Impaired Anti-Tumor T cell Response in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. Lymphocyte Recruitment into HCC Tumor and Peritumor Site
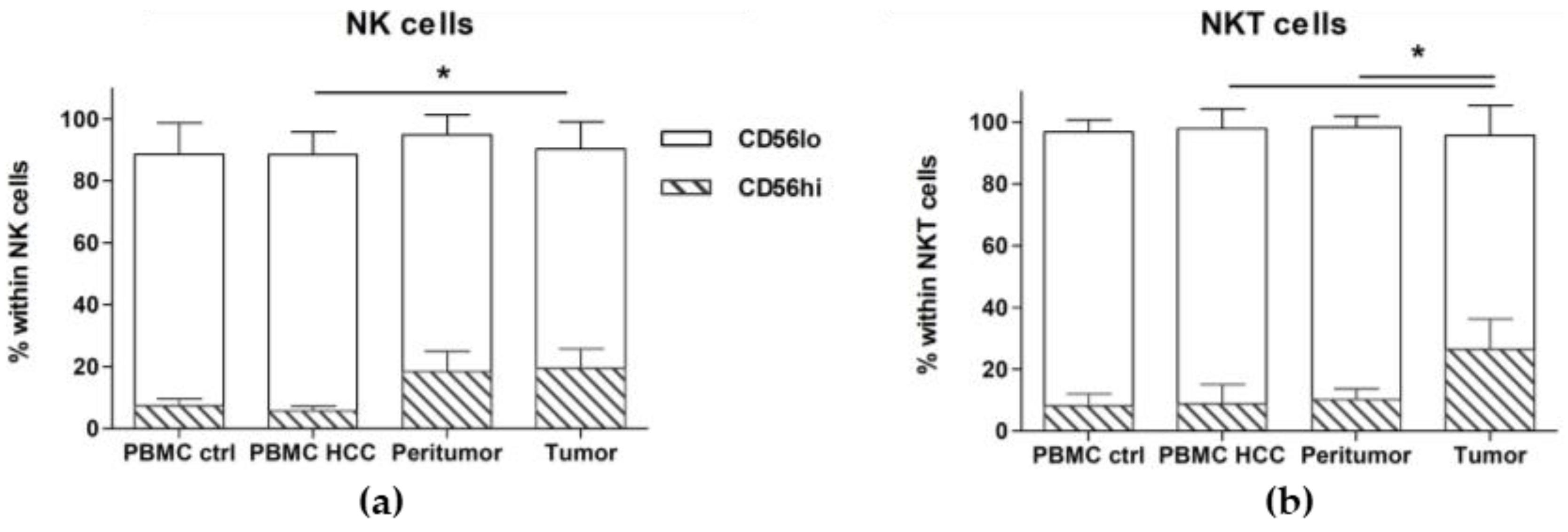
2.2. CD56hi NK and NKT Cells Are Increased in the Tumor
2.3. Cytotoxic T Cells Are Reduced at Tumor Site, While Tregs Accumulate
2.4. Terminally Differentiated Effector T Cells Are Strongly Reduced at Tumor Site
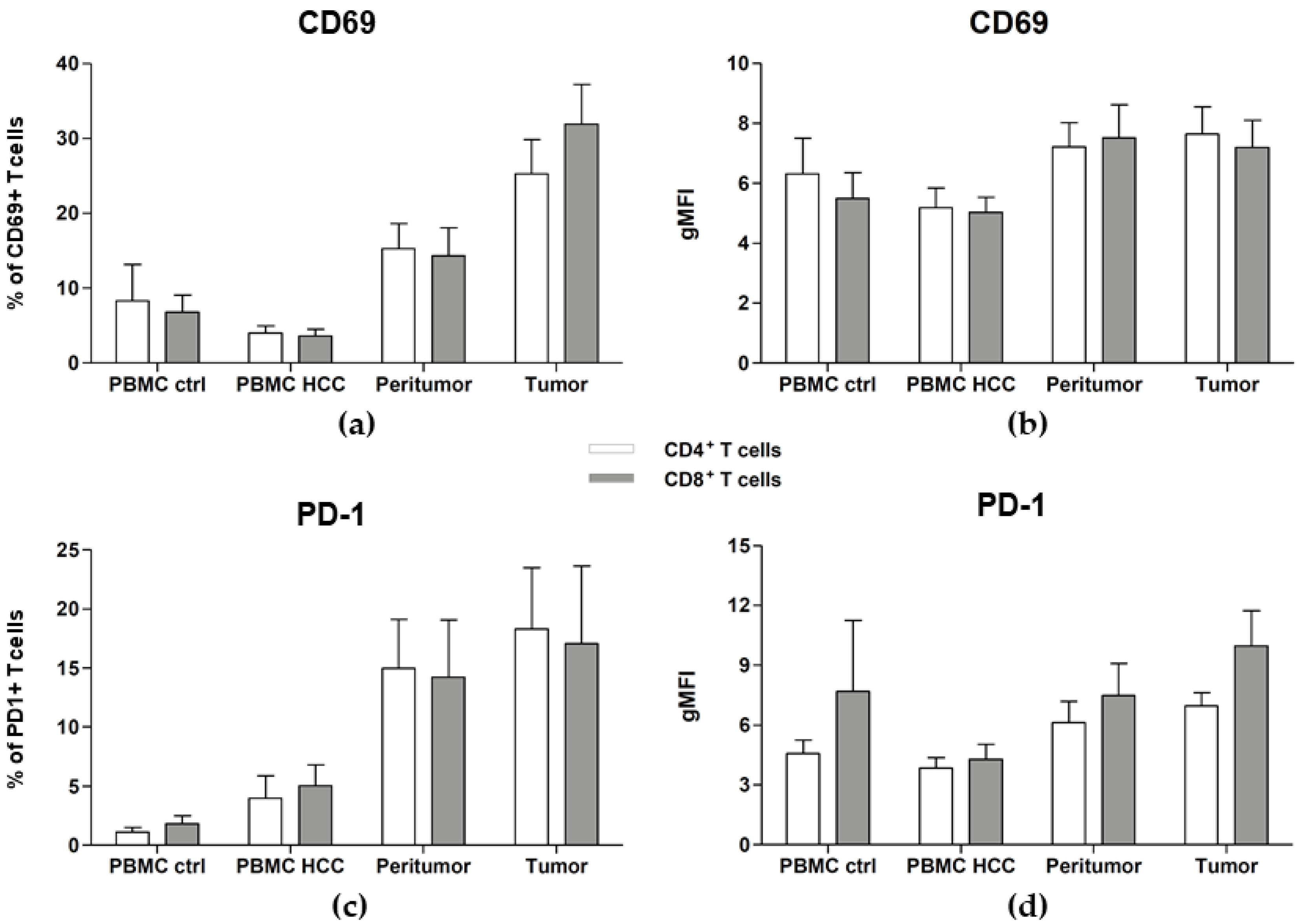
2.5. Tumor- and Peritumor-Infiltrating T Cells Are Activated and Exhausted
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Isolation of Patients’ Peripheral Blood Mononuclear Cells (PBMC), Tumor-Infiltrating Lymphocytes (TILs) and Lymphocytes from the Peritumor Site
4.3. Flow Cytometry Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nordenstedt, H.; White, D.L. El-Serag HB: The changing pattern of epidemiology in hepatocellular carcinoma. Dig. Liver Dis. 2010, 42, S206–S214. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Lang, L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology 2008, 134, 379. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Marinelli, S.; Negrini, G.; Menetti, S.; Benevento, F.; Bolondi, L. Prognostic significance of adverse events in patients with hepatocellular carcinoma treated with sorafenib. Ther. Adv. Gastroenterol. 2016, 9, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, M.; Cabibbo, G.; Piscaglia, F.; Zavaglia, C.; Grieco, A.; Villa, E.; Camma, C.; Colombo, M.; Group, S.S. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology 2011, 54, 2055–2063. [Google Scholar] [CrossRef]
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018, 18, 44. [Google Scholar] [CrossRef]
- Wen, L.; Liang, C.; Chen, E.; Chen, W.; Liang, F.; Zhi, X.; Wei, T.; Xue, F.; Li, G.; Yang, Q.; et al. Regulation of Multi-drug Resistance in hepatocellular carcinoma cells is TRPC6/Calcium Dependent. Sci. Rep. 2016, 6, 23269. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Busund, L.T.; Kilvaer, T.L.; Andersen, S.; Richardsen, E.; Paulsen, E.E.; Hald, S.; Khanehkenari, M.R.; Cooper, W.A.; Kao, S.C.; et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 789–800. [Google Scholar] [CrossRef]
- Hiraoka, K.; Kimura, T.; Logg, C.R.; Kasahara, N. Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin. Cancer Res. 2006, 12, 7108–7116. [Google Scholar] [CrossRef]
