Development and Validation of a Clinically Relevant Workflow for MR-Guided Volumetric Arc Therapy in a Rabbit Model of Head and Neck Cancer
Abstract
:1. Introduction
2. Results
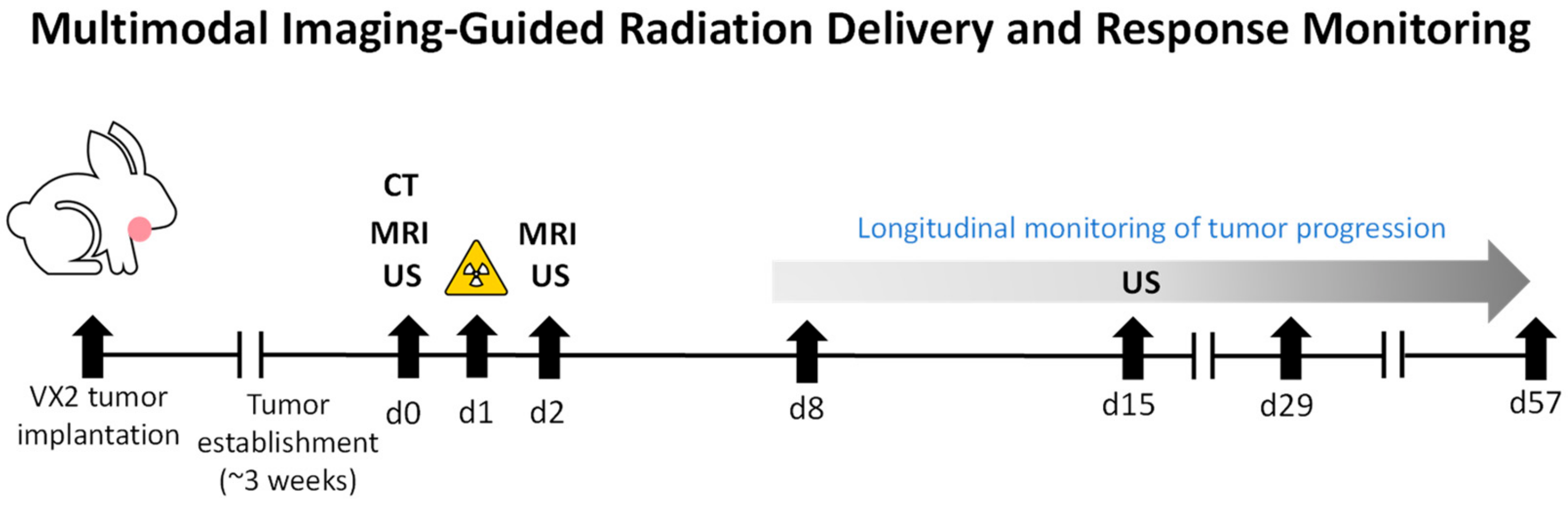
2.1. MR-CT and US Imaging of VX2 Neck Tumors—Study Design and Workflow
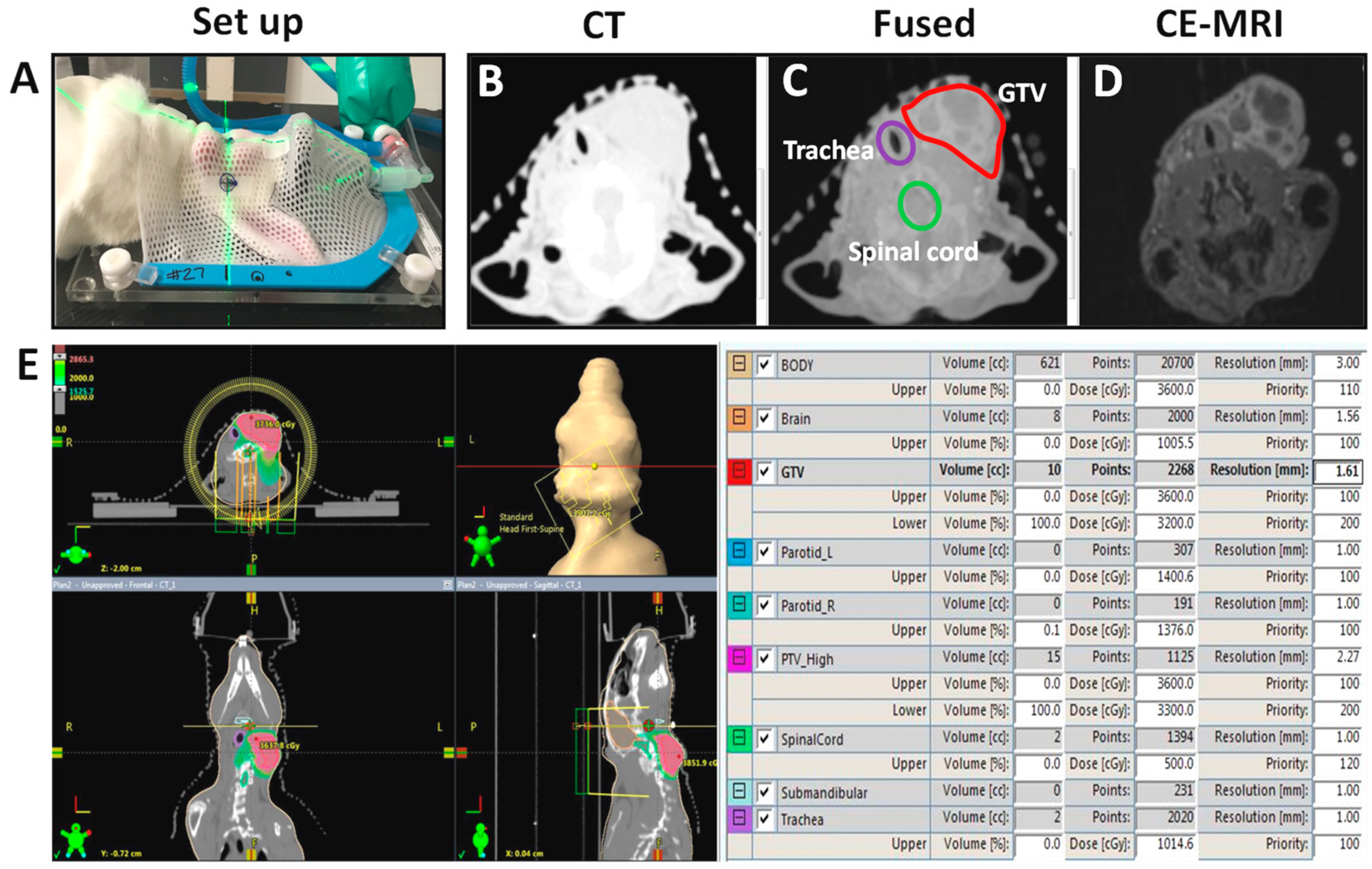
2.2. MR-Guided VMAT of VX2 Tumors
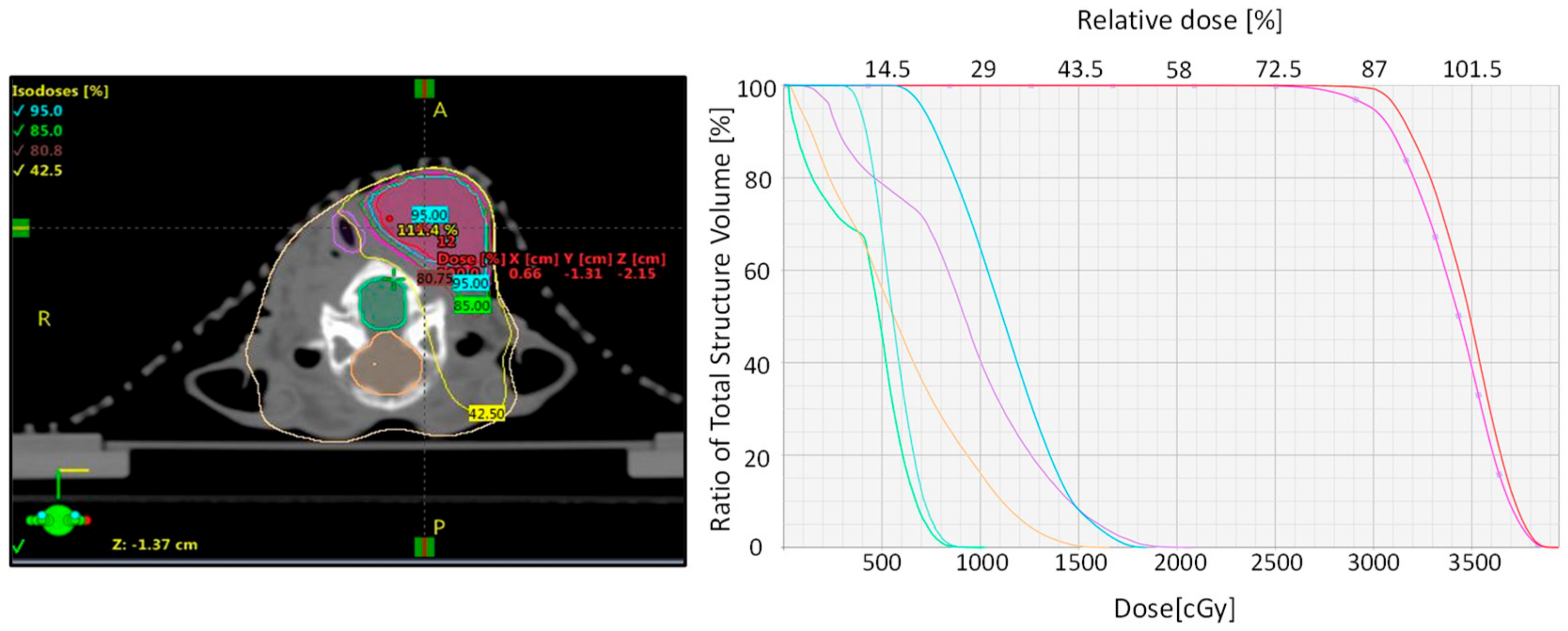
2.3. Treatment Planning and Dosimetry
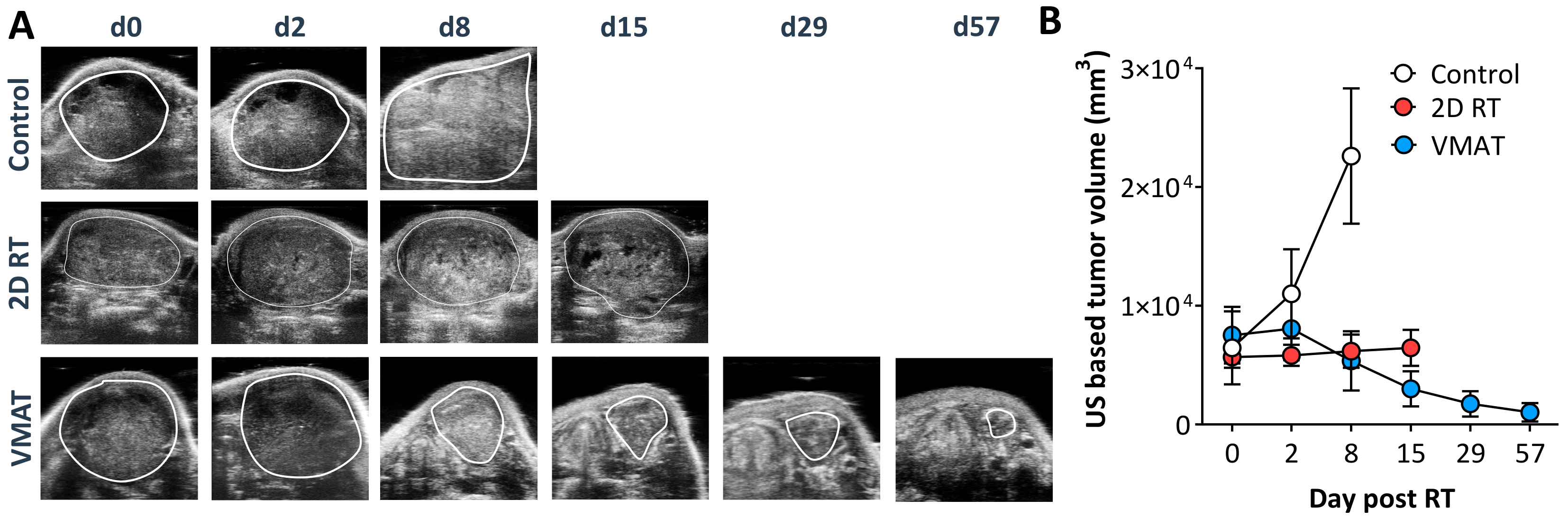
2.4. Treatment Outcome and Toxicity—MR-Guided VMAT vs. 2D RT
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure for Establishing VX2 Tumors in the Neck
4.3. Magnetic Resonance Imaging
4.4. Radiation Therapy
4.5. Ultrasound
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanetti, E.; Clivio, A.; Nicolini, G.; Fogliata, A.; Ghosh-Laskar, S.; Agarwal, J.P.; Upreti, R.R.; Budrukkar, A.; Murthy, V.; Deshpande, D.D.; et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: A treatment planning comparison with fixed field IMRT. Radiother. Oncol. 2009, 92, 111–117. [Google Scholar] [CrossRef]
- Verbakel, W.F.; Cuijpers, J.P.; Hoffmans, D.; Bieker, M.; Slotman, B.J.; Senan, S. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: A comparative planning and dosimetric study. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Syam Kumar, S.A.; Vivekanandan, N.; Sriram, P. A study on conventional IMRT and RapidArc treatment planning techniques for head and neck cancers. Rep. Pract. Oncol. Radiother. 