Animal Models in Microbeam Radiation Therapy: A Scoping Review
Abstract
1. Introduction
1.1. Background
1.2. Rationale
1.3. Objective
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
- Medline (Ovid) (incl. Epub Ahead of Print, In-Process and Other Non-Indexed Citations, Medline Daily and Ovid Medline Versions (1946–October 02, 2019)
- Embase (Ovid) (1947–October 02, 2019)
- Cochrane Library (1996–October 02, 2019)
- Scopus (1788–October 02, 2019)
- ICTRP Trial Register (WHO)
- ClinicalTrials.gov
- Livivo Search portal
2.4. Search Strategy
2.5. Selection of Sources of Evidence
2.6. Data Charting and Synthesis of Results
3. Results
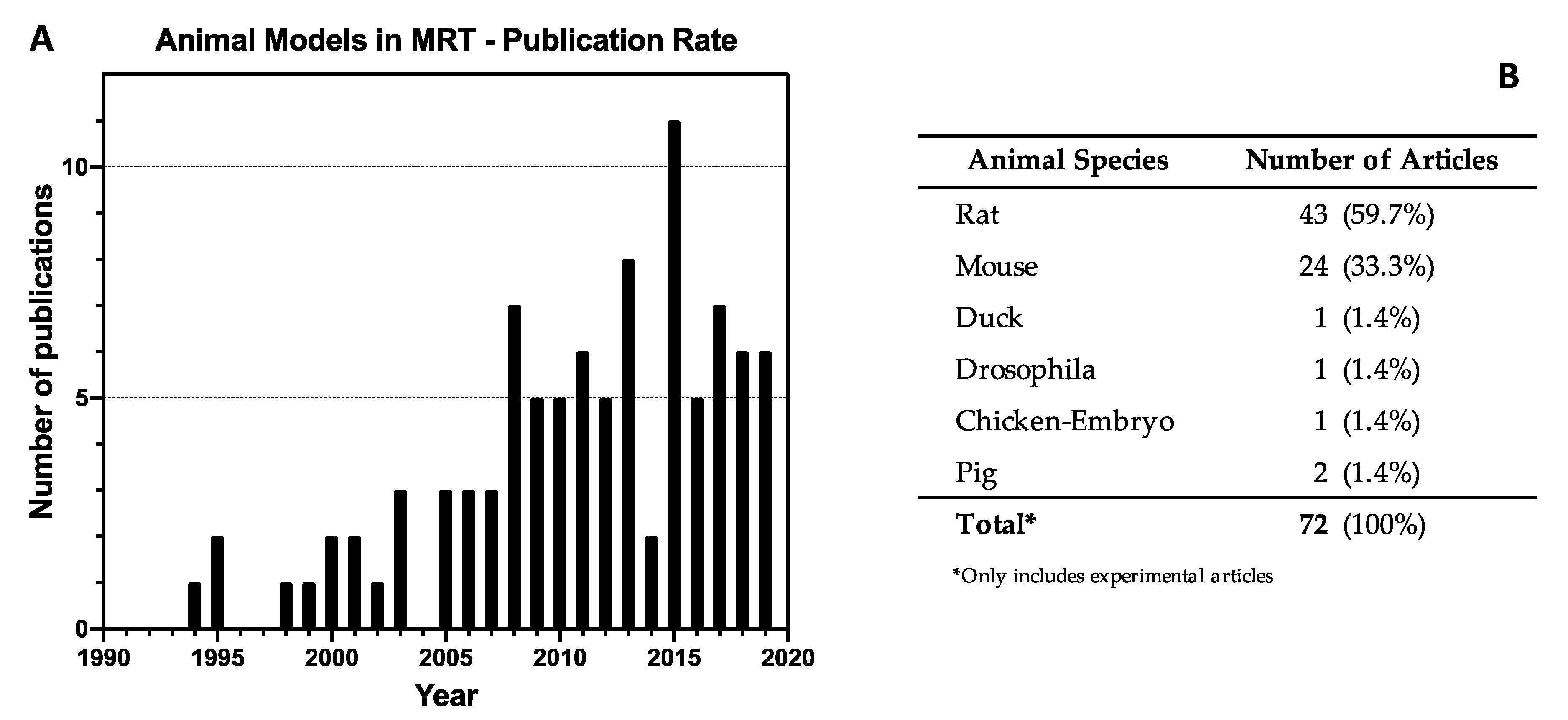
3.1. Literature Selection
3.2. Study Characteristics
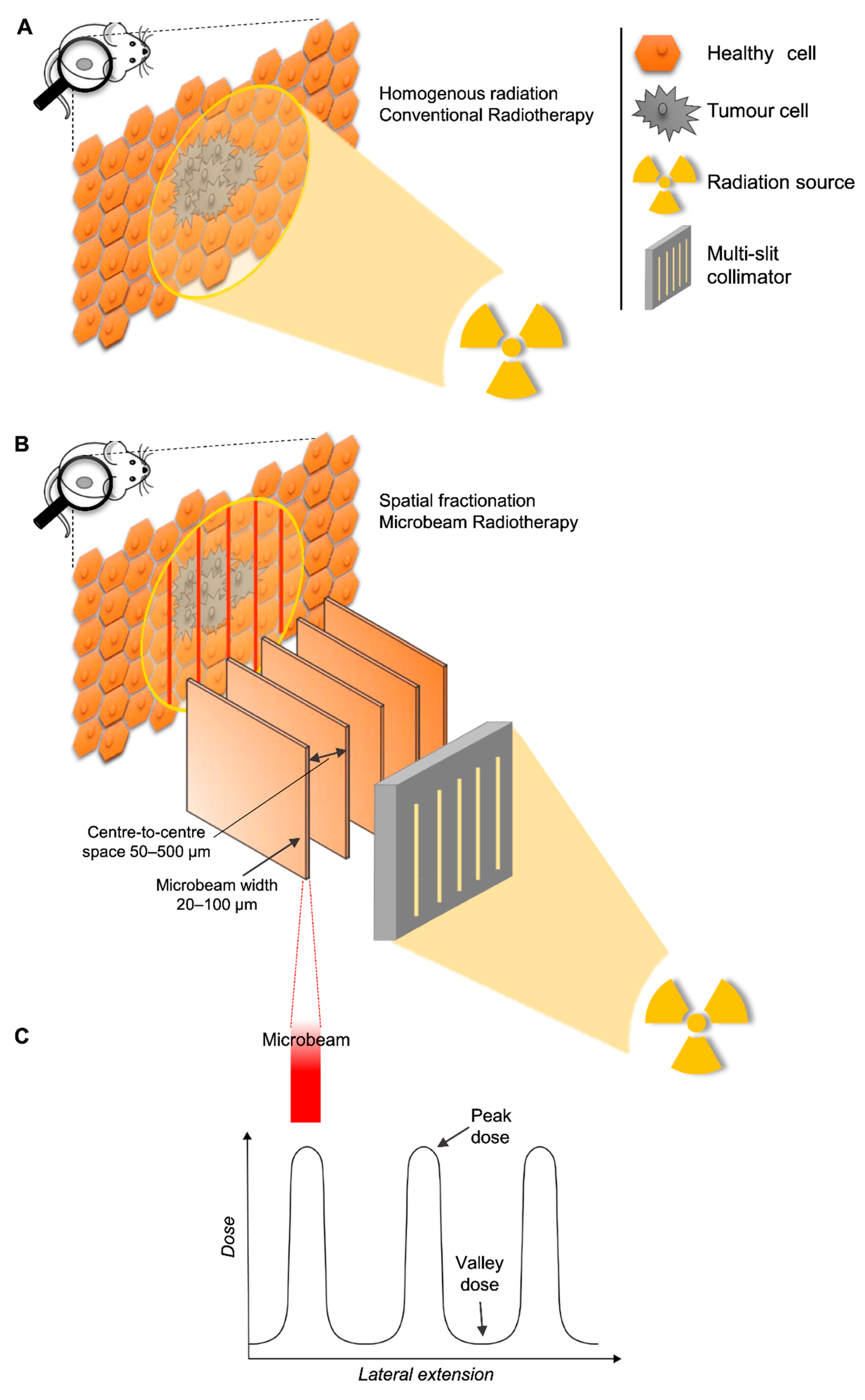
3.3. Technical Parameters
- Trajectory of delivery: if two or more arrays were delivered in the same trajectory or direction, they were called unidirectional. However, if the arrays were delivered in two or more trajectories, they were referred to as bidirectional or multidirectional.