- Choi, H.S.; Ha, S.Y.; Kim, H.M.; Ahn, S.M.; Kang, M.S.; Kim, K.M.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; et al. The prognostic effects of tumor infiltrating regulatory T cells and myeloid derived suppressor cells assessed by multicolor flow cytometry in gastric cancer patients. Oncotarget 2016, 7, 7940–7951. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009, 58, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Hiraoka, N.; Yamagami, W.; Ojima, H.; Kanai, Y.; Kosuge, T.; Nakajima, A.; Hirohashi, S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin. Cancer Res. 2007, 13, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.P.; Campa, M.J.; Sperlazza, J.; Conlon, D.; Joshi, M.B.; Harpole, D.H., Jr.; Patz, E.F., Jr. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006, 107, 2866–2872. [Google Scholar] [CrossRef]
- Schouppe, E.; Mommer, C.; Movahedi, K.; Laoui, D.; Morias, Y.; Gysemans, C.; Luyckx, A.; De Baetselier, P.; Van Ginderachter, J.A. Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T-cell activation events. Eur. J. Immunol. 2013, 43, 2930–2942. [Google Scholar] [CrossRef]
- Fu, J.; Xu, D.; Liu, Z.; Shi, M.; Zhao, P.; Fu, B.; Zhang, Z.; Yang, H.; Zhang, H.; Zhou, C.; et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007, 132, 2328–2339. [Google Scholar] [CrossRef]
- Zahran, A.M.; Nafady-Hego, H.; Mansor, S.G.; Abbas, W.A.; Abdel-Malek, M.O.; Mekky, M.A.; Hetta, H.F. Increased frequency and FOXP3 expression of human CD8(+)CD25(High+) T lymphocytes and its relation to CD4 regulatory T cells in patients with hepatocellular carcinoma. Hum. Immunol. 2019, 80, 510–516. [Google Scholar] [CrossRef]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014, 59, 1415–1426. [Google Scholar] [CrossRef]
- Dudley, M.E.; Wunderlich, J.R.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Restifo, N.P.; Royal, R.E.; Kammula, U.; White, D.E.; Mavroukakis, S.A.; et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005, 23, 2346–2357. [Google Scholar] [CrossRef]