2012, 17, 168–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzese, C.; Fogliata, A.; Clerici, E.; Franceschini, D.; Villa, E.; D’Agostino, G.; Navarria, P.; Mancosu, P.; Tomatis, S.; Cozzi, L.; et al. Toxicity profile and early clinical outcome for advanced head and neck cancer patients treated with simultaneous integrated boost and volumetric modulated arc therapy. Radiat. Oncol. 2015, 10, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.M.; Cao, M.; Hsu, S.; Lamb, J.; Mikaeilian, A.; Yang, Y.; Agazaryan, N.; Low, D.A.; Steinberg, M.L. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Adv. Radiat. Oncol. 2017, 2, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.M.; Hsu, S.; Lamb, J.; Yang, Y.; Agazaryan, N.; Steinberg, M.L.; Low, D.A.; Cao, M. MRI-guided radiotherapy for head and neck cancer: Initial clinical experience. Clin. Transl. Oncol. 2018, 20, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kiser, K.; Meheissen, M.A.M.; Mohamed, A.S.R.; Kamal, M.; Ng, S.P.; Elhalawani, H.; Jethanandani, A.; He, R.; Ding, Y.; Rostom, Y.; et al. Prospective quantitative quality assurance and deformation estimation of MRI-CT image registration in simulation of head and neck radiotherapy patients. Clin. Transl. Radiat. Oncol. 2019, 18, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Tillner, F.; Thute, P.; Butof, R.; Krause, M.; Enghardt, W. Pre-clinical research in small animals using radiotherapy technology—A bidirectional translational approach. Z. Med. Phys. 2014, 24, 335–351. [Google Scholar] [CrossRef]
