Long-Term Deleterious Effects of Short-term Hyperoxia on Cancer Progression—Is Brain-Derived Neurotrophic Factor an Important Mediator? An Experimental Study
Abstract
1. Introduction
2. Results
2.1. In Vitro Model—Breast Cancer Cell Cultures
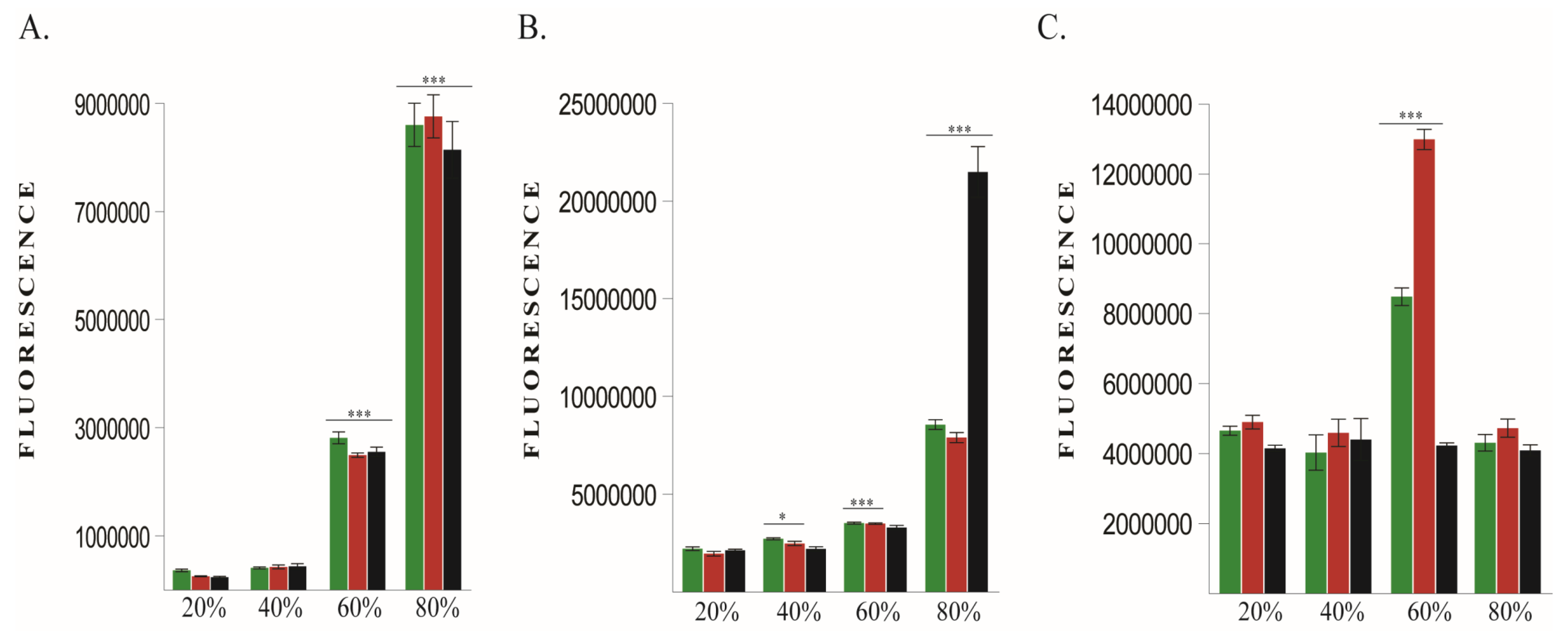
2.1.1. ROS Levels and Cell Viability
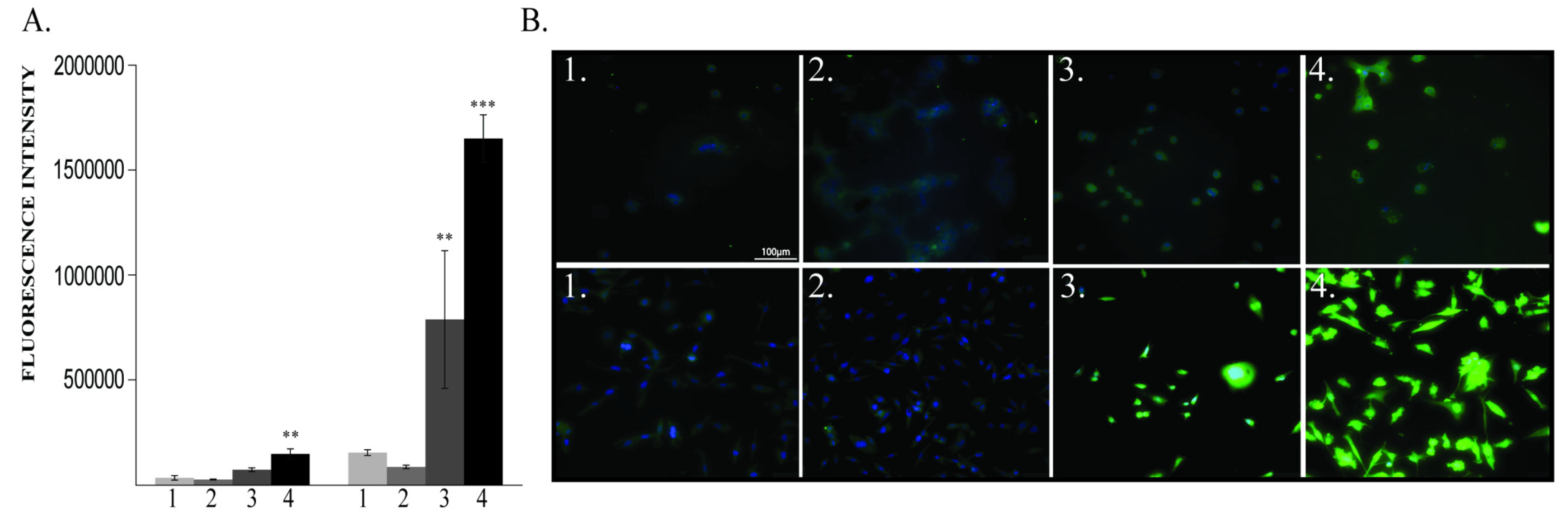
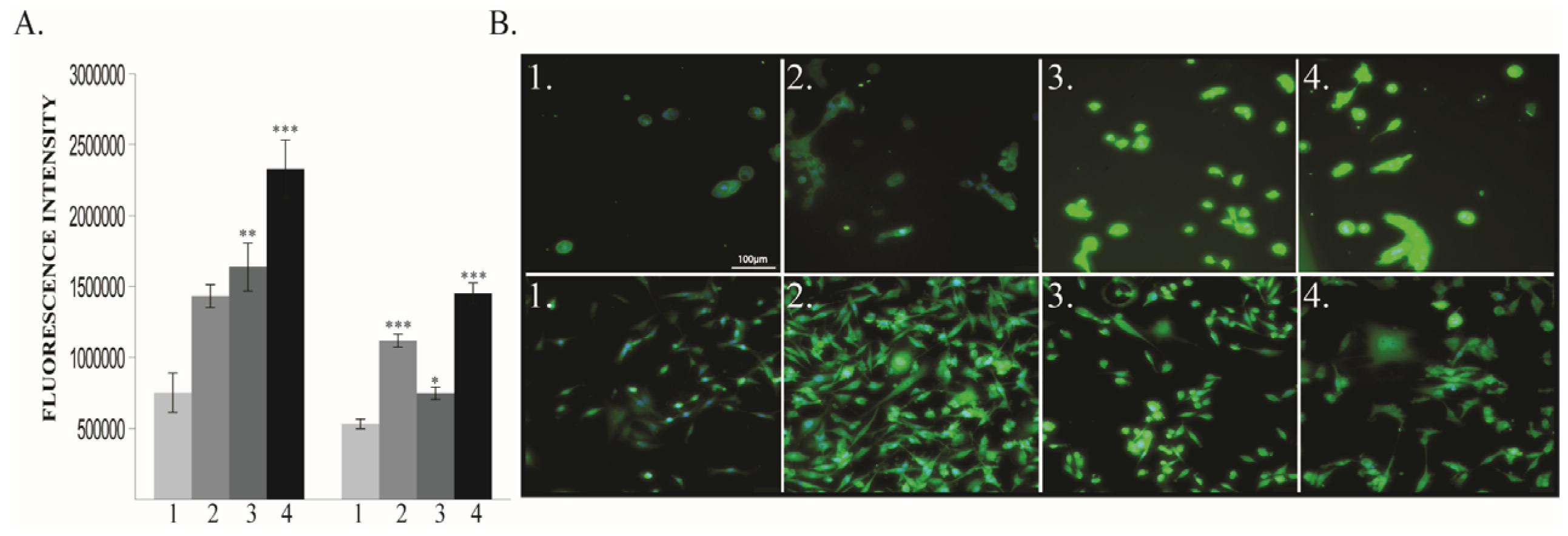
2.1.2. BDNF Expression
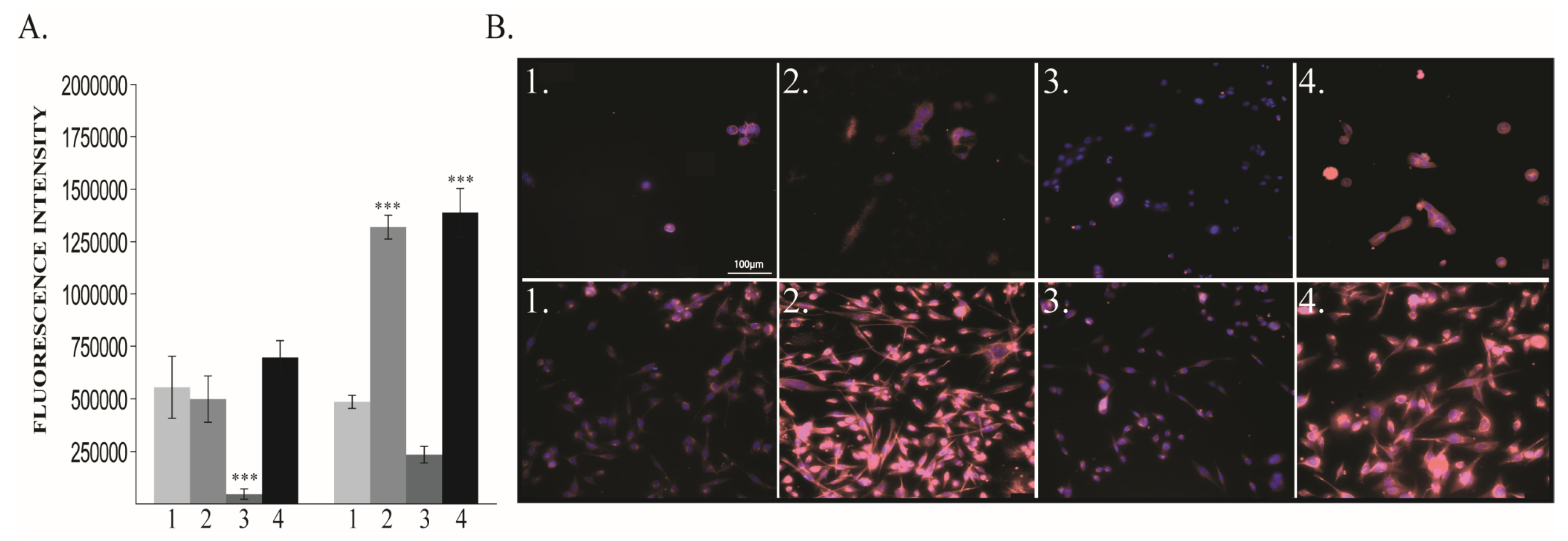
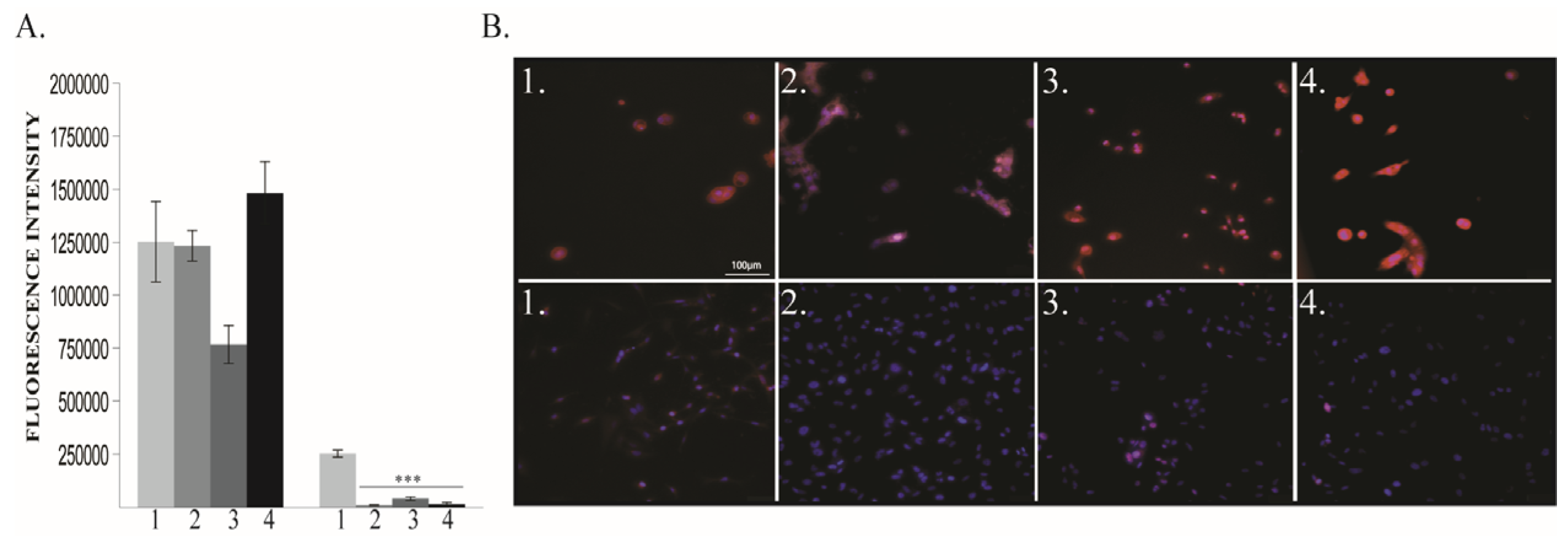
2.1.3. Vimentin and E-Cadherin Expression
2.1.4. VEGF-R2 Expression
2.1.5. HIF-1α Expression
2.2. In vivo Model—Murine Breast Cancer
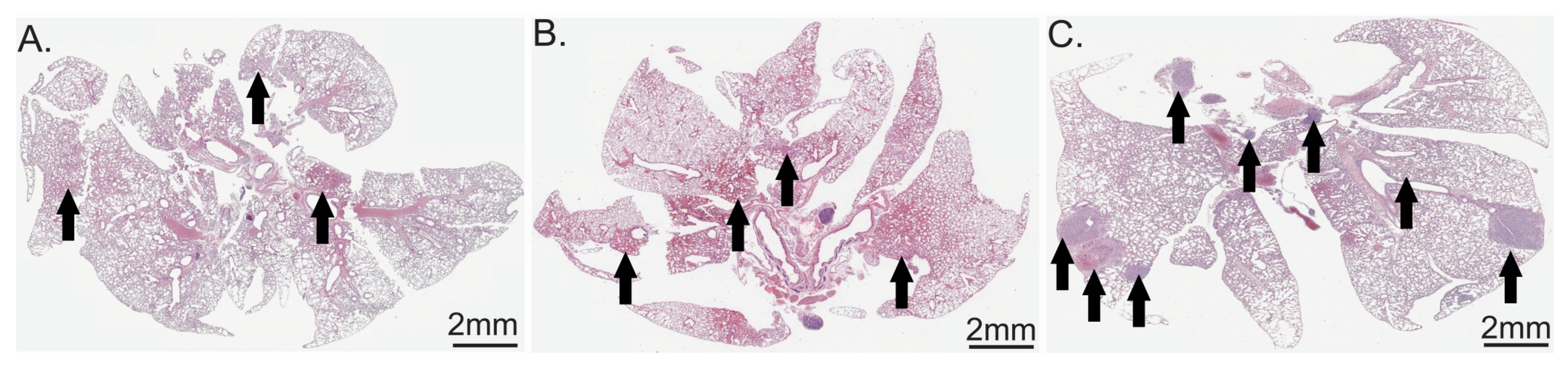
2.2.1. Lung Metastasis
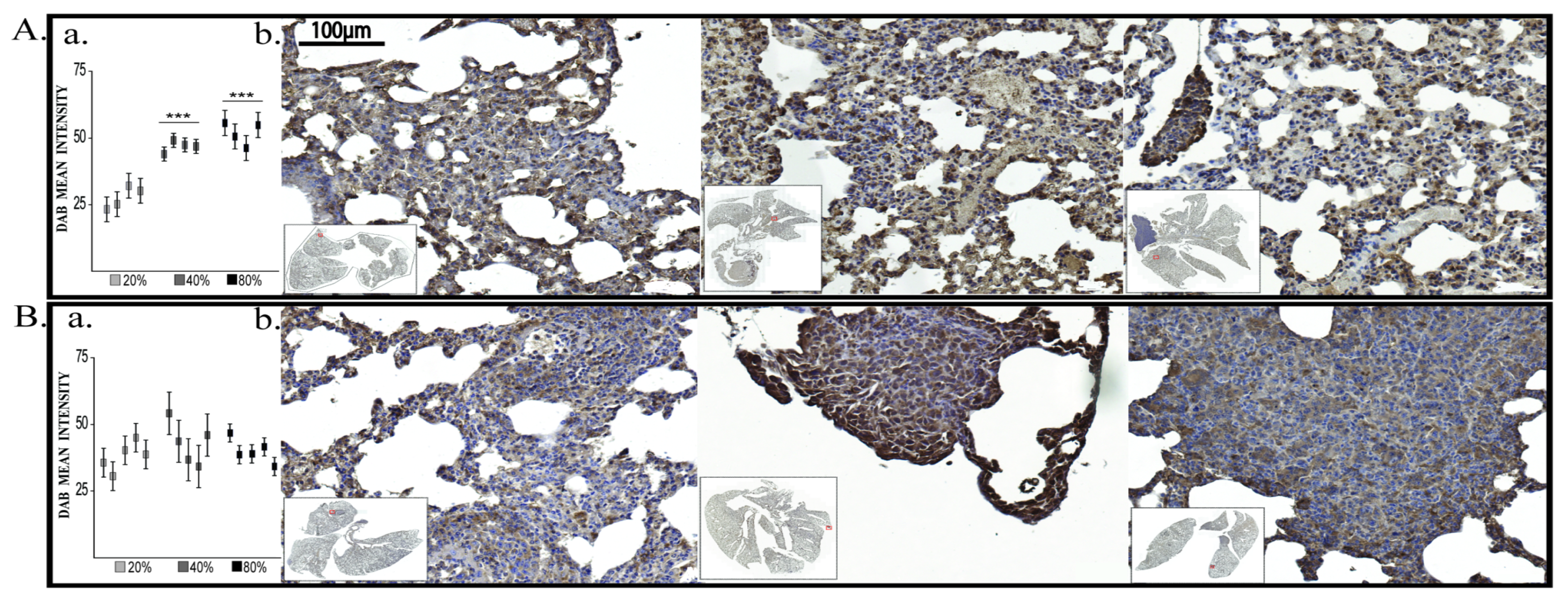
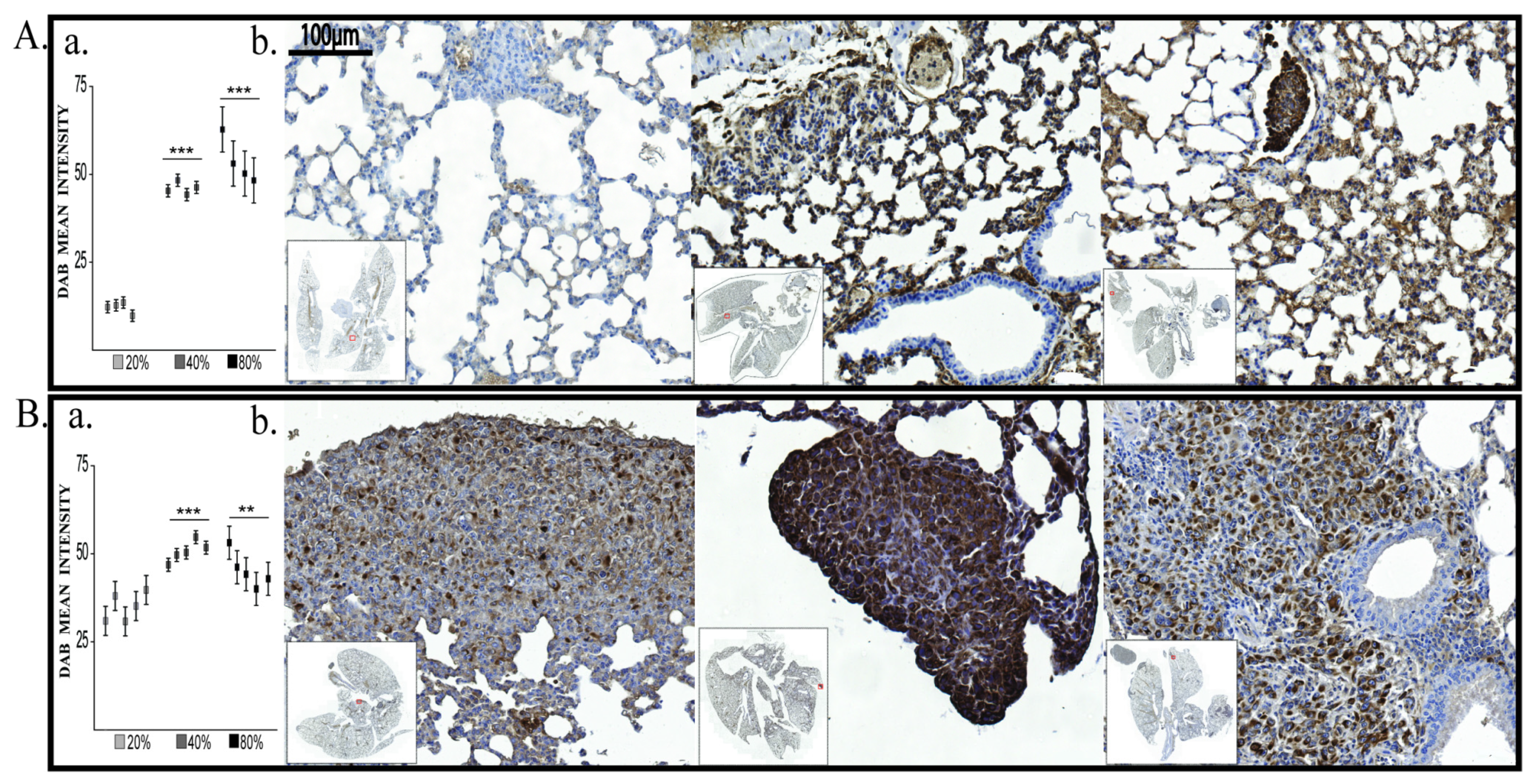
2.2.2. BDNF Expression
2.2.3. Vimentin Expression
2.2.4. E-Cadherin, VEGF-R2 and HIF-1α Evaluation
3. Discussion
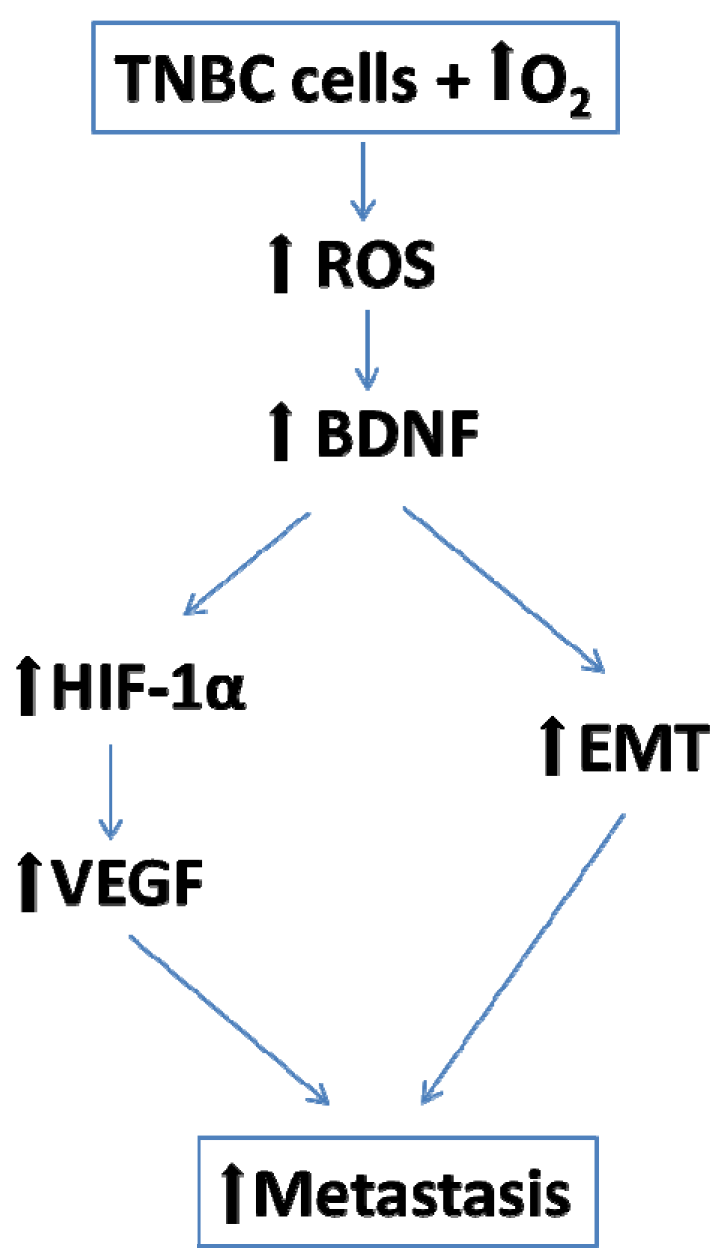
3.1. BDNF Expression is Enhanced by 6 h O2 Exposure in Triple Negative Breast Cancer Cells
3.2. EMT is Enhanced by 6 h O2 Exposure in Triple Negative Breast Cancer Cells
3.3. Angiogenesis is Enhanced by 6 h O2 Exposure in Triple Negative Breast Cancer Cells
3.4. Hyperoxia Effects are Regimen-Dependent
3.5. Study Limitations
4. Materials and Methods
4.1. In vitro Model—Breast Cancer Cell Cultures
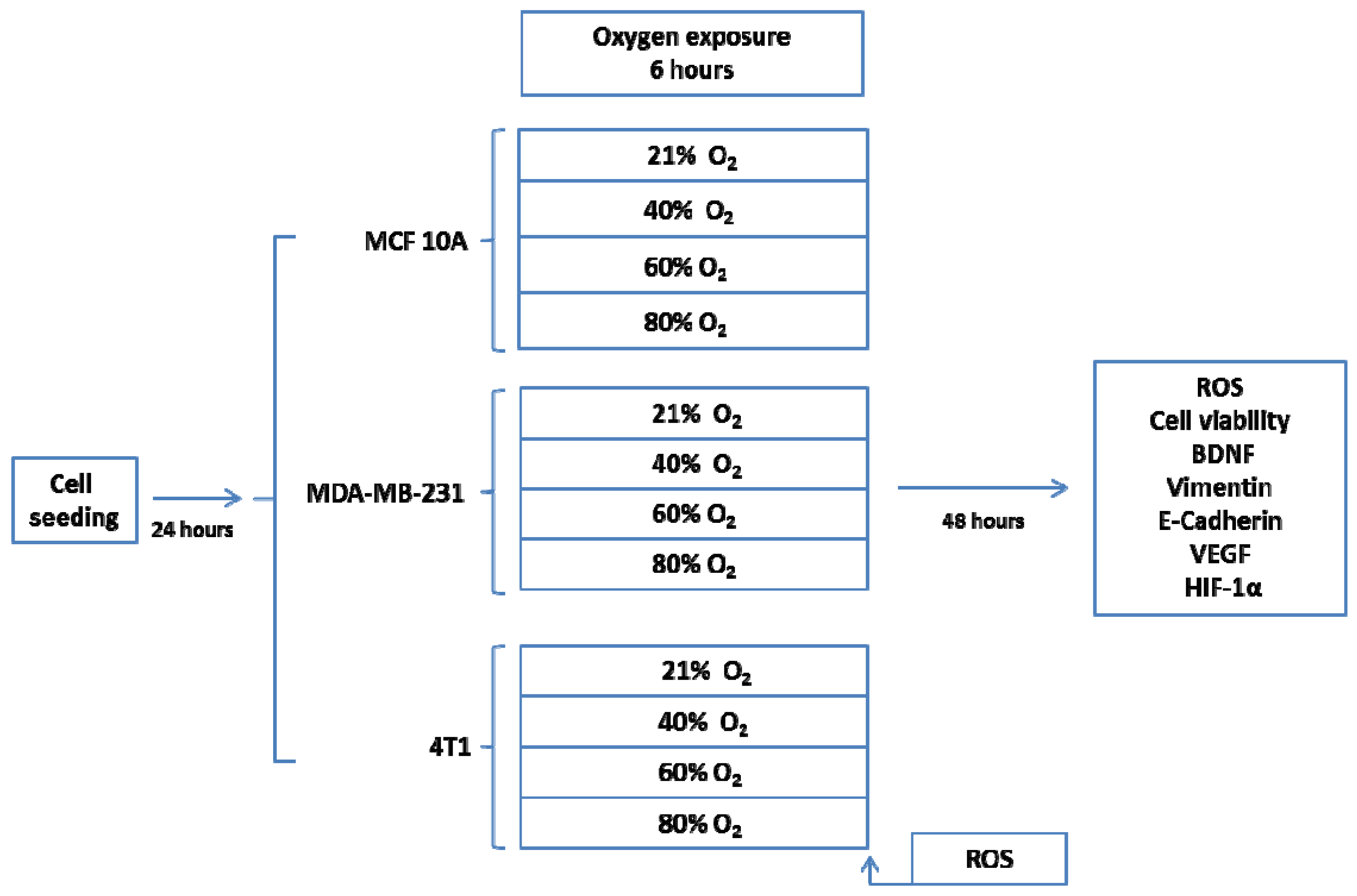
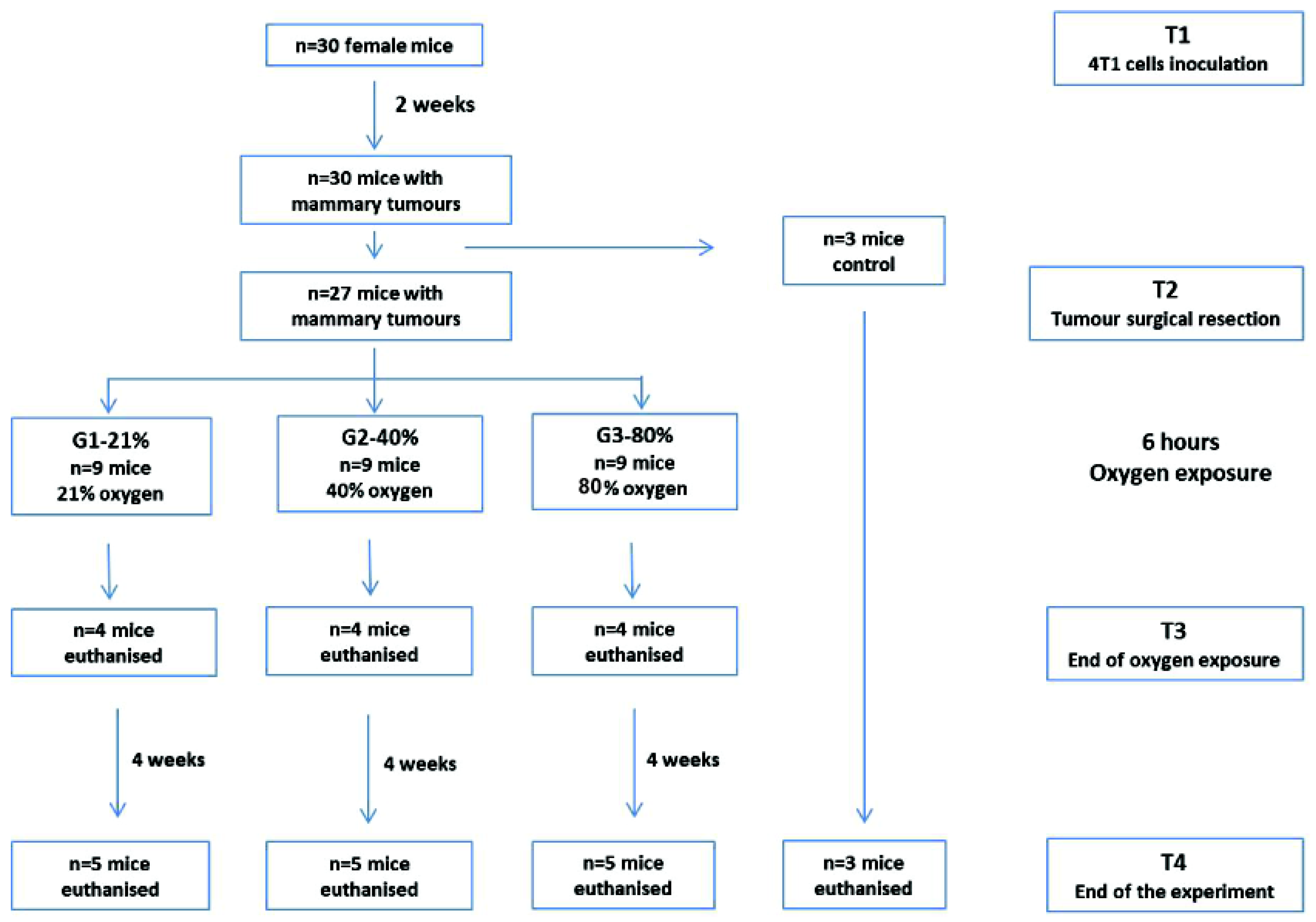
4.1.1. Experimental Design
4.1.2. Cell Lines and Cultures
4.1.3. Oxygen Treatment
4.1.4. Fluorescence Measurements
4.1.5. Immunofluorescence Staining
4.2. In Vivo Model—Murine Breast Cancer
4.2.1. Tumor Model
4.2.2. Tested Animals
4.2.3. Tumor Engraftment and Monitoring
4.2.4. Tumor Surgical Resection and Oxygen Exposure