- Mackensen, A.; Meidenbauer, N.; Vogl, S.; Laumer, M.; Berger, J.; Andreesen, R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J. Clin. Oncol. 2006, 24, 5060–5069. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Dudley, M.E. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 2009, 21, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Gill, D.M.; Pal, S.K.; Agarwal, N. The future of immune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy 2017, 9, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Peggs, K.S. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br. J. Cancer 2013, 108, 1560–1565. [Google Scholar] [CrossRef]
- Gardiner, D.; Lalezari, J.; Lawitz, E.; DiMicco, M.; Ghalib, R.; Reddy, K.R.; Chang, K.M.; Sulkowski, M.; Marro, S.O.; Anderson, J.; et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE 2013, 8, e63818. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Inarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef]
- Mossanen, J.C.; Tacke, F. Role of lymphocytes in liver cancer. Oncoimmunology 2013, 2, e26468. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Theresine, M.; Andres, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Gao, Q.; Qiu, S.J.; Fan, J.; Zhou, J.; Wang, X.Y.; Xiao, Y.S.; Xu, Y.; Li, Y.W.; Tang, Z.Y. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007, 25, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Menetrier-Caux, C.; Gobert, M.; Caux, C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009, 69, 7895–7898. [Google Scholar] [CrossRef] [PubMed]
- Tzankov, A.; Meier, C.; Hirschmann, P.; Went, P.; Pileri, S.A.; Dirnhofer, S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 2008, 93, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.R.; Ochoa, S.; Prins, P.A.; He, A.R. Systemic therapy for advanced hepatocellular carcinoma: An update. J. Gastrointest. Oncol. 2017, 8, 243–255. [Google Scholar] [CrossRef]
- Phan, G.Q.; Yang, J.C.; Sherry, R.M.; Hwu, P.; Topalian, S.L.; Schwartzentruber, D.J.; Restifo, N.P.; Haworth, L.R.; Seipp, C.A.; Freezer, L.J.; et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 2003, 100, 8372–8377. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Byun, D.J.; Wolchok, J.D.; Rosenberg, L.M.; Girotra, M. Cancer immunotherapy—Immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef]
- Friedman, C.F.; Snyder, A. Atypical autoimmune adverse effects with checkpoint blockade therapies. Ann. Oncol. 2017, 28, 206–207. [Google Scholar] [CrossRef]
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Chiu, D.K.; Tse, A.P.; Xu, I.M.; Di Cui, J.; Lai, R.K.; Li, L.L.; Koh, H.Y.; Tsang, F.H.; Wei, L.L.; Wong, C.M.; et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 2017, 8, 517. [Google Scholar] [CrossRef]