- Anatomical plane: if two or more MRT arrays were delivered in the same anatomical plane they were also called co-planar. When two co-planar arrays were delivered without overlap of the peak-dose regions, they were referred to as interlaced. Delivery in different planes was referred to as cross-planar where irradiation with a single unidirectional MRT array was followed by a rotation of the animal and a second (or third) MRT array was delivered (creating a grid-like pattern in the target volume). When arrays in this manner intersect at 90°, they are orthogonal (irrespective of the anatomical plane or trajectory).
3.4. Animal Models in MRT
3.4.1. MRT for Cancer
3.4.2. Glioma
3.4.3. Mammary Tumours
3.4.4. Melanoma
3.4.5. Squamous Cell Carcinoma
3.4.6. MRT for non-neoplastic diseases
Epilepsy:
Restenosis:
Spinal cord Injury:
3.4.7. Normal Tissue Effects of MRT
Central Nervous System, Adults:
Developing CNS:
Eye:
Vasculature:
Skin:
Other tissues:
Importance of Valley-Doses:
4. Discussion
4.1. Potential Underlying Mechanisms of Microbeam Radiation Therapy
4.1.1. Targeted and Non-Targeted Effects of MRT
4.1.2. MRT Selectively Disrupts Immature Blood Vessel
4.1.3. MRT Transiently Increases Tumour Blood Vessel Permeability
4.1.4. MRT Boosts the Recruitment of Circulating Immune Cells into Tumours
4.2. Clinical Translation
4.2.1. Comparative Therapeutic Effects of MRT and BB Radiotherapy
4.2.2. Identifying Optimal Targets and Strategies for MRT
4.2.3. Candidate Animal Models
4.3. Limitations of this Study
5. Conclusion and Recommendations
5.1. Appropriate Controls
5.2. Standardization of the MRT Array and Doses
5.3. Preclinical Animal Models
5.4. Veterinary Trials
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Causes of death statistics - Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Causes_of_death_statistics#Main_findings (accessed on 17 January 2020).
- Barnett, G.C.; West, C.M.L.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.P.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, D.N.; Spanne, P.; Dilmanian, F.A.; Sandborg, M. Microbeam radiation therapy. Med. Phys. 1992, 19, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Bräuer-Krisch, E.; Serduc, R.; Siegbahn, E.A.; Le Duc, G.; Prezado, Y.; Bravin, A.; Blattmann, H.; Laissue, J.A. Effects of pulsed, spatially fractionated, microscopic synchrotron X-ray beams on normal and tumoral brain tissue. Mutat. Res. 2010, 704, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Potez, M.; Bouchet, A.; Wagner, J.; Donzelli, M.; Bräuer-Krisch, E.; Hopewell, J.W.; Laissue, J.; Djonov, V. Effects of Synchrotron X-Ray Micro-beam Irradiation on Normal Mouse Ear Pinnae. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron Microbeam Radiation Therapy as a New Approach for the Treatment of Radioresistant Melanoma: Potential Underlying Mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136. [Google Scholar] [CrossRef]
- Peters, M.; Godfrey, C.; McInerney, P.; Baldini Soares, C.; Khalil, H.; Parker, D. Chapter 11: Scoping Reviews. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute, 2017; Available online: https://reviewersmanual.joannabriggs.org/ (accessed on 25 February 2020).
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K.; et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016, 16, 15. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467. [Google Scholar] [CrossRef]
- Bartzsch, S.; Lott, J.; Welsch, K.; Bräuer-Krisch, E.; Oelfke, U. Micrometer-resolved film dosimetry using a microscope in microbeam radiation therapy. Med. Phys. 2015, 42, 4069–4079. [Google Scholar] [CrossRef]
- Bräuer-Krisch, E.; Requardt, H.; Brochard, T.; Berruyer, G.; Renier, M.; Laissue, J.A.; Bravin, A. New technology enables high precision multislit collimators for microbeam radiation therapy. Rev. Sci. Instrum. 2009, 80, 074301. [Google Scholar] [CrossRef] [PubMed]
- Bravin, A.; Olko, P.; Schültke, E.; Wilkens, J.J. SYRA3 COST Action - Microbeam radiation therapy: Roots and prospects. Physica. Medica. 2015, 31, 561–563. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Button, T.M.; Le Duc, G.; Zhong, N.; Peña, L.A.; Smith, J.A.L.; Martinez, S.R.; Bacarian, T.; Tammam, J.; Ren, B.; et al. Response of rat intracranial 9L gliosarcoma to microbeam radiation therapy. Neuro. Oncol. 2002, 4, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Rothkamm, K.; Crosbie, J.C.; Daley, F.; Bourne, S.; Barber, P.R.; Vojnovic, B.; Cann, L.; Rogers, P.a.W. In situ biological dose mapping estimates the radiation burden delivered to “spared” tissue between synchrotron X-ray microbeam radiotherapy tracks. PLoS ONE 2012, 7, e29853. [Google Scholar] [CrossRef]
- Slatkin, D.N.; Spanne, P.; Dilmanian, F.A.; Gebbers, J.O.; Laissue, J.A. Subacute neuropathological effects of microplanar beams of x-rays from a synchrotron wiggler. Proc. Natl. Acad. Sci. USA 1995, 92, 8783–8787. [Google Scholar] [CrossRef]
- Fernandez-Palomo, C.; Schültke, E.; Smith, R.; Bräuer-Krisch, E.; Laissue, J.; Schroll, C.; Fazzari, J.; Seymour, C.; Mothersill, C. Bystander effects in tumor-free and tumor-bearing rat brains following irradiation by synchrotron X-rays. Int. J. Radiat. Biol. 2013, 89, 445–453. [Google Scholar] [CrossRef]
- Régnard, P.; Duc, G.L.; Bräuer-Krisch, E.; Troprès, I.; Siegbahn, E.A.; Kusak, A.; Clair, C.; Bernard, H.; Dallery, D.; Laissue, J.A.; et al. Irradiation of intracerebral 9L gliosarcoma by a single array of microplanar x-ray beams from a synchrotron: Balance between curing and sparing. Phys. Med. Biol. 2008, 53, 861–878. [Google Scholar] [CrossRef]