4.2.5. Tissue Collection
4.2.6. Studies in Sacrificed Animals
4.2.7. Immunohistochemistry (IHC)
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.; Levins, K.J.; Buggy, D.J. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can. J. Anesth/J. Can. Anesth. 2016, 63, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 4, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Aguirre, J.A.; Bencic, I.; Borgeat, A.; Cama, A.; Condron, C.; Ionescu, D.; Buggy, D.J. How Anesthetic, Analgesic and Other Non-Surgical Techniques During Cancer Surgery Might Affect Postoperative Oncologic Outcomes: A Summary of Current State of Evidence. Cancers 2019, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Siemieniuk, R.A.; Chu, D.K.; Kim, L.H.Y.; Güell-Rous, M.R.; Alhazzani, W.; Soccal, P.M.; Irusen, E.M. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. Br. Med. J. 2018, 363, k4169. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.; Chien, J.; Douglas, J.; Eastlake, L.; Farah, C.; King, G.; Walters, H. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology 2015, 20, 1182–1191. [Google Scholar] [CrossRef]
- Meyhoff, C.S.; Jorgensen, L.N.; Wetterslev, J.; Christensen, K.B.; Rasmussen, L.S.; PROXI Trial Group. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: Follow-up of a randomized clinical trial. Anesth. Analg. 2012, 115, 849–854. [Google Scholar] [CrossRef]
- Meyhoff, C.S.; Jorgensen, L.N.; Wetterslev, J.; Siersma, V.D.; Rasmussen, L.S.; PROXI Trial Group. Risk of new or recurrent cancer after a high perioperative inspiratory oxygen fraction during abdominal surgery. Br. J. Anaesth. 2014, 113 (Suppl. 1), i74–i81. [Google Scholar] [CrossRef]