- Ghita, M.; Brown, K.H.; Kelada, O.J.; Graves, E.E.; Butterworth, K.T. Integrating Small Animal Irradiators with Functional Imaging for Advanced Preclinical Radiotherapy Research. Cancers 2019, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Medina, L.A.; Herrera-Penilla, B.I.; Castro-Morales, M.A.; García-López, P.; Jurado, R.; Pérez-Cárdenas, E.; Chanona-Vilchis, J.; Brandan, M.E. Use of an orthovoltage X-ray treatment unit as a radiation research system in a small-animal cancer model. J. Exp. Clin. Cancer Res. 2008, 27, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Steen, S.; Green, D.J.; Gopal, A.K.; Orozco, J.J.; Kenoyer, A.L.; Lin, Y.; Wilbur, D.S.; Hamlin, D.K.; Fisher, D.R.; Hylarides, M.D.; et al. Venetoclax Synergizes with Radiotherapy for Treatment of B-cell Lymphomas. Cancer Res. 2017, 77, 3885–3893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizumi, T.; Brady, S.L.; Robbins, M.E.; Bourland, J.D. Specific issues in small animal dosimetry and irradiator calibration. Int. J. Radiat. Biol. 2011, 87, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Molloy, J.; Izumi, T.; Sterpin, E. Impact of backscatter material thickness on the depth dose of orthovoltage irradiators for radiobiology research. Phys. Med. Biol. 2019, 64, 055001. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, B.J.; Vargo, J.A.; Ling, D.; Jones, B.; Mohney, M.; Clump, D.A.; Ohr, J.P.; Ferris, R.L.; Heron, D.E. Carotid Dosimetry and the Risk of Carotid Blowout Syndrome After Reirradiation With Head and Neck Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Koontz, B.F.; Verhaegen, F.; De Ruysscher, D. Tumour and normal tissue radiobiology in mouse models: How close are mice to mini-humans? Br. J. Radiol. 2017, 90, 20160441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaegen, F.; Granton, P.; Tryggestad, E. Small animal radiotherapy research platforms. Phys. MedBiol. 2011, 56, R55–R83. [Google Scholar] [CrossRef]