- Bräuer-Krisch, E.; Requardt, H.; Régnard, P.; Corde, S.; Siegbahn, E.; LeDuc, G.; Brochard, T.; Blattmann, H.; Laissue, J.; Bravin, A. New irradiation geometry for microbeam radiation therapy. Phys. Med. Biol. 2005, 50, 3103–3111. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef]
- Laissue, J.A.; Geiser, G.; Spanne, P.O.; Dilmanian, F.A.; Gebbers, J.O.; Geiser, M.; Wu, X.Y.; Makar, M.S.; Micca, P.L.; Nawrocky, M.M.; et al. Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated X rays. Int. J. Cancer 1998, 78, 654–660. [Google Scholar] [CrossRef]
- Laissue, J.A.; Lyubimova, N.; Wagner, H.-P.; Archer, D.W.; Slatkin, D.N.; Di Michiel, M.; Nemoz, C.; Renier, M.; Brauer, E.; Spanne, P.O.; et al. Microbeam radiation therapy. In Proceedings of the Medical Applications of Penetrating Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1485–1494. [Google Scholar] [CrossRef]
- Rothman, E.Z.; Hulbert, S.L.; Lazarz, N.M. Microplanar Beam Radiotherapy. Proc. SPIE 1999. [Google Scholar] [CrossRef]
- Slatkin, D.N.; Dilmanian, F.A.; Nawrocky, M.M.; Spanne, P.; Gebbers, J.-O.; Archer, D.W.; Laissue, J.A. Design of a multislit, variable width collimator for microplanar beam radiotherapy. Rev. Sci. Instru. 1995, 66, 1459–1460. [Google Scholar] [CrossRef][Green Version]
- Bouchet, A.; Sakakini, N.; El Atifi, M.; Le Clec’h, C.; Brauer, E.; Moisan, A.; Deman, P.; Rihet, P.; Le Duc, G.; Pelletier, L. Early gene expression analysis in 9L orthotopic tumor-bearing rats identifies immune modulation in molecular response to synchrotron microbeam radiation therapy. PLoS ONE 2013, 8, e81874. [Google Scholar] [CrossRef]
- Bouchet, A.; Sakakini, N.; El Atifi, M.; Le Clec’h, C.; Bräuer-Krisch, E.; Rogalev, L.; Laissue, J.; Rihet, P.; Le Duc, G.; Pelletier, L. Identification of AREG and PLK1 pathway modulation as a potential key of the response of intracranial 9L tumor to microbeam radiation therapy. Int. J. cancer 2015, 136, 2705–2716. [Google Scholar] [CrossRef]
- Bouchet, A.; Bräuer-Krisch, E.; Prezado, Y.; El Atifi, M.; Rogalev, L.; Le Clec’h, C.; Laissue, J.A.; Pelletier, L.; Le Duc, G. Better Efficacy of Synchrotron Spatially Microfractionated Radiation Therapy Than Uniform Radiation Therapy on Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1485–1494. [Google Scholar] [CrossRef]
- Bouchet, A.; Lemasson, B.; Le Duc, G.; Maisin, C.; Bräuer-Krisch, E.; Siegbahn, E.A.; Renaud, L.; Khalil, E.; Rémy, C.; Poillot, C.; et al. Preferential effect of synchrotron microbeam radiation therapy on intracerebral 9l gliosarcoma vascular networks. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1503–1512. [Google Scholar] [CrossRef]
- Bouchet, A.; Lemasson, B.; Christen, T.; Potez, M.; Rome, C.; Coquery, N.; Le Clec’H, C.; Moisan, A.; Bräuer-Krisch, E.; Leduc, G.; et al. Synchrotron microbeam radiation therapy induces hypoxia in intracerebral gliosarcoma but not in the normal brain. Radiot. Oncol. 2013, 108, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Serduc, R.; Bouchet, A.; Bräuer-Krisch, E.; Laissue, J.A.; Spiga, J.; Sarun, S.; Bravin, A.; Fonta, C.; Renaud, L.; Boutonnat, J.; et al. Synchrotron microbeam radiation therapy for rat brain tumor palliation - Influence of the microbeam width at constant valley dose. Phys. Med. Biol. 2009, 54, 6711–6724. [Google Scholar] [CrossRef] [PubMed]
- Serduc, R.; Bräuer-Krisch, E.; Bouchet, A.; Renaud, L.; Brochard, T.; Bravin, A.; Laissue, J.A.; Le Duc, G. First trial of spatial and temporal fractionations of the delivered dose using synchrotron microbeam radiation therapy. J. Synchrotron. Radiat. 2009, 16, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Régnard, P.; Bräuer-Krisch, E.; Troprès, I.; Keyriläinen, J.; Bravin, A.; Le Duc, G. Enhancement of survival of 9L gliosarcoma bearing rats following intracerebral delivery of drugs in combination with microbeam radiation therapy. Eur. J. Radiol. 2008, 68, S151–S155. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, A.; Boumendjel, A.; Khalil, E.; Serduc, R.; Brauer, E.; Siegbahn, E.A.; Laissue, J.A.; Boutonnat, J. Chalcone JAI-51 improves efficacy of synchrotron microbeam radiation therapy of brain tumors. J. Synchrotron. Radiat. 2012, 19, 478–482. [Google Scholar] [CrossRef]
- Lemasson, B.; Bouchet, A.; Maisin, C.; Christen, T.; Le Duc, G.; Rémy, C.; Barbier, E.L.; Serduc, R. Multiparametric MRI as an early biomarker of individual therapy effects during concomitant treatment of brain tumours. NMR Biomed. 2015, 28, 1163–1173. [Google Scholar] [CrossRef]
- Smilowitz, H.M.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Di Michiel, M.; Gebbers, J.O.; Hanson, A.L.; Lyubimova, N.; Slatkin, D.N.; Stepanek, J.; et al. Synergy of gene-mediated immunoprophylaxis and microbeam radiation therapy for advanced intracerebral rat 9L gliosarcomas. J. Neurooncol. 2006, 78, 135–143. [Google Scholar] [CrossRef]
- Schültke, E.; Juurlink, B.H.J.; Ataelmannan, K.; Laissue, J.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Minczewska, J.; Crosbie, J.; Taherian, H.; et al. Memory and survival after microbeam radiation therapy. Eur. J. Radiol. 2008, 68, S142–S146. [Google Scholar] [CrossRef]
- Schültke, E.; Bräuer-Krisch, E.; Blattmann, H.; Requardt, H.; Laissue, J.A.; Hildebrandt, G. Survival of rats bearing advanced intracerebral F 98 tumors after glutathione depletion and microbeam radiation therapy: Conclusions from a pilot project. Radiat. Oncol. 2018, 13, 89. [Google Scholar] [CrossRef]