- Numakawa, T.; Matsumoto, T.; Numakawa, Y.; Richards, M.; Yamawaki, S.; Kunugi, H. Protective action of neurotrophic factors and estrogen against oxidative stress-mediated neurodegeneration. J. Toxicol. 2011, 2011, 405194. [Google Scholar] [CrossRef]
- Bruna, B.; Lobos, P.; Herrera-Molina, R.; Hidalgo, C.; Paula-Lima, A.; Adasme, T. The signaling pathways underlying BDNF-induced Nrf2 hippocampal nuclear translocation involve ROS, RyR-Mediated Ca2+ signals, ERK and PI3K. Biochem. Biophys. Res. Commun. 2018, 505, 201–207. [Google Scholar] [CrossRef]
- Lee, B.; Cao, R.; Choi, Y.S.; Cho, H.Y.; Rhee, A.D.; Hah, C.K.; Obrietan, K. The CREB/CRE transcriptional pathway: Protection against oxidative stress-mediated neuronal cell death. J. Neurochem. 2009, 108, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhou, C.; Sun, Y.; Di, W.; Scheffler, E.; Healey, S.; Wan, Y. Crosstalk between EGFR and TrkB enhances ovarian cancer cell migration and proliferation. Int. J. Oncol. 2006, 29, 1003–1011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakamoto, Y.; Kitajima, Y.; Edakuni, G.; Sasatomi, E.; Mori, M.; Kitahara, K.; Miyazaki, K. Expression of Trk tyrosine kinase receptor is a biologic marker for cell proliferation and perineural invasion of human pancreatic ductal adenocarcinoma. Oncol. Rep. 2001, 8, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Roesler, R.; De Farias, C.B.; Abujamra, A.L.; Brunetto, A.L.; Schwartsmann, G. BDNF/TrkB signaling as an anti-tumor target. Expert Rev. Anticancer Ther. 2011, 11, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.; Won, K.Y.; Lee, J.Y.; Kang, I.; Lee, S.K.; Lee, J. Expression of BDNF, TrkB and p53 in early-stage squamous cell carcinoma of the uterine cervix. Pathology 2011, 43, 453–458. [Google Scholar] [CrossRef]