- Wong, J.; Armour, E.; Kazanzides, P.; Iordachita, I.; Tryggestad, E.; Deng, H.; Matinfar, M.; Kennedy, C.; Liu, Z.; Chan, T.; et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1591–1599. [Google Scholar] [CrossRef] [Green Version]
- Grover, A.R.; Kimler, B.F.; Duncan, F.E. Use of a Small Animal Radiation Research Platform (SARRP) facilitates analysis of systemic versus targeted radiation effects in the mouse ovary. J. Ovarian Res. 2018, 11, 72. [Google Scholar] [CrossRef]
- Gutierrez, S.; Descamps, B.; Vanhove, C. MRI-Only Based Radiotherapy Treatment Planning for the Rat Brain on a Small Animal Radiation Research Platform (SARRP). PLoS ONE 2015, 10, e0143821. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, C.L.; Monroe, M.M.; Hunt, J.P.; Buchmann, L.; Baker, O.J. Comparing human and mouse salivary glands: A practice guide for salivary researchers. Oral Dis. 2019, 25, 403–415. [Google Scholar] [CrossRef]
- Supsavhad, W.; Dirksen, W.P.; Martin, C.K.; Rosol, T.J. Animal models of head and neck squamous cell carcinoma. Vet. J. 2016, 210, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xiong, H.; Ellis, A.E.; Northrup, N.C.; Dobbin, K.K.; Shin, D.M.; Zhao, S. Canine spontaneous head and neck squamous cell carcinomas represent their human counterparts at the molecular level. PLoS Genet. 2015, 11, e1005277. [Google Scholar] [CrossRef] [PubMed]