- Bouchet, A.; Potez, M.; Coquery, N.; Rome, C.; Lemasson, B.; Bräuer-Krisch, E.; Rémy, C.; Laissue, J.; Barbier, E.L.; Djonov, V.; et al. Permeability of Brain Tumor Vessels Induced by Uniform or Spatially Microfractionated Synchrotron Radiation Therapies. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1174–1182. [Google Scholar] [CrossRef]
- Fernandez-Palomo, C.; Bräuer-Krisch, E.; Laissue, J.; Vukmirovic, D.; Blattmann, H.; Seymour, C.; Schültke, E.; Mothersill, C. Use of synchrotron medical microbeam irradiation to investigate radiation-induced bystander and abscopal effects in vivo. Phys. Med. 2015, 31, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Palomo, C.; Mothersill, C.; Laissue, J.; Seymour, C.; Schültke, E.; Seymour, C.; Schültke, E. γ-H2AX as a marker for dose deposition in the brain of wistar rats after synchrotron microbeam radiation. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Worobec, S.; Schultke, E. Assessment of rat optic nerve damage due to microbeam radiation therapy in the treatment of glioblastomas. Biomed. Sci. Instr. 2008, 44, 501–506. [Google Scholar]
- Serduc, R.; Christen, T.; Laissue, J.A.; Farion, R.R.; Bouchet, A.; Van der Sanden, B.; Segebarth, C.; Brauer-Krisch, E.; Le Duc, G.G.; Bravin, A.; et al. Brain tumor vessel response to synchrotron microbeam radiation therapy: A short-term in vivo study. Phys. Med. Boil. 2008, 53, 3609–3622. [Google Scholar] [CrossRef]
- Fernandez-Palomo, C.; Schültke, E.; Bräuer-Krisch, E.; Laissue, J.A.; Blattmann, H.; Seymour, C.; Mothersill, C. Investigation of abscopal and bystander effects in immunocompromised mice after exposure to pencilbeam and microbeam synchrotron radiation. Heal. Phys. 2016, 111, 149–159. [Google Scholar] [CrossRef]
- Uyama, A.; Kondoh, T.; Nariyama, N.; Umetani, K.; Fukumoto, M.; Shinohara, K.; Kohmura, E. A narrow microbeam is more effective for tumor growth suppression than a wide microbeam: An in vivo study using implanted human glioma cells. J. Synchrotron Radiat. 2011, 18, 671–678. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Morris, G.M.; Zhong, N.; Bacarian, T.; Hainfeld, J.F.; Kalef-Ezra, J.; Brewington, L.J.; Tammam, J.; Rosen, E.M. Murine EMT-6 carcinoma: High therapeutic efficacy of microbeam radiation therapy. Radiat. Res. 2003, 159, 632–641. [Google Scholar] [CrossRef]
- Crosbie, J.C.; Anderson, R.L.; Rothkamm, K.; Restall, C.M.; Cann, L.; Ruwanpura, S.; Meachem, S.; Yagi, N.; Svalbe, I.; Lewis, R.A.; et al. Tumor cell response to synchrotron microbeam radiation therapy differs markedly from cells in normal tissues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 886–894. [Google Scholar] [CrossRef]
- Sprung, C.N.; Yang, Y.; Forrester, H.B.; Li, J.; Zaitseva, M.; Cann, L.; Restall, T.; Anderson, R.L.; Crosbie, J.C.; Rogers, P.A.W. Genome-Wide Transcription Responses to Synchrotron Microbeam Radiotherapy. Radiat. Res. 2012, 178, 249. [Google Scholar] [CrossRef]
- Ibahim, M.J.; Yang, Y.; Crosbie, J.C.; Stevenson, A.; Cann, L.; Paiva, P.; Rogers, P.A. Eosinophil-Associated Gene Pathways but not Eosinophil Numbers are Differentially Regulated between Synchrotron Microbeam Radiation Treatment and Synchrotron Broad-Beam Treatment by 48 Hours Postirradiation. Radiat. Res. 2015, 185, 60. [Google Scholar] [CrossRef]
- Yang, Y.; Swierczak, A.; Ibahim, M.; Paiva, P.; Cann, L.; Stevenson, A.W.; Crosbie, J.C.; Anderson, R.L.; Rogers, P.A.W. Synchrotron microbeam radiotherapy evokes a different early tumor immunomodulatory response to conventional radiotherapy in EMT6.5 mammary tumors. Radiot. Oncol. 2019, 133, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Crosbie, J.C.; Puskar, L.; Rogers, P.A.W. Microbeam-irradiated tumour tissue possesses a different infrared absorbance profile compared to broad beam and sham-irradiated tissue. Int. J. Radiat. Biol. 2013, 89, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.J.; Koonce, N.A.; Dings, R.P.M.; Siegel, E.; Moros, E.G.; Bräuer-Krisch, E.; Corry, P.M. Microbeam Radiation Therapy Alters Vascular Architecture and Tumor Oxygenation and is Enhanced by a Galectin-1 Targeted Anti-Angiogenic Peptide. Radiat. Res. 2012, 177, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Hanson, A.L.; Nawrocky, M.M.; Micca, P.L.; Slatkin, D.N.; Laissue, J.A. Radiosurgical palliation of aggressive murine SCCVII squamous cell carcinomas using synchrotron-generated X-ray microbeams. Br. J. Radiol. 2006, 79, 71–75. [Google Scholar] [CrossRef]
- Fardone, E.; Bravin, A.; Conti, A.; Bräuer-Krisch, E.; Requardt, H.; Bucci, D.; Le Duc, G.; Battaglia, G.; Romanelli, P. Rat sensorimotor cortex tolerance to parallel transections induced by synchrotron-generated X-ray microbeams. Sci. Rep. 2017, 7, 14290. [Google Scholar] [CrossRef]
- Fardone, E.; Pouyatos, B.; Bräuer-Krisch, E.; Bartzsch, S.; Mathieu, H.; Requardt, H.; Bucci, D.; Barbone, G.; Coan, P.; Battaglia, G.; et al. Synchrotron-generated microbeams induce hippocampal transections in rats. Sci. Rep. 2018, 8, 184. [Google Scholar] [CrossRef]
- Romanelli, P.; Fardone, E.; Battaglia, G.; Brauer-Krisch, E.; Prezado, Y.; Requardt, H.; Le Duc, G.; Nemoz, C.; Anschel, D.J.; Spiga, J.; et al. Synchrotron-generated microbeam sensorimotor cortex transections induce seizure control without disruption of neurological functions. PLoS ONE 2013, 8, e53549. [Google Scholar] [CrossRef][Green Version]