- Wang, P.; Wan, W.; Xiong, S.; Wang, J.; Zou, D.; Lan, C.; Wu, N. HIF1α regulates glioma chemosensitivity through the transformation between differentiation and dedifferentiation in various oxygen levels. Sci. Rep. 2017, 7, 7965. [Google Scholar] [CrossRef]
- Chen, C.C.; Hsia, C.W.; Ho, C.W.; Liang, C.M.; Chen, C.M.; Huang, K.L.; Chen, Y.H. Hypoxia and hyperoxia differentially control proliferation of rat neural crest stem cells via distinct regulatory pathways of the HIF1α–CXCR4 and TP53–TPM1 proteins. Dev. Dynam. 2017, 246, 162–185. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Taccone, F.S.; He, X. Effects of Hyperoxia in Postcardiac Arrest, Sepsis, Traumatic Brain Injury or Stroke: The Importance of Individualized Oxygen Therapy in Critically Ill Patients. Can. Respir. J. 2017, 2017, 2834956. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.; Schultz, M.J.; Van der Voort, P.H.; De Jonge, E.; Van Westerloo, D.J. Bench-to-bedside review: The effects of hyperoxia during critical illness. Crit. Care 2015, 19, 284. [Google Scholar] [CrossRef]
- Moen, I.; Stuhr, L.E. Hyperbaric oxygen therapy and cancer—A review. Target. Oncol. 2012, 7, 233–242. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, I.K.; Ha, J.H.; Yeo, C.D.; Kang, H.H.; Kim, J.W.; Lee, S.H. Normobaric hyperoxia in-hibits the progression of lung cancer by inducing apoptosis. Exp. Biol. Med. 2018, 243, 739–748. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.H.; Belikoff, B.; Abbott, R.; Thayer, M. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015, 7, 277ra30. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.; Ranzani, O.T. Hyperoxia following cardiac arrest. Intensiv. Care Med. 2015, 41, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Del Portillo, I.P.; Vázquez, S.T.; Mendoza, J.B.; Moreno, R.V. Oxygen therapy in critical care: A double edged sword. Health 2014, 6, 2035–2046. [Google Scholar] [CrossRef]