- Chiu, D.K.; Xu, I.M.; Lai, R.K.; Tse, A.P.; Wei, L.L.; Koh, H.Y.; Li, L.L.; Lee, D.; Lo, R.C.; Wong, C.M.; et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology 2016, 64, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Tucker-Heard, G.; Perdue, N.R.; Killebrew, J.R.; Urdahl, K.B.; Campbell, D.J. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009, 10, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sharma, S.; Edwards, J.; Feigenbaum, L.; Zhu, J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat. Immunol. 2015, 16, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Caceres, J.; Sanchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten Dijke, P.; Consortium, I.-L. TGF-beta signalling and liver disease. FEBS. J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Mikulits, W.; Dooley, S.; Fabregat, I.; Moustakas, A.; ten Dijke, P.; Portincasa, P.; Winter, P.; Janssen, R.; Leporatti, S.; et al. The rationale for targeting TGF-beta in chronic liver diseases. Eur. J. Clin. Investig. 2016, 46, 349–361. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, L.; Yoo, J.K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356. [Google Scholar] [CrossRef]
- Matzinger, P.; Kamala, T. Tissue-based class control: The other side of tolerance. Nat. Rev. Immunol. 2011, 11, 221–230. [Google Scholar] [CrossRef]
- Angelin, A.; Gil-de-Gomez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J.; Kopinski, P.K.; Wang, L.; et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017, 25, 1282–1293. [Google Scholar] [CrossRef]
- Galon, J.; Angell, H.K.; Bedognetti, D.; Marincola, F.M. The continuum of cancer immunosurveillance: Prognostic, predictive, and mechanistic signatures. Immunity 2013, 39, 11–26. [Google Scholar] [CrossRef]


| % Within CD45+ Cells | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| T cells | 61.52 ± 5.16 | 37.89 ± 4.59 | 0.0071 | 49.79 ± 5.76 | 0.22 | 0.11 | 42.29 ± 7.12 | 0.09 | 0.38 | 0.51 |
| NKT cells | 5.94 ± 1.64 | 10.32 ± 2.48 | 0.36 | 7.001 ± 2.45 | 0.95 | 0.47 | 6.53 ± 2.34 | 0.97 | 0.31 | 0.70 |
| NK cells | 9.28 ± 1.15 | 15.72 ± 3.41 | 0.60 | 8.133 ± 1.76 | 0.68 | 0.15 | 9.80 ± 2.97 | 0.40 | 0.41 | 0.76 |