- Pouyatos, B.; Serduc, R.; Chipaux, M.; Chabrol, T.; Brauer-Krisch, E.; Nemoz, C.; Mathieu, H.; David, O.; Renaud, L.; Prezado, Y.; et al. Synchrotron X-ray interlaced microbeams suppress paroxysmal oscillations in neuronal networks initiating generalized epilepsy. Neurobiol. Dis. 2013, 51, 152–160. [Google Scholar] [CrossRef]
- Studer, F.; Serduc, R.; Pouyatos, B.; Chabrol, T.; Bräuer-Krisch, E.; Donzelli, M.; Nemoz, C.; Laissue, J.A.; Estève, F.; Depaulis, A. Synchrotron X-ray microbeams: A promising tool for drug-resistant epilepsy treatment. Phys. Med. 2015, 31, 607–614. [Google Scholar] [CrossRef]
- Pouyatos, B.; Nemoz, C.; Chabrol, T.; Potez, M.; Bräuer, E.; Renaud, L.; Pernet-Gallay, K.; Estève, F.; David, O.; Kahane, P.; et al. Synchrotron X-ray microtransections: A non invasive approach for epileptic seizures arising from eloquent cortical areas. Sci. Rep. 2016, 6, 27250. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Kalef-Ezra, J.; Petersen, M.J.; Bozios, G.; Vosswinkel, J.; Giron, F.; Ren, B.; Yakupov, R.; Antonakopoulos, G. Could X-ray microbeams inhibit angioplasty-induced restenosis in the rat carotid artery? Cardiovasc. Radiat. Med. 2003, 4, 139–145. [Google Scholar] [CrossRef]
- Ishii, T.; Ueyama, T.; Shigyo, M.; Kohta, M.; Kondoh, T.; Kuboyama, T.; Uebi, T.; Hamada, T.; Gutmann, D.H.; Aiba, A.; et al. A novel Rac1-GSPT1 signaling pathway controls astrogliosis following central nervous system injury. J. Biol. Chem. 2017, 292, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Laissue, J.A.; Bartzsch, S.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Dalléry, D.; Djonov, V.; Hanson, A.L.; Hopewell, J.W.; Kaser-Hotz, B.; et al. Response of the rat spinal cord to X-ray microbeams. Radiot. Oncol. 2013, 106, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Laissue, J.A.; Blattmann, H.; Michiel, M.D.; M.d, D.N.S.; Lyubimova, N.; Guzman, R.; Zimmermann, W.; Birrer, S.; Bley, T.; Kircher, P.; et al. Weanling piglet cerebellum: A surrogate for tolerance to MRT (microbeam radiation therapy) in pediatric neuro-oncology. Proc. SPIE 2001. [Google Scholar] [CrossRef]
- Dilmanian, F.A.; Morris, G.M.; Le Duc, G.; Huang, X.; Ren, B.; Bacarian, T.; Allen, J.C.; Kalef-Ezra, J.; Orion, I.; Rosen, E.M.; et al. Response of avian embryonic brain to spatially segmented x-ray microbeams. Cell Mol. Biol. (Noisy-le-grand) 2001, 47, 485–493. [Google Scholar]
- Sandmeyer, L.S.; Sheikh, A.; Schültke, E.; Fourney, D.; Grahn, B.H. Chronic ocular lesions associated with bi-directional microbeam radiation therapy in an experimental rat study for therapy of C6 and F98 gliomas. Vet. Ophthalmol. 2008, 11, 290–298. [Google Scholar] [CrossRef]
- Laissue, J.A.; Blattmann, H.; Siegbahn, E.A.; Slatkin, D.N. A misprint in a description of microbeam irradiations of rats’ heads. Vet. Ophthalmol. 2012, 15, 210–211. [Google Scholar] [CrossRef]
- Brönnimann, D.; Bouchet, A.; Schneider, C.; Potez, M.; Serduc, R.; Bräuer-Krisch, E.; Graber, W.; Von Gunten, S.; Laissue, J.A.; Djonov, V. Synchrotron microbeam irradiation induces neutrophil infiltration, thrombocyte attachment and selective vascular damage in vivo. Sci. Rep. 2016, 6, 33601. [Google Scholar] [CrossRef]
- Sabatasso, S.; Laissue, J.A.; Hlushchuk, R.; Graber, W.; Bravin, A.; Bräuer-Krisch, E.; Corde, S.; Blattmann, H.; Gruber, G.; Djonov, V. Microbeam radiation-induced tissue damage depends on the stage of vascular maturation. Int. J. Radiat. Biol. Phys. 2011, 80, 1522–1532. [Google Scholar] [CrossRef]
- Serduc, R.; Vérant, P.; Vial, J.C.; Farion, R.; Rocas, L.; Rémy, C.; Fadlallah, T.; Brauer, E.; Bravin, A.; Laissue, J.; et al. In vivo two-photon microscopy study of short-term effects of microbeam irradiation on normal mouse brain microvasculature. Int. J. Radiat. Biol. Phys. 2006, 64, 1519–1527. [Google Scholar] [CrossRef]
- Dilmanian, F.a.; Qu, Y.; Liu, S.; Cool, C.D.; Gilbert, J.; Hainfeld, J.F.; Kruse, C.A.; Laterra, J.; Lenihan, D.; Nawrocky, M.M.; et al. X-ray microbeams: Tumor therapy and central nervous system research. Nucl. Instrum. Methods Phys. Res. A 2005, 548, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Serduc, R.; Van De Looij, Y.; Francony, G.; Verdonck, O.; Van Der Sanden, B.; Laissue, J.; Farion, R.; Bräuer-Krisch, E.; Siegbahn, E.A.; Bravin, A.; et al. Characterization and quantification of cerebral edema induced by synchrotron x-ray microbeam radiation therapy. Phys. Med. Biol. 2008, 53, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sanden, B.; Bräuer-Krisch, E.; Siegbahn, E.A.; Ricard, C.; Vial, J.C.; Laissue, J. Tolerance of arteries to microplanar X-ray beams. Int. J. Radiat. Biol. Phys. 2010, 77, 1545–1552. [Google Scholar] [CrossRef]
- Zhong, N.; Morris, G.M.; Bacarian, T.; Rosen, E.M.; Avraham Dilmanian, F. Response of Rat Skin to High-Dose Unidirectional X-Ray Microbeams: A Histological Study. Radiat. Res. 2003, 160, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.; Lobachevsky, P.N.; Palazzolo, J.S.; Forrester, H.; Haynes, N.M.; Ivashkevich, A.; Stevenson, A.W.; Hall, C.J.; Ntargaras, A.; Kotsaris, V.; et al. Localized synchrotron irradiation of mouse skin induces persistent systemic genotoxic and immune responses. Cancer Res. 2017, 77, 6389–6399. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshika, R.C.U.; Crosbie, J.C.; Kumar, B.; Rogers, P.A.W. Biodosimetric quantification of short-term synchrotron microbeam versus broad-beam radiation damage to mouse skin using a dermatopathological scoring system. Br. J. Radiol. 2011, 84, 833–842. [Google Scholar] [CrossRef]
