
| NSs | 28 (53.8%) |
| Mixed | 24 (46.2%) |
| EC | 3 (5.8%) |
| C | 1 (1.9%) |
| T | - |
| Unknown | 1 (1.9%) |
| STAGE | |
| GCNIS | 1 (1.9%) |
| CS I | 30 (57.7%) |
| CS II | 18 (34.6%) |
| CS III | 3 (5.8%) |
| Prognostic group CS II/III | 21 |
| Good | 17 (81.0%) |
| Intermediate | 3 (14.3%) |
| Poor | 1 (4.8%) |
| Patients with relapse | 14 |
| Patients receiving chemotherapy | 32 |
| Clinical Cohort | Number of Patients with miRNAs Measured (%) | Number of Patients with STMs Measured (%) |
|---|---|---|
| Before orchiectomy | 40 | 39* |
| CS I | 25 (62.5%) | 24 (61.5%) |
| CS II | 14 (35.0%) | 14 (35.9%) |
| CS III | 1 (2.5%) | 1 (2.6%) |
| Before and after orchiectomy | 8 | 8 (100%) |
| CS I | 5 (62.5%) | 5 (62.5%) |
| CS II | 3 (37.5%) | 3 (37.5%) |
| Patients with relapse | 3/14 (21.4%) | 14 |
| Patients receiving chemotherapy | 29/32 (90.6%) |
| Tumor Markers | All Patients | CS I | CS II | CS III | S | NS |
|---|---|---|---|---|---|---|
| Number of patients with miRs measured | 40 | 25 | 14 | 1 | 17 | 23 |
| At least one measurable miRNA | 31 (77.5%) | 17 (68%) | 13 (92.9%) | 1 (100%) | 12 (70.6%) | 19 (82.6%) |
| miR-367-3p | 8 (20%) | 2 (8%) | 6 (42.9%) | 0 | 1 (5.9%) | 7 (30.4%) |
| miR-371a-3p | 27 (67.5%) | 13 (52%) | 13 (92.9%) | 1 (100%) | 9 (52.9%) | 18 (78.3%) |
| miR-372-3p | 22 (55%) | 10 (40%) | 12 (85.7%) | 0 | 7 (41.2%) | 15 (65.2%) |
| miR-373-3p | 25 (62.5%) | 13 (52%) | 12 (85.7%) | 0 | 10 (58.8%) | 15 (65.2%) |
| Number of patients with STMs measured | 39 | 24 | 14 | 1 | 16 | 23 |
| β-HCG and/or AFP | 19 (48.7%) | 12 (50%) | 7 (50%) | 0 | 5 (31.3%) | 14 (60.9%) |
| LDH | 12 (30.8%) | 5 (20.8%) | 7 (50%) | 1 (100%) | 6 (37.5%) | 6 (26.1%) |
| β-HCG | 18 (46.2%) | 11 (45.8%) | 7 (50%) | 0 | 5 (31.3%) | 13 (56.5%) |
| AFP | 11 (28.2%) | 7 (29.2%) | 4 (28.6%) | 0 | 2 (12.5%) | 9 (39.1%) |
| Clinical Stage and Histology | miR Detected | AFP and/or β-HCG Elevated | miR Detected and/or AFP Elevated and/or β-HCG Elevated |
|---|---|---|---|
| All patients at primary diagnosis | 31/40 (77.5%) | 19/39 (48.7%) | 33/39 (84.6%) |
| S | 12/17 (70.6%) | 5/16 (31.3%) | 12/16 (75.0%) |
| NS | 19/23 (82.6%) | 14/23 (60.9%) | 21/23 (91.3%) |
| CS I | 17/25 (68%) | 12/24 (50%) | 19/24 (79.2%) |
| S | 9/13 (69.2) | 3/12 (25.0%) | 9/12 (75%) |
| NS | 8/12 (66.7%) | 9/12 (75%) | 10/12 (83.3%) |
| CS II | 13/14 (92.9%) | 7/14 (50%) | 13/14 (92.9%) |
| S | 3/4 (75%) | 2/4 (50%) | 3/4 (75%) |
| NS | 10/10 (100%) | 5/10 (50%) | 10/10 (100%) |
| CS III | 1/1 (100%) | 0/1 (0%) | 1/1 (100%) |
| S | - | - | - |
| NS | 1/1 (100%) | 0/1 (0%) | 1/1 (100%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mørup, N.; Rajpert-De Meyts, E.; Juul, A.; Daugaard, G.; Almstrup, K. Evaluation of Circulating miRNA Biomarkers of Testicular Germ Cell Tumors during Therapy and Follow-up―A Copenhagen Experience. Cancers 2020, 12, 759. https://doi.org/10.3390/cancers12030759
Mørup N, Rajpert-De Meyts E, Juul A, Daugaard G, Almstrup K. Evaluation of Circulating miRNA Biomarkers of Testicular Germ Cell Tumors during Therapy and Follow-up―A Copenhagen Experience. Cancers. 2020; 12(3):759. https://doi.org/10.3390/cancers12030759
Chicago/Turabian StyleMørup, Nina, Ewa Rajpert-De Meyts, Anders Juul, Gedske Daugaard, and Kristian Almstrup. 2020. "Evaluation of Circulating miRNA Biomarkers of Testicular Germ Cell Tumors during Therapy and Follow-up―A Copenhagen Experience" Cancers 12, no. 3: 759. https://doi.org/10.3390/cancers12030759
APA StyleMørup, N., Rajpert-De Meyts, E., Juul, A., Daugaard, G., & Almstrup, K. (2020). Evaluation of Circulating miRNA Biomarkers of Testicular Germ Cell Tumors during Therapy and Follow-up―A Copenhagen Experience. Cancers, 12(3), 759. https://doi.org/10.3390/cancers12030759