- Jiang, J.M.; Zhou, C.F.; Gao, S.L.; Tian, Y.; Wang, C.Y.; Wang, L.; Tang, X.Q. BDNF-TrkB pathway me-diates neuroprotection of hydrogen sulfide against formaldehyde-induced toxicity to PC12 cells. PLoS ONE 2015, 10, e0119478. [Google Scholar]
- Nakamura, K.; Martin, K.C.; Jackson, J.K.; Beppu, K.; Woo, C.W.; Thiele, C.J. Brain-derived neu-rotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006, 66, 4249–4255. [Google Scholar] [CrossRef]
- Yin, B.; Ma, Z.Y.; Zhou, Z.W.; Gao, W.C.; Du, Z.G.; Zhao, Z.H.; Li, Q.Q. The TrkB+ cancer stem cells contribute to post-chemotherapy recurrence of triple-negative breast cancers in an orthotopic mouse model. Oncogene 2015, 34, 761–770. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Okumura, T.; Hirano, K.; Watanabe, T.; Nagata, T.; Shimada, Y.; Tsukada, K. p75 neu-rotrophin receptor expression is a characteristic of the mitotically quiescent cancer stem cell population present in esophageal squamous cell carcinoma. Int. J. Oncol. 2016, 48, 1943–1954. [Google Scholar] [CrossRef]
- Wickman, G.R.; Julian, L.; Mardilovich, K.; Schumacher, S.; Munro, J.; Rath, N.; Bienvenut, W.V. Blebs produced by actin–myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 2013, 20, 1293–1305. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Tokunaga, E.; Kitao, H.; Hisamatsu, Y.; Taketani, K.; Akiyoshi, S.; Maehara, Y. Vimentin as a poor prognostic factor for triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, A.; Kanaji, N.; Liu, D.; Yokomise, H.; Haba, R.; Ishii, T.; Bandoh, S. Vimentin regulates invasiveness and is a poor prognostic marker in non-small cell lung cancer. Anticancer Res. 2016, 36, 1545–1551. [Google Scholar] [PubMed]