- Fukunaga, H.; Kaminaga, K.; Sato, T.; Butterworth, K.T.; Watanabe, R.; Usami, N.; Ogawa, T.; Yokoya, A.; Prise, K.M. High-precision microbeam radiotherapy reveals testicular tissue-sparing effects for male fertility preservation. Sci. Rep. 2019, 9, 12618. [Google Scholar] [CrossRef]
- Mukumoto, N.; Nakayama, M.; Akasaka, H.; Shimizu, Y.; Osuga, S.; Miyawaki, D.; Yoshida, K.; Ejima, Y.; Miura, Y.; Umetani, K.; et al. Sparing of tissue by using micro-slit-beam radiation therapy reduces neurotoxicity compared with broad-beam radiation therapy. J. Radiat. Res. 2017, 58, 17–23. [Google Scholar] [CrossRef]
- Romanelli, P.; Bravin, A. Synchrotron-generated microbeam radiosurgery: A novel experimental approach to modulate brain function. Neurol. Res. 2011, 33, 825–831. [Google Scholar] [CrossRef]
- Laissue, J.A.; Blattmann, H.; Wagner, H.P.; Grotzer, M.A.; Slatkin, D.N. Prospects for microbeam radiation therapy of brain tumours in children to reduce neurological sequelae. Dev. Med. Child. Neurol. 2007, 49, 577–581. [Google Scholar] [CrossRef]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, P.M.; Spanne, P.; Di Michiel, M.; Jauch, U.; Blattmann, H.; Laissue, J.A. Tissue lesions caused by microplanar beams of synchrotron-generated X-rays in Drosophila melanogaster. Int. J. Radiat. Biol. 2000, 76, 567–574. [Google Scholar] [PubMed]
- Dilmanian, F.A.; Qu, Y.; Feinendegen, L.E.; Peña, L.A.; Bacarian, T.; Henn, F.A.; Kalef-Ezra, J.; Liu, S.; Zhong, Z.; McDonald, J.W. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: Is a bystander effect involved? Exp. Hematol. 2007, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lobachevsky, P.N.; Ventura, J.; Giannak; Ropoulou, L.; Forrester, H.; Palazzolo, J.S.; Haynes, N.M.; Stevenson, A.W.; Hall, C.J.; Mason, J.; et al. A Functional Immune System Is Required for the Systemic Genotoxic Effects of Localized Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1184–1193. [Google Scholar] [CrossRef]
- Bouchet, A.; Serduc, R.; Laissue, J.A.; Djonov, V. Effects of microbeam radiation therapy on normal and tumoral blood vessels. Phys. Med. 2015, 31, 634–641. [Google Scholar] [CrossRef]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef]
- Livingstone, J.; Adam, J.F.; Crosbie, J.C.; Hall, C.J.; Lye, J.E.; McKinlay, J.; Pelliccia, D.; Pouzoulet, F.; Prezado, Y.; Stevenson, A.W.; et al. Preclinical radiotherapy at the Australian Synchrotron’s Imaging and Medical Beamline: Instrumentation, dosimetry and a small-animal feasibility study. J. Synchrotron Radiat. 2017, 24, 854–865. [Google Scholar] [CrossRef]
- Stevenson, A.W.; Crosbie, J.C.; Hall, C.J.; Häusermann, D.; Livingstone, J.; Lye, J.E. Quantitative characterization of the X-ray beam at the Australian Synchrotron Imaging and Medical Beamline (IMBL). J. Synchrotron Radit. 2017, 24, 110–141. [Google Scholar] [CrossRef]
- Smyth, L.M.L.; Day, L.R.; Woodford, K.; Rogers, P.A.W.; Crosbie, J.C.; Senthi, S. Identifying optimal clinical scenarios for synchrotron microbeam radiation therapy: A treatment planning study. Phys. Med. 2019, 60, 111–119. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but Not Single-Dose Radiotherapy Induces an Immune-Mediated Abscopal Effect when Combined with Anti–CTLA-4 Antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Eling, L.; Bouchet, A.; Nemoz, C.; Djonov, V.; Balosso, J.; Laissue, J.; Bräuer-Krisch, E.; Adam, J.F.; Serduc, R. Ultra high dose rate Synchrotron Microbeam Radiation Therapy. Preclinical evidence in view of a clinical transfer. Radiot. Oncol. 2019, 139, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Grotzer, M.A.; Schültke, E.; Bräuer-Krisch, E.; Laissue, J.A. Microbeam radiation therapy: Clinical perspectives. Phys. Med. 2015, 31, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Balosso, J.; Breslin, T.; Cavaletti, G.; Djonov, V.; Esteve, F.; Grotzer, M.; Hildebrandt, G.; Valdman, A.; Laissue, J. Microbeam radiation therapy - Grid therapy and beyond: A clinical perspective. Br. J. Radiol. 2017, 90, 20170073. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Kitadate, A.; Ikeda, S.; Yamashita, J.; Nakanishi, H.; Takahashi, N.; Asaka, C.; Teshima, K.; Miyagaki, T.; Sugaya, M.; et al. Histone deacetylase inhibitors inhibit metastasis by restoring a tumor suppressive microRNA-150 in advanced cutaneous T-cell lymphoma. Oncotarget 2016, 8, 7572–7585. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sells, R.E.; Hwang, S.T. Upregulation of Inflammatory Cytokines and Oncogenic Signal Pathways Preceding Tumor Formation in a Murine Model of T-Cell Lymphoma in Skin. J. Investig. Dermatol. 2011, 131, 1727–1734. [Google Scholar] [CrossRef]
- Wu, X.; Hwang, S.T. A Microbiota-Dependent, STAT3-Driven Mouse Model of Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2018, 138, 1022–1026. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ayoub, N.M.; Tajmim, A.; Meyer, S.A.; Hill, R.A.; El Sayed, K.A. (−)-Oleocanthal Prevents Breast Cancer Locoregional Recurrence After Primary Tumor Surgical Excision and Neoadjuvant Targeted Therapy in Orthotopic Nude Mouse Models. Cancers 2019, 11, 637. [Google Scholar] [CrossRef]
- Buchakjian, M.R.; Merritt, N.M.; Moose, D.L.; Dupuy, A.J.; Tanas, M.R.; Henry, M.D. A Trp53fl/flPtenfl/fl mouse model of undifferentiated pleomorphic sarcoma mediated by adeno-Cre injection and in vivo bioluminescence imaging. PLoS ONE 2017, 12, e0183469. [Google Scholar] [CrossRef]