- Oak, C.; Ahn, Y.C.; Nam, S.J.; Jung, M.H.; Hwang, S.S.; Chae, Y.G.; Lee, H.S.; Lee, K.D.; Jung, M.J.; Chun, B.K.; et al. Multimodal imaging using optical coherence tomography and endolaryngeal ultrasonography in a new rabbit VX2 laryngeal cancer model. Lasers Surg. Med. 2015, 47, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.J.; Sexton, S.; Curtin, L.; Seshadri, M. Spatiotemporal Optoacoustic Mapping of Tumor Hemodynamics in a Clinically Relevant Orthotopic Rabbit Model of Head and Neck Cancer. Transl. Oncol. 2017, 10, 839–845. [Google Scholar] [CrossRef]
- Lijowski, M.; Caruthers, S.; Hu, G.; Zhang, H.; Scott, M.J.; Williams, T.; Erpelding, T.; Schmieder, A.H.; Kiefer, G.; Gulyas, G.; et al. High sensitivity: High-resolution SPECT-CT/MR molecular imaging of angiogenesis in the Vx2 model. Investig. Radiol. 2009, 44, 15–22. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F.Y.; Fu, J.X.; Duan, F.; Fan, Q.S.; Wang, M.Q. Hepatic arterial administration of sorafenib and iodized oil effectively attenuates tumor growth and intrahepatic metastasis in rabbit VX2 hepatocellular carcinoma model. Int. J. Clin. Exp. Pathol. 2014, 7, 7775–7781. [Google Scholar]
- Lam, M.K.; Oerlemans, C.; Froeling, M.; Deckers, R.; Barten-Van Rijbroek, A.D.; Viergever, M.A.; Moonen, C.T.; Bos, C.; Bartels, L.W. DCE-MRI and IVIM-MRI of rabbit Vx2 tumors treated with MR-HIFU-induced mild hyperthermia. J. Ther. Ultrasound 2016, 4, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov NCT02364557. Available online: https://clinicaltrials.gov/ct2/show/NCT02364557 (accessed on 1 March 2020).