- Du, L.; Li, J.; Lei, L.; He, H.; Chen, E.; Dong, J.; Yang, J. High vimentin expression predicts a poor prognosis and progression in colorectal cancer: A study with meta-analysis and TCGA data-base. BioMed. Res. Int. 2018, 2018, 6387810. [Google Scholar] [CrossRef] [PubMed]
- Chaw, S.Y.; Majeed, A.A.; Dalley, A.J.; Chan, A.; Stein, S.; Farah, C.S. Epithelial to mesenchymal transition (EMT) biomarkers–E-cadherin, beta-catenin, APC and Vimentin–in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012, 48, 997–1006. [Google Scholar] [CrossRef]
- Aiello, N.M.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef]
- Moen, I.; Øyan, A.M.; Kalland, K.H.; Tronstad, K.J.; Akslen, L.A.; Chekenya, M.; Stuhr, L.E.B. Hyperoxic treatment induces mesenchymal-to-epithelial transition in a rat adenocarcinoma model. PLoS ONE 2009, 4, e6381. [Google Scholar] [CrossRef]
- Crowley, P.D.; Stuttgen, V.; O’Carroll, E.; Ash, S.A.; Buggy, D.J.; Gallagher, H.C. Exposure to 60% oxygen promotes migration and upregulates angiogenesis factor secretion in breast cancer cells. Med. Gas. Res. 2017, 7, 226–235. [Google Scholar] [CrossRef]
- Kubis, H.P.; Hanke, N.; Scheibe, R.J.; Gros, G. Accumulation and nuclear import of HIF1 alpha during high and low oxygen concentration in skeletal muscle cells in primary culture. BBA Mol. Cell Res. 2005, 1745, 187–195. [Google Scholar] [CrossRef]
- Terraneo, L.; Virgili, E.; Caretti, A.; Bianciardi, P.; Samaja, M. In vivo hyperoxia induces hypoxia-inducible factor-1α overexpression in LNCaP tumors without affecting the tumor growth rate. Int. J. Biochem. Cell Biol. 2014, 51, 65–74. [Google Scholar] [CrossRef]