- Stewart, E.; Federico, S.M.; Chen, X.; Shelat, A.A.; Bradley, C.; Gordon, B.; Karlstrom, A.; Twarog, N.R.; Clay, M.R.; Bahrami, A.; et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature 2017, 549, 96–100. [Google Scholar] [CrossRef]
- Dass, C.R.; Ek, E.T.; Contreras, K.G.; Choong, P.F. A novel orthotopic murine model provides insights into cellular and molecular characteristics contributing to human osteosarcoma. Clin. Exp. Metastasis 2006, 23, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Lussier, D.M.; Johnson, J.L.; Hingorani, P.; Blattman, J.N. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J. Immunother. Cancer 2015, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yasui, T.; Tamari, K.; Minami, K.; Otani, K.; Isohashi, F.; Seo, Y.; Kambe, R.; Koizumi, M.; Ogawa, K. Radiation enhanced the local and distant anti-tumor efficacy in dual immune checkpoint blockade therapy in osteosarcoma. PLoS ONE 2017, 12, e0189697. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.M.; Selting, K.A. Exploring the Potential Utility of Pet Dogs With Cancer for Studying Radiation-Induced Immunogenic Cell Death Strategies. Front. Oncol. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Rowell, J.L.; McCarthy, D.O.; Alvarez, C.E. Dog models of naturally occurring cancer. Trends Mol. Med. 2011, 17, 380–388. [Google Scholar] [CrossRef] [PubMed]

| Animal | Cancer Type | Number of Arrays | Microbeam | Peak Dose (Gy) | Valley Dose (Gy) | Evaluated Criteria | |
|---|---|---|---|---|---|---|---|
| Width (µm) | Spacing (µm) | ||||||
| Rat [32] | Gliosarcoma (9L) | 1 and 2 | 50 | 200 | 400 entrance dose; 350 @ 1 cm depth | 12.5 @ 1 cm depth | Animal survival, tumour growth, tumour vasculature, and cell proliferation |
| Rat [37] | Gliosarcoma (9L) | 2 | 50 | 200 | 480 entrance dose; 418 @ 1 cm depth | 18.6 @ 1 cm depth | Animal survival, cell cycle, and DNA distribution patterns |
| Rat [33] | Gliosarcoma (9L) | 2 | 50 | 200 | 400 entrance dose; 350 @ 1 cm depth | 12.5 @ 1 cm depth | Tumour vasculature and tumour oxygenation |
| Rat [29] | Gliosarcoma (9L) | 1 | 50 | 200 | 400 dose @ tumour (i.e., @ 7 mm depth) | 18 dose @ tumour (i.e., @ 7 mm depth) | Animal survival and transcriptomics |
| Rat [30] | Gliosarcoma (9L) | 1 | 50 | 200 | 400 dose @ tumour (i.e., @ 7 mm depth) | 8 dose @ tumour (i.e., @ 7 mm depth) | Tumour growth, transcriptomics, and histopathology |
| Rat [31] | Gliosarcoma (9L) | 1 | 50 | 200 | 400 dose @ tumour (i.e., @ 7 mm depth) | 17.4 dose @ tumour (i.e., @ 7 mm depth) | Animal survival, tumour growth, cell proliferation, and gene expression |
| 200 dose @ tumour (i.e., @ 7 mm depth) | 8.7 dose @ tumour (i.e., @ 7 mm depth) | ||||||
| Rat [42] | Glioma (F98) | 2 | 50 | 200 | 241.4 entrance dose | 10.5 @ 9 mm depth | Tumour vasculature and tumour oxygenation |
| Mouse [50] | Mammary (EMT6.5/67NR) | 1 | 25 | 200 | 560 entrance dose | 8.5 @ centre of brain | Animal survival, DNA damage, cell proliferation, and apoptosis |
| 800 entrance dose | 12 @ centre of brain | ||||||
| 2 | 280 entrance dose | 8.5 @ centre of brain | |||||
| 560 entrance dose | 17 @ centre of brain | ||||||
| Rat [15] | Gliosarcoma (9L) | 1 | 27 | 50 | 150 entrance dose; 108 @ centre of brain | 20 @ centre of brain | Animal survival and histopathology |
| 250 entrance dose; 179 @ centre of brain | 34 @ centre of brain | ||||||
| 300 entrance dose; 215 @ centre of brain | 40 @ centre of brain | ||||||
| 75 | 250 entrance dose; 179 @ centre of brain | 17 @ centre of brain | |||||
| 300 entrance dose; 215 @ centre of brain | 20 @ centre of brain | ||||||
| 500 entrance dose; 359 @ centre of brain | 33 @ centre of brain | ||||||
| 100 | 500 entrance dose; 359 @ centre of brain | 19 @ centre of brain | |||||
| Mouse [49] | Mammary (EMT6.5) | 1 | 90 | 300 | 800 dose @ tumour | 16 dose @ tumour | Tumour ablation |
| 890 dose @ tumour | 18 dose @ tumour | ||||||
| 970 dose @ tumour | 19 dose @ tumour | ||||||
| 1740 dose @ tumour | 35 dose @ tumour | ||||||
| 1820 dose @ tumour | 36 dose @ tumour | ||||||
| 1900 dose @ tumour | 38 dose @ tumour | ||||||
| 2 | 90 | 300 | 410 dose @ tumour | 16 dose @ tumour | |||
| 520 dose @ tumour | 21 dose @ tumour | ||||||
| 650 dose @ tumour | 26 dose @ tumour | ||||||
| Rat [19] | Glioma (C6) | 1 | 25 | 200 | 17.5, 35, 70, 350 entrance dose | 0.51, 1.03, 2, 10.3 | Bystander effects in vivo by clonogenic cell survival |
| Rat [44] | Glioma (C6) | 1 | 25 | 200 | 35, 70, 350 entrance dose | NR | DNA damage |
| Rat [43] | Glioma (F98) | 1 | 25 | 200 | 20, 200 entrance dose | NR | Bystander effects in vivo by clonogenic cell survival and cellular calcium fluxes |
| Mouse nude [47] | Glioma (F98) | 50 | 400 | 22, 110 entrance dose | 0.5, 2.5 | Bystander effects in vivo by clonogenic cell survival and cellular calcium fluxes | |
| Mouse [55] | Mammary (4T1) | 1 | 50 | 200 | 150 @ 5 mm depth | 7.5 in a 10-mm solid water phantom | Tumour growth, tumour vasculature, and tumour hypoxia |
| Mouse [52] | Mammary (EMT6.5) | 1 | 25 | 200 | 112, 560 | NR | Immune response by gene expression and histopathology |
| Rat [38] | Gliosarcoma (9L) | 1 | 50 | 200 | 400 entrance dose | NR | Tumour vasculature, and tumour hypoxia |
| Rat [25] | Gliosarcoma (9L) | 1 | 25 | 100 | 625 entrance dose | NR | Animal survival, tumour growth, and histopathology |
