Changes in Functional Outcome and Quality of Life in Soft Tissue Sarcoma Patients within the First Year after Surgery: A Prospective Observational Study
Abstract
1. Introduction
2. Results
2.1. Functional Outcome Measures
2.2. Distress Measures
2.3. Associations between Self-Reported Outcomes and Objective Measurements
3. Discussion
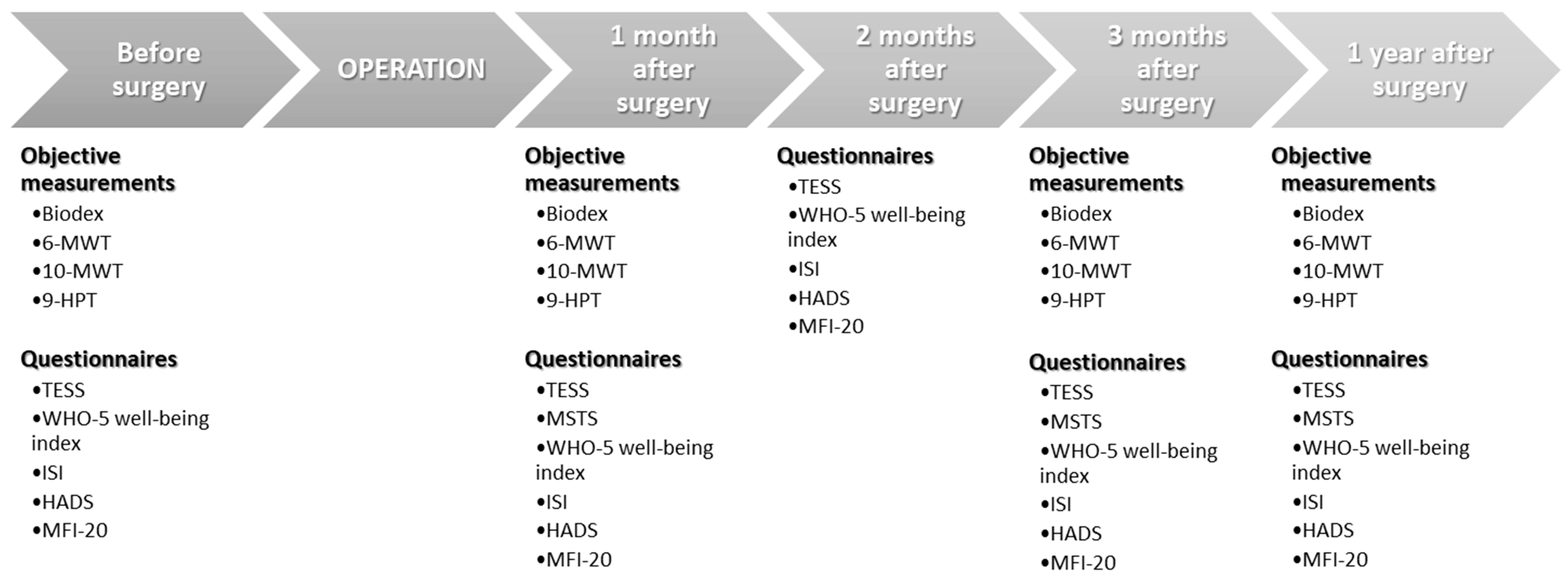
4. Materials and Methods
4.1. Study Population
4.2. Outcome Measures
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Storey, L.; Fern, L.A.; Martins, A.; Wells, M.; Bennister, L.; Gerrand, C.; Onasanya, M.; Whelan, J.S.; Windsor, R.; Woodford, J.; et al. A Critical Review of the Impact of Sarcoma on Psychosocial Wellbeing. Sarcoma 2019, 2019, 9730867. [Google Scholar] [CrossRef]
- Davis, A.M.; Devlin, M.; Griffin, A.M.; Wunder, J.S.; Bell, R.S. Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: A matched case−control study. Arch. Phys. Med. Rehabil. 1999, 80, 615–618. [Google Scholar] [CrossRef]
- Malek, F.; Somerson, J.S.; Mitchel, S.; Williams, R.P. Does limb−salvage surgery offer patients better quality of life and functional capacity than amputation? Clin. Orthop. Relat. Res. 2012, 470, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.H.; Pan, D.J.; Castle, D.J.; Choong, P.F. A systematic review of the recent quality of life studies in adult extremity sarcoma survivors. Sarcoma 2012, 2012, 171342. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Davis, A.M.; Wright, J.G.; Williams, J.I.; Bombardier, C.; Griffin, A.; Bell, R.S. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual. Life Res. 1996, 5, 508–516. [Google Scholar] [CrossRef]
- Marchese, V.G.; Rai, S.N.; Carlson, C.A.; Hinds, P.S.; Spearing, E.M.; Zhang, L.; Callaway, L.; Neel, M.D.; Rao, B.N.; Ginsberg, J.P. Assessing functional mobility in survivors of lower−extremity sarcoma: Reliability and validity of a new assessment tool. Pediatr. Blood Cancer 2007, 49, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Yoshimura, Y.; Aoki, K.; Okamoto, M.; Kito, M.; Suzuki, S.; Takazawa, A.; Ishida, T.; Kato, H. Prediction of muscle strength and postoperative functionafter knee flexor muscle resection for soft tissue sarcoma of the lower limbs. Orthop. Traumatol. Surg. Res. 2017, 1081–1085. [Google Scholar] [CrossRef]
- Tanaka, A.; Yoshimura, Y.; Aoki, K.; Kito, M.; Okamoto, M.; Suzuki, S.; Momose, T.; Kato, H. Knee extension strength and post−operative functional prediction in quadriceps resection for soft−tissue sarcoma of the thigh. Bone Joint Res. 2016, 5, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Harbo, T.; Brincks, J.; Andersen, H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur. J. Appl. Physiol. 2012, 112, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Saebye, C.; Fugloe, H.M.; Nymark, T.; Safwat, A.; Petersen, M.M.; Baad−Hansen, T.; Krarup−Hansen, A.; Keller, J. Factors associated with reduced functional outcome and quality of life in patients having limb-sparing surgery for soft tissue sarcomas—A national multicenter study of 128 patients. Acta Oncol. 2017, 56, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, D.; Bell, R.S.; Wunder, J.S.; O‘Sullivan, B.; Turcotte, R.; Masri, B.A.; Davis, A.M. Evaluating function and health related quality of life in patients treated for extremity soft tissue sarcoma. Qual. Life Res. 2006, 15, 1439–1446. [Google Scholar] [CrossRef]