- Xia, C.; Meng, Q.; Liu, L.Z.; Rojanasakul, Y.; Wang, X.R.; Jiang, B.H. Reactive oxygen species regu-late angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007, 67, 10823–10830. [Google Scholar] [CrossRef]
- Yamamoto, N.; Oyaizu, T.; Enomoto, M.; Horie, M.; Yuasa, M.; Okawa, A.; Yagishita, K. VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration. Sci. Rep. 2020, 17, 2744. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.A.; Geiger, T.R.; Song, J.Y.; Gitelman, I.; Peeper, D.S. A Twist-Snail Axis Critical for TrkB-Induced Epithelial-Mesenchymal Transition-Like Transformation, Anoikis Resistance and Metastasis. Mol. Cell. Biol. 2009, 29, 3722–3737. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Wu, D.; Pal, B.; Bouras, T.; Asselin-Labat, M.L.; Vaillant, F.; Visvader, J.E. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010, 12, R21. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.E.; Hornig, Y.S.; Oei, Y.; Dusich, J.; Purchio, T. Bioluminescent human breast cancer cell lines that permit rapid and sensitive in vivo detection of mammary tumors and multiple metastases in immune deficient mice. Breast Cancer Res. 2005, 7, R444–R454. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.H.; Tanese de Souza, C.; Sahi, S.; Zhang, J.; Alkayyal, A.A.; Ananth, A.A.; Auer, R.A. A Mouse Tumor Model of Surgical Stress to Explore the Mechanisms of Postoperative Immunosuppression and Evaluate Novel Perioperative Immunotherapies. J. Vis. Exp. 2014, 85, e51253. [Google Scholar] [CrossRef] [PubMed]

| MDA-MB–231 | MCF 10A | |||||
|---|---|---|---|---|---|---|
| 40% O2 | 60% O2 | 80% O2 | 40% O2 | 60% O2 | 80% O2 | |