| Mouse [56] | Squamous cell carcinoma (SCCVII) | 1 | 35 | 200 | 442, 625, 884 entrance dose | NR | Animal survival, tumour growth, and tumour ablation |
| 70 | 200 | 442 entrance dose | |||||
| Rat [45] | Glioma (C6) | 2 | 25 | 200 | 350 entrance dose | NR | Optic nerve damage by histopathology |
| Mouse [7] | Melanoma (B16F10) | 1 | 50 | 200 | 407.6 dose @ tumour | 6.2 dose @ tumour | Tumour growth, tumour vasculature, cell proliferation, cell senescence, and immune response |
| Rat [20] | Gliosarcoma (9L) | 1 | 25 | 200 | 625 entrance dose | 12.1 dose @ tumour | Animal survival, tumour growth, and histopathology |
| 100 | 625 entrance dose | 36 dose @ tumour | |||||
| Rat [36] | Gliosarcoma (9L) | 1 | 25 | 200 | 625 entrance dose | NR | Animal survival, tumour growth, and histopathology |
| Mouse [54] | Mammary (EMT6.5) | 1 | 25 | 200 | 560 entrance dose | 11 | Biochemical changes by synchrotron Fourier-transform infrared microspectroscopy |
| Rat [40] | Glioma (C6, F98) | 2 | 25 | 211 | 350 entrance dose | NR | Animal survival and object recognition |
| Rat [41] | Glioma (F98) | 2 | 28 | 400 | 350 | 18 dose @ tumour | Animal survival and cognitive dysfunction |
| Mouse nude [46] | Gliosarcoma (9L) | 1 | 25 | 211 | 500 entrance dose | 24 (cross-fired) | Animal survival, tumour growth, and tumour vasculature |
| Rat [34] | Gliosarcoma (9L) | 2 | 25 | 211 | 860 entrance dose | 18 @ 1 cm depth | Animal survival and histopathology |
| 50 | 480 entrance dose | ||||||
| 75 | 320 entrance dose | ||||||
| Rat [35] | Gliosarcoma (9L) | 3 | 50 | 211 | 400, 360 (+24 h), 400 (+48 h) entrance dose | 15 | Animal survival and histopathology |
| Rat [39] | Gliosarcoma (9L) | 1 | 27 | 211 | 625 entrance dose | NR | Animal survival, histopathology, and immune response |
| Mouse [51] | Mammary (EMT6.5) | 1 | 25 | 200 | 560 | 11 | Transcriptomics |
| Mouse nude [48] | Glioma (U251) | 1 | 100 | 500 | 124 | 4.8 | Tumour growth, histopathology, and apoptosis |
| 2 | 20 | 100 | 111 | 8.2 | |||
| 100 | 500 | 124 | 9.6 | ||||
| Mouse [53] | Mammary (EMT6.5) | 1 | 25 | 200 | 112, 560 | NR | Immune response |
| Animal | Tissue | Microbeam | Pathology | Threshold Peak-Dose (Gy) | Valley-Dose (Gy) | |
|---|---|---|---|---|---|---|
| Width (µm) | Spacing (µm) | |||||
| Duck [67] | Immature CNS | 27 | 100 | Ataxia | 160 | NR |
| Rat [15] | Brain | 27 | 75 | Necrosis, oedema | 250 | 17 |
| 27 | 100 | 500 | 33 | |||
| Rat [73] | Brain | 27 | 200 | Cell loss, demyelination | 1000 | NR |
| Rat [25] | Brain | 25 | 100 | Tissue damage (loss of tissue structure; vascular damage) | 625 uni- & bidirectional | NR |
| 312 bidirectional | ||||||
| Rat [65] | Spinal cord | 35 | 210 | Paresis/Paralysis (over 383 dpi) | 357 (19mm transverse depth) | 12.7 |
| Mouse [72] | Brain | 25 | 211 | Damage to microvasculature/vasogenic oedema (up to 1 mpi) | 312–1000 | 5.8 |
| Rat (11–13 day old) [26] | Brain | 28 | 105 | Neurological dysfunction | 150 | >5 Gy [69] |
| 28 | 105 | 50 | ||||
| 25 | 210 | 300 bidirectional | ||||
| Piglet [66] | Brain | 20–30 | 210 | Neurological function (465 dpi) | 600 peak-entry 263 @ cerebellum | 12.6 @ cerebellum |
| Rat [18] | Brain | 20 | 200 | Necrosis (14 dpi) | 5000 | NR |
| 37 | 75 | Tissue damage (14–31dpi) | 625 | |||
| 20 | 200 | Loss of nuclei (30-31dpi) | 2000 | |||
| 37 | 75 | |||||
| Skin | 37 | 200 | Epilation (2–4 weeks dpi) | 1250–2500 | ||
| 37 | 75 | 1250–2500 | ||||
| Mouse [74] | Brain | 25 | 211 | Cerebral oedema (up to 28 dpi) | <1000 | 10.5 |
| Mouse [49] | Skin | 90 | 300 | Moist desquamation | 1740 co-planar | 35 |
| 650 cross-planar | 26 | |||||
| Mouse [76] | Skin | 90 | 300 | Moist desquamation | 1005-1170 | 14-17 |
| Mouse [83] | Abdomen | 25 | 400 | GI syndrome | 249 (257 TD50) | 7.5 (7.7) |
| Head | Neurological dysfunction | 255 (268 TD50) | 6.8 (7.2) | |||
| Thorax | Neurological dysfunction, pulmonary damage | 391 (lowest administered dose) | 9.5 | |||
| Total body | Weight loss, moribund behaviour | 88.9 (120 TD50) | 2.8 (3.8) | |||
| Mouse [75] | Saphenous artery | 50 | 400 | Functional deficits, atrophy, arterial damage | 2000 | 17.6 |
| Rat [34] | Brain | 50 | 211 | Neurological dysfunction | 480 | 18 |
| Mouse [79] | Testes (ex vivo) | 50 | 100 | Spermatogenesis | 5 | NR |
| Rat [68] | Brain (+eye) | 25 | 211 | Retinal degeneration (12 dpi) | 350 * (bidirectional) | NR |
| Rat [45] | Brain (+eye) | 25 | 200 | Optic nerve damage | 350 * (bidirectional) | NR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Palomo, C.; Fazzari, J.; Trappetti, V.; Smyth, L.; Janka, H.; Laissue, J.; Djonov, V. Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers 2020, 12, 527. https://doi.org/10.3390/cancers12030527
Fernandez-Palomo C, Fazzari J, Trappetti V, Smyth L, Janka H, Laissue J, Djonov V. Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers. 2020; 12(3):527. https://doi.org/10.3390/cancers12030527
Chicago/Turabian StyleFernandez-Palomo, Cristian, Jennifer Fazzari, Verdiana Trappetti, Lloyd Smyth, Heidrun Janka, Jean Laissue, and Valentin Djonov. 2020. "Animal Models in Microbeam Radiation Therapy: A Scoping Review" Cancers 12, no. 3: 527. https://doi.org/10.3390/cancers12030527
APA StyleFernandez-Palomo, C., Fazzari, J., Trappetti, V., Smyth, L., Janka, H., Laissue, J., & Djonov, V. (2020). Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers, 12(3), 527. https://doi.org/10.3390/cancers12030527