- Davis, A.M.; Sennik, S.; Griffin, A.M.; Wunder, J.S.; O’Sullivan, B.; Catton, C.N.; Bell, R.S. Predictors of functional outcomes following limb salvage surgery for lower−extremity soft tissue sarcoma. J. Surg. Oncol. 2000, 73, 206–211. [Google Scholar] [CrossRef]
- Siracuse, B.L.; Gorgy, G.; Ruskin, J.; Beebe, K.S. What is the Incidence of Suicide in Patients with Bone and Soft Tissue Cancer? Suicide and Sarcoma. Clin. Orthop. Relat. Res. 2017, 475, 1439–1445. [Google Scholar] [CrossRef]
- Davis, A.M.; Punniyamoorthy, S.; Griffin, A.M.; Wunder, J.S.; Bell, R.S. Symptoms and their Relationship to Disability Following Treatment for Lower Extremity Tumours. Sarcoma 1999, 3, 73–77. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Bell, R.S.; Turcotte, R.; Catton, C.N.; Wunder, J.S.; Chabot, P.; Hammond, A.; Benk, V.; Isler, M.; et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J. Clin. Oncol. 2002, 20, 4472–4477. [Google Scholar] [CrossRef]
- Davidson, D.; Barr, R.D.; Riad, S.; Griffin, A.M.; Chung, P.W.; Catton, C.N.; O’Sullivan, B.; Ferguson, P.C.; Davis, A.M.; Wunder, J.S. Health−related quality of life following treatment for extremity soft tissue sarcoma. J. Surg. Oncol. 2016, 114, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Davidge, K.; Bell, R.; Ferguson, P.; Turcotte, R.; Wunder, J.; Davis, A.M. Patient expectations for surgical outcome in extremity soft tissue sarcoma. J. Surg. Oncol. 2009, 100, 375–381. [Google Scholar] [CrossRef]
- de Visser, E.; Pauwels, J.; Duysens, J.E.; Mulder, T.; Veth, R.P. Gait adaptations during walking under visual and cognitive constraints: A study of patients recovering from limb−saving surgery of the lower limb. Am. J. Phys. Med. Rehabil. 1998, 77, 503–509. [Google Scholar] [CrossRef]
- Carmody Soni, E.E.; Miller, B.J.; Scarborough, M.T.; Parker Gibbs, C. Functional outcomes and gait analysis of patients after periacetabular sarcoma resection with and without ischiofemoral arthrodesis. J. Surg. Oncol. 2012, 106, 844–849. [Google Scholar] [CrossRef]
- Ven Fong, Z.; Chang, D.C.; Lillemoe, K.D.; Nipp, R.D.; Tanabe, K.K.; Qadan, M. Contemporary Opportunity for Prehabilitation as Part of an Enhanced Recovery after Surgery Pathway in Colorectal Surgery. Clin. Colon. Rectal. Surg. 2019, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, L.H.; Bauer, H.C.; Jebsen, N.L.; Folleras, G.; Allert, C.; Haugen, G.S.; Hall, K.S. Limb-sparing surgery preserves more function than amputation: A Scandinavian sarcoma group study of 118 patients. J. Bone Joint Surg. Br. 2008, 90, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Snaith, R.P. The Hospital Anxiety And Depression Scale. Health Qual. Life Outcomes 2003, 1, 29. [Google Scholar] [CrossRef]
- Paredes, T.; Canavarro, M.C.; Simoes, M.R. Anxiety and depression in sarcoma patients: Emotional adjustment and its determinants in the different phases of disease. Eur. J. Oncol. Nurs. 2011, 15, 73–79. [Google Scholar] [CrossRef]
- Paredes, T.; Pereira, M.; Simoes, M.R.; Canavarro, M.C. A longitudinal study on emotional adjustment of sarcoma patients: The determinant role of demographic, clinical and coping variables. Eur. J. Cancer Care (Engl.) 2012, 21, 41–51. [Google Scholar] [CrossRef]