| ROS, after exposure | ↔ | ↑↑↑ | ↑↑↑ | ↔ | ↑↑↑ | ↑↑↑ |
| ROS at 48h | ↑ | ↑↑↑ | ↑↑↑ | ↑ | ↑↑↑ | ↑↑↑ |
| Cell viability | ↔ | ↑↑↑ | ↔ | ↔ | ↑↑↑ | ↔ |
| BDNF | ↔ | ↑↑ | ↑↑↑ | ↔ | ↔ | ↑↑↑ |
| Vimentin | ↑↑↑ | ↔ | ↑↑↑ | ↔ | ↓↓↓ | ↔ |
| E-Cadherin | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↔ | ↔ | ↔ |
| VEGF-R | ↑↑↑ | ↑ | ↑↑↑ | ↔ | ↑↑ | ↑↑↑ |
| HIF-1α | ↑↑↑ | ↑ | ↑↑ | ↔ | ↔ | ↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiron, A.; Ristescu, I.; Postu, P.A.; Tiron, C.E.; Zugun-Eloae, F.; Grigoras, I. Long-Term Deleterious Effects of Short-term Hyperoxia on Cancer Progression—Is Brain-Derived Neurotrophic Factor an Important Mediator? An Experimental Study. Cancers 2020, 12, 688. https://doi.org/10.3390/cancers12030688
Tiron A, Ristescu I, Postu PA, Tiron CE, Zugun-Eloae F, Grigoras I. Long-Term Deleterious Effects of Short-term Hyperoxia on Cancer Progression—Is Brain-Derived Neurotrophic Factor an Important Mediator? An Experimental Study. Cancers. 2020; 12(3):688. https://doi.org/10.3390/cancers12030688
Chicago/Turabian StyleTiron, Adrian, Irina Ristescu, Paula A. Postu, Crina E. Tiron, Florin Zugun-Eloae, and Ioana Grigoras. 2020. "Long-Term Deleterious Effects of Short-term Hyperoxia on Cancer Progression—Is Brain-Derived Neurotrophic Factor an Important Mediator? An Experimental Study" Cancers 12, no. 3: 688. https://doi.org/10.3390/cancers12030688
APA StyleTiron, A., Ristescu, I., Postu, P. A., Tiron, C. E., Zugun-Eloae, F., & Grigoras, I. (2020). Long-Term Deleterious Effects of Short-term Hyperoxia on Cancer Progression—Is Brain-Derived Neurotrophic Factor an Important Mediator? An Experimental Study. Cancers, 12(3), 688. https://doi.org/10.3390/cancers12030688