- Saw, C.B.; Li, S. 3D treatment planning systems. Med. Dosim. 2018, 43, 103–105. [Google Scholar] [CrossRef]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Brown, M.L.; Glanzmann, C.; Huber, G.; Bredell, M.; Rordorf, T.; Studer, G. IMRT/VMAT for malignancies in the head-and-neck region: Outcome in patients aged 80. Strahlenther. Onkol. 2016, 192, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Fung-Kee-Fung, S.D.; Hackett, R.; Hales, L.; Warren, G.; Singh, A.K. A prospective trial of volumetric intensity-modulated arc therapy vs. conventional intensity modulated radiation therapy in advanced head and neck cancer. World J. Clin. Oncol. 2012, 3, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, D.J., 3rd.; Singh, A.K. Single-fraction stereotactic body radiation therapy for sinonasal malignant melanoma. Head Neck 2015, 37, E34–E37. [Google Scholar] [CrossRef] [PubMed]
- Bolcaen, J.; Descamps, B.; Deblaere, K.; Boterberg, T.; Hallaert, G.; Van den Broecke, C.; Decrock, E.; Vral, A.; Leybaert, L.; Vanhove, C.; et al. MRI-guided 3D conformal arc micro-irradiation of a F98 glioblastoma rat model using the Small Animal Radiation Research Platform (SARRP). J. Neurooncol. 2014, 120, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.D.; Arai, T.J.; Campbell, I.J.; Jiang, S.B.; Mason, R.P.; Stojadinovic, S. MR-CBCT image-guided system for radiotherapy of orthotopic rat prostate tumors. PLoS ONE 2018, 13, e0198065. [Google Scholar] [CrossRef]
- Dobiasch, S.; Kampfer, S.; Habermehl, D.; Duma, M.N.; Felix, K.; Strauss, A.; Schilling, D.; Wilkens, J.J.; Combs, S.E. MRI-based high-precision irradiation in an orthotopic pancreatic tumor mouse model: A treatment planning study. Strahlenther. Onkol. 2018, 194, 944–952. [Google Scholar] [CrossRef]
- Wang, C.W.; Zhou, Y.; Bai, J.P.; Liu, H.; Liu, Y.; Shi, G.L.; Ding, J.J.; Ma, D.H.; Li, W.T.; Xie, P.M.; et al. Application of Volumetric Modulated Arc Therapy and Simultaneous Integrated Boost Techniques to Prepare “Safe Margin” in the Rabbit VX2 Limb Tumor Model. Med. Sci. Monit. 2015, 21, 2397–2405. [Google Scholar] [CrossRef] [Green Version]
- Dolera, M.; Malfassi, L.; Mazza, G.; Urso, G.; Sala, M.; Marcarini, S.; Carrara, N.; Pavesi, S.; Finesso, S.; Kent, M.S. Feasibility for Using Hypofractionated Stereotactic Volumetric Modulated Arc Radiotherapy (Vmat) with Adaptive Planning for Treatment of Thymoma in Rabbits: 15 Cases. Vet. Radiol. Ultrasound 2016, 57, 313–320. [Google Scholar] [CrossRef]

| Prescription Dose Constraints | ||||||||
|---|---|---|---|---|---|---|---|---|
| PTV (cc) | Rb1 | 8 | Rx IDL (%): | Rb1 | 80 | |||
| Rb2 | 10 | Rb2 | 80 | |||||
| Rb3 | 19.3 | Rb3 | 90 | |||||
| 100% Rx Dose | 50% Rx Dose | 105% Rx Dose outside PTV | ||||||
| 30 Gy vol (cc) | Rb1 | 7.5 | 15Gy vol (cc) | Rb1 | 8 | 31.5 Gy vol (cc) | Rb1 | 0 |