- Refaat, Y.; Gunnoe, J.; Hornicek, F.J.; Mankin, H.J. Comparison of quality of life after amputation or limb salvage. Clin. Orthop. Relat. Res. 2002, 397, 298–305. [Google Scholar] [CrossRef]
- Savard, M.H.; Savard, J.; Simard, S.; Ivers, H. Empirical validation of the Insomnia Severity Index in cancer patients. Psycho−Oncology 2005, 14, 429–441. [Google Scholar] [CrossRef]
- Cha, K.M.; Chung, Y.K.; Lim, K.Y.; Noh, J.S.; Chun, M.; Hyun, S.Y.; Kang, D.R.; Oh, M.J.; Kim, N.H. Depression and insomnia as mediators of the relationship between distress and quality of life in cancer patients. J. Affect Disord. 2017, 217, 260–265. [Google Scholar] [CrossRef]
- Eiser, C.; Darlington, A.S.; Stride, C.B.; Grimer, R. Quality of life implications as a consequence of surgery: Limb salvage, primary and secondary amputation. Sarcoma 2001, 5, 189–195. [Google Scholar] [CrossRef]
- Saebye, C.K.P.; Keller, J.; Baad−Hansen, T. Validation of the Danish version of the musculoskeletal tumour society score questionnaire. World J. Orthop. 2019, 10, 23–32. [Google Scholar] [CrossRef]
- Saebye, C.; Safwat, A.; Kaa, A.K.; Pedersen, N.A.; Keller, J. Validation of a Danish version of the Toronto Extremity Salvage Score questionnaire for patients with sarcoma in the extremities. Dan. Med. J. 2014, 61, A4734. [Google Scholar]
- Drouin, J.M.; Valovich−mcLeod, T.C.; Shultz, S.J.; Gansneder, B.M.; Perrin, D.H. Reliability and validity of the Biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur. J. Appl. Physiol. 2004, 91, 22–29. [Google Scholar] [CrossRef]
- Nordez, A.; Casari, P.; Cornu, C. Accuracy of Biodex system 3 pro computerized dynamometer in passive mode. Med. Eng. Phys. 2008, 30, 880–887. [Google Scholar] [CrossRef]
- Enright, P.L. The six−minute walk test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- Schmidt, K.; Vogt, L.; Thiel, C.; Jager, E.; Banzer, W. Validity of the six−minute walk test in cancer patients. Int. J. Sports Med. 2013, 34, 631–636. [Google Scholar] [CrossRef]
- Eden, M.M.P.D.; Tompkins, J.P.D.; Verheijde, J.L.P.P.M. Reliability and a correlational analysis of the 6MWT, ten−meter walk test, thirty second sit to stand, and the linear analog scale of function in patients with head and neck cancer. Physiother. Theory. Pract. 2017, 34, 202–211. [Google Scholar] [CrossRef]
- Gibbons, W.J.; Fruchter, N.; Sloan, S.; Levy, R.D. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J. Cardiopulm. Rehabil. 2001, 21, 87–93. [Google Scholar] [CrossRef]
- Laboratories ATSCoPSfCPF. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Critic. Care Med. 2002, 166, 111–117. [CrossRef]
- Bohannon, R.W.; Andrews, A.W.; Thomas, M.W. Walking speed: Reference values and correlates for older adults. J. Orthop. Sports Phys. Ther. 1996, 24, 86–90. [Google Scholar] [CrossRef]
- Watson, M.J. Refining the Ten−metre Walking Test for Use with Neurologically Impaired People. Physiotherapy 2002, 88, 386–397. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Weber, K.; Kashman, N.; Volland, G. Adult Norms For The Nine Hole Peg Test Of Finger Dexterity. Occup. Ther. J. Res. 1985, 5, 24. [Google Scholar] [CrossRef]