| B cells | 9.76 ± 2.30 | 3.00 ± 0.58 | 0.0046 | 4.569 ± 1.72 | 0.032 | 0.67 | 1.81 ± 0.45 | 0.002 | 0.28 | 0.39 |
| % Within NK Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| CD56hi | 7.47 ± 2.21 | 5.71 ± 1.59 | 0.47 | 18.49 ± 6.51 | 0.55 | 0.13 | 19.60 ± 6.13 | 0.07 | 0.014 | 0.78 |
| CD56low | 81.15 ± 10.16 | 82.73 ± 7.43 | 0.78 | 76.45 ± 6.36 | 0.25 | 0.21 | 70.76 ± 8.65 | 0.11 | 0.10 | 1.00 |
| % Within NKT Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| CD56hi | 8.27 ± 3.87 | 8.83 ± 6.38 | 0.35 | 10.28 ± 3.56 | 0.93 | 0.29 | 26.49 ± 9.90 | 0.11 | 0.013 | 0.14 |
| CD56low | 88.49 ± 3.98 | 88.99 ± 6.42 | 0.43 | 88.15 ± 3.49 | 1.00 | 0.28 | 69.19 ± 9.61 | 0.08 | 0.003 | 0.04 |
| % Within CD3+ T Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| CD4+ T cells | 70.29 ± 2.94 | 54.45 ± 4.97 | 0.036 | 48.13 ± 6.03 | 0.0056 | 0.74 | 48.07 ± 5.22 | 0.006 | 0.74 | 0.78 |
| CD8+ T cells | 19.20 ± 2.08 | 30.97 ± 4.06 | 0.0498 | 29.514 ± 4.94 | 0.25 | 0.81 | 31.95 ± 3.65 | 0.017 | 0.48 | 0.72 |
| % Within CD4+ T Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| Tregs | 2.28 ± 0.44 | 4.88 ± 0.97 | 0.60 | 5.32 ± 2.50 | 0.833 | 0.596 | 5.63 ± 1.59 | 0.024 | 0.840 | 0.463 |
| Th1 | 0.04 ± 0.02 | 2.41 ± 1.60 | 0.12 | 4.35 ± 1.49 | 0.017 | 0.120 | 12.97 ± 7.39 | 0.024 | 0.229 | 0.779 |
| T-bet+ Tregs | 0.09 ± 0.01 | 0.40 ± 0.19 | 0.88 | 0.56 ± 0.28 | 0.617 | 0.410 | 3.90 ± 1.90 | 0.2788 | 0.16 | 0.462 |
| % Within CD4+ T Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| TN | 14.24 ± 5.80 | 18.06 ± 5.21 | 0.65 | 2.02 ± 0.66 | 0.0096 | 0.005 | 5.55 ± 3.22 | 0.030 | 0.015 | 0.69 |
| TCM | 15.36 ± 6.87 | 10.24 ± 1.84 | 0.65 | 8.99 ± 3.27 | 0.46 | 0.52 | 14.69 ± 4.14 | 0.84 | 0.69 | 0.35 |
| TEM | 46.54 ± 5.58 | 44.41 ± 6.02 | 0.92 | 68.80 ± 8.11 | 0.012 | 0.012 | 69.75 ± 6.98 | 0.020 | 0.007 | 0.92 |
| TEFF | 26.82 ± 6.79 | 26.22 ± 6.52 | 0.60 | 11.22 ± 2.80 | 0.10 | 0.015 | 9.95 ± 1.95 | 0.09 | 0.018 | 0.97 |
| % Within CD8+ T Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| TN | 19.90 ± 8.58 | 19.47 ± 4.99 | 0.43 | 11.56 ± 4.20 | 0.90 | 0.15 | 10.45 ± 4.61 | 0.94 | 0.06 | 0.70 |
| TCM | 9.36 ± 4.39 | 4.62 ± 2.10 | 0.19 | 9.86 ± 3.99 | 0.50 | 0.05 | 14.30 ± 5.88 | 0.90 | 0.31 | 0.76 |
| TEM | 40.31 ± 9.17 | 28.98 ± 4.64 | 0.32 | 51.91 ± 9.23 | 0.36 | 0.049 | 49.77 ± 8.16 | 0.36 | 0.10 | 0.85 |