| Rb2 | 9.5 | Rb2 | 10 | Rb2 | 0 | |||
| Rb3 | 18.4 | Rb3 | 19.3 | Rb3 | 0 | |||
| Rx Isodose Coverage | ||||||||
| Parameter | Limit | Actual | Protocol Limit Met? | |||||
| Rx Isodose (%) | ≥ 60%, ≤ 90% | Rb1 | 80 | YES | ||||
| Rb2 | 80 | YES | ||||||
| Rb3 | 90 | YES | ||||||
| % PTV covered by 30 Gy | ≥ 95 | Rb1 | 95 | YES | ||||
| Rb2 | 95 | YES | ||||||
| Rb3 | 95 | YES | ||||||
| Vol outside PTV ≥ 105% Rx dose (cc) | 0 | Rb1 | 0 | YES | ||||
| Rb2 | 0 | YES | ||||||
| Rb3 | 0 | YES | ||||||
| Conformality - Vol rx IDL / Vol PTV | ≤ 1.2 | Rb1 | 0.95 | YES | ||||
| Rb2 | 0.95 | YES | ||||||
| Rb3 | 0.96 | YES | ||||||
| Max dose beyond PTV + 2.0 cm (% Rx Dose) | Rb1 | ≤ 58.52 | 56.6 | YES | ||||
| Rb2 | ≤ 60.24 | 56.0 | YES | |||||
| Rb3 | ≤ 68.26 | 53.3 | YES | |||||
| Vol 50% Rx Dose / Vol PTV | Rb1 | ≤ 5.77 | 1 | YES | ||||
| Rb2 | ≤ 5.67 | 1 | YES | |||||
| Rb3 | ≤ 5.18 | 1 | YES | |||||
| Dose Constraints for OAR. | |||||
|---|---|---|---|---|---|
| Organ | Parameter | Limit | Actual | Protocol Limit Met? | |
| Spinal Cord | Volume > 8.0 Gy | ≤ 1.20 cc | Rb1 | 0.00 cc | Yes |
| Rb2 | 0.00 cc | Yes | |||
| Rb3 | 0.00 cc | Yes | |||
| Volume > 10.0 Gy | ≤ 0.35 cc | Rb1 | 0.00 cc | Yes | |
| Rb2 | 0.00 cc | Yes | |||
| Rb3 | 0.00 cc | Yes | |||
| Trachea | Volume > 17.4 Gy | ≤ 4.00 cc | Rb1 | 1.00 cc | Yes |
| Rb2 | 0.00 cc | Yes | |||
| Rb3 | 0.00 cc | Yes | |||
| Skin (surface − 2 mm) | Volume > 25.5 Gy | ≤ 10.00 cc | Rb1 | 7.69 cc | Yes |
| Rb2 | 7.20 cc | Yes | |||
| Rb3 | 4.37 cc | Yes | |||
| Volume > 27.5 Gy | ≤ 0.03 cc | Rb1 | 0.00 cc | Yes | |
| Rb2 | 0.00 cc | Yes | |||
| Rb3 | 0.02 cc | Yes | |||
| Brain | Volume = 10 Gy | < 10 cc | Rb1 | 0.00 cc | Yes |
| Rb2 | 1.50 cc | Yes | |||
| Rb3 | 2.02 cc | Yes | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajab Bolookat, E.; Malhotra, H.; Rich, L.J.; Sexton, S.; Curtin, L.; Spernyak, J.A.; Singh, A.K.; Seshadri, M. Development and Validation of a Clinically Relevant Workflow for MR-Guided Volumetric Arc Therapy in a Rabbit Model of Head and Neck Cancer. Cancers 2020, 12, 572. https://doi.org/10.3390/cancers12030572
Rajab Bolookat E, Malhotra H, Rich LJ, Sexton S, Curtin L, Spernyak JA, Singh AK, Seshadri M. Development and Validation of a Clinically Relevant Workflow for MR-Guided Volumetric Arc Therapy in a Rabbit Model of Head and Neck Cancer. Cancers. 2020; 12(3):572. https://doi.org/10.3390/cancers12030572
Chicago/Turabian StyleRajab Bolookat, Eftekhar, Harish Malhotra, Laurie J. Rich, Sandra Sexton, Leslie Curtin, Joseph A. Spernyak, Anurag K. Singh, and Mukund Seshadri. 2020. "Development and Validation of a Clinically Relevant Workflow for MR-Guided Volumetric Arc Therapy in a Rabbit Model of Head and Neck Cancer" Cancers 12, no. 3: 572. https://doi.org/10.3390/cancers12030572