| TEFF | 30.43 ± 6.32 | 46.93 ± 5.90 | 0.09 | 26.68 ± 5.78 | 0.72 | 0.021 | 25.49 ± 7.26 | 0.41 | 0.023 | 0.70 |
| % of CD69+ Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| CD4+ T cells | 8.31 ± 4.83 | 4.01 ± 0.96 | 0.64 | 15.25 ± 3.38 | 0.0057 | 0.014 | 25.26 ± 4.55 | 0.0039 | 0.010 | 0.65 |
| CD8+ T cells | 6.79 ± 2.30 | 3.60 ± 0.92 | 0.38 | 14.30 ± 3.74 | 0.0006 | 0.026 | 31.87 ± 5.34 | 0.037 | 0.12 | 0.55 |
| gMFI of CD69+ Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| CD4+ T cells | 6.31 ± 1.18 | 5.19 ± 0.65 | 0.55 | 7.20 ± 0.82 | 0.70 | 0.10 | 7.65 ± 0.90 | 0.36 | 0.054 | 0.74 |
| CD8+ T cells | 5.50 ± 0.87 | 5.04 ± 0.50 | 0.59 | 7.52 ± 1.10 | 0.16 | 0.05 | 7.19 ± 0.90 | 0.18 | 0.099 | 1.00 |
| % of PD1+ Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| CD4+ T cells | 1.12 ± 0.39 | 3.97 ± 1.95 | 0.92 | 14.98 ± 4.14 | 0.058 | 0.0045 | 18.30 ± 5.20 | 0.0033 | <0.0001 | 0.20 |
| CD8+ T cells | 1.83 ± 0.67 | 5.04 ± 1.76 | 0.26 | 14.24 ± 4.82 | 0.09 | 0.017 | 17.07 ± 6.59 | 0.0004 | 0.0001 | 0.035 |
| gMFI of PD1+ Cells | ||||||||||
| Mean ± SEM | ||||||||||
| PBMC Ctrl | PBMC HCC | p Value 1 | Peritumor Lymphocytes | p Value 1 | p Value 2 | TILs HCC | p Value 1 | p Value 2 | p Value 3 | |
| CD4+ T cells | 4.58 ± 0.65 | 3.85 ± 0.51 | 0.30 | 6.12 ± 1.08 | 0.15 | 0.11 | 6.97 ± 0.67 | 0.0016 | 0.005 | 0.23 |
| CD8+ T cells | 7.69 ± 3.56 | 4.28 ± 0.75 | 0.19 | 7.48 ± 1.63 | 0.21 | 0.05 | 9.97 ± 1.76 | 0.010 | 0.001 | 0.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaoul, N.; Mancarella, S.; Lupo, L.; Giannelli, G.; Dituri, F. Impaired Anti-Tumor T cell Response in Hepatocellular Carcinoma. Cancers 2020, 12, 627. https://doi.org/10.3390/cancers12030627
Chaoul N, Mancarella S, Lupo L, Giannelli G, Dituri F. Impaired Anti-Tumor T cell Response in Hepatocellular Carcinoma. Cancers. 2020; 12(3):627. https://doi.org/10.3390/cancers12030627
Chicago/Turabian StyleChaoul, Nada, Serena Mancarella, Luigi Lupo, Gianluigi Giannelli, and Francesco Dituri. 2020. "Impaired Anti-Tumor T cell Response in Hepatocellular Carcinoma" Cancers 12, no. 3: 627. https://doi.org/10.3390/cancers12030627
APA StyleChaoul, N., Mancarella, S., Lupo, L., Giannelli, G., & Dituri, F. (2020). Impaired Anti-Tumor T cell Response in Hepatocellular Carcinoma. Cancers, 12(3), 627. https://doi.org/10.3390/cancers12030627