- Oxford Grice, K.; Vogel, K.A.; Le, V.; Mitchell, A.; Muniz, S.; Vollmer, M.A. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am. J. Occup. Ther. 2003, 57, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Gorter, R.R.; Vos, C.G.; Halmans, J.; Hartemink, K.J.; Paul, M.A.; Oosterhuis, J.W. Evaluation of arm function and quality of life after trimodality treatment for superior sulcus tumours. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Topp, C.W.; Ostergaard, S.D.; Sondergaard, S.; Bech, P. The WHO-5 Well-being Index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Ibbotson, T.; Maguire, P.; Selby, P.; Priestman, T.; Wallace, L. Screening for anxiety and depression in cancer patients: The effects of disease and treatment. Eur. J. Cancer 1994, 30, 37–40. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Smets, E.M.; Garssen, B.; Bonke, B.; De Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Smets, E.M.; Garssen, B.; Cull, A.; de Haes, J.C. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br. J. Cancer 1996, 73, 241–245. [Google Scholar] [CrossRef]
| Characteristics | Patients (n = 29) | Characteristics | Patients (n = 29) |
|---|---|---|---|
| Age (in years + SD) | 63 ± 17.1 | Tumor grade (n, %) | |
| Range in age | 25–86 | Grade 1 | 4 (14%) |
| Gender (n, %) | Grade 2 | 11 (38%) | |
| Male | 15 (52%) | Grade 3 | 14 (48%) |
| Female | 14 (48%) | Tumor margin (n, %) | |
| Location (n, %) | Wide | 24 (83%) | |
| Upper extremity | 8 (28%) | Marginal | 5 (17%) |
| Lower extremity | 21 (72%) | Adjuvant treatment (n, %) | |
| Tumor size (in cm + SD) | 8.85 ± 4.81 | None | 17 (59%) |
| Range in size | 1–18 | Radiotherapy (RT) | 11 (38%) |
| Tumor depth (n, %) | RT + chemotherapy | 1 (3%) | |
| Subcutaneous | 13 (45%) | ||
| Subfascial | 16 (55%) |
| Method | Before Surgery | 1 Month after Surgery | 2 Months after Surgery | 3 Months after Surgery | 1 Year after Surgery |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Objective measurements | 28/29 (97%) | 15/29 (52%) | N/A | 19/27 (66%) | 13/24 (45%) |
| Questionnaires | 28/29 (97%) | 20/29 (69%) | 25/29 (86%) | 24/27 (83%) | 16/24 (55%) |
| Method | Score Range | Before Surgery (Baseline) | 1 Month after Surgery (MSTS Baseline) | 2 Months after Surgery | 3 Months after Surgery | 1 Year after Surgery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | ||
| TESS | 0–100 | 96.5 | 88–100 | 78.5 | 57–93.5 | <0.01 * | 88 | 77–94 | 0.01 * | 94 | 83.5–100 | 0.26 | 88 | 76–97.5 | 0.08 |
| MSTS | 0–100 | N/A | N/A | 76.5 | 51.5–95 | N/A | N/A | N/A | N/A | 97 | 85–100 | 0.03 * | 93 | 80–100 | 0.11 |
| WHO-5 well-being | 0–100 | 70 | 42–92 | 68 | 54–74 | 0.36 | 76 | 64–84 | 0.63 | 74 | 56–96 | 0.47 | 80 | 68–92 | 0.24 |
| HADS | |||||||||||||||
| Anxiety score | 0–21 | 5 | 3–7.5 | 4 | 1–5 | 0.12 | 2 | 0–5 | 0.03* | 2 | 0.5–5 | 0.03* | 2 | 1–5 | 0.03 * |
| Depression score | 0–21 | 1 | 0–3 | 1 | 0–3 | 0.72 | 1 | 0–2 | 0.44 | 1 | 0–2 | 0.50 | 1 | 0–1 | 0.52 |
| ISI | 0–28 | 7 | 3–13 | 7 | 4–9 | 0.80 | 3 | 2–9 | 0.21 | 6 | 1–10 | 0.46 | 3.5 | 0.5–10 | 0.19 |
| MFI-20 | |||||||||||||||
| General fatigue score | 4–20 | 9 | 5.5–12.5 | 10.5 | 8–12 | 0.31 | 8 | 6–12 | 0.84 | 7 | 4.5–14.5 | 0.94 | 10 | 4.5–11.5 | 0.96 |
| Physical fatigue score | 4–20 | 8 | 6.5–13 | 13 | 11–18 | <0.01 * | 13 | 10–14 | 0.01 * | 10 | 7.5–14.5 | 0.36 | 10 | 7–14.5 | 0.35 |
| Mental fatigue score | 4–20 | 8 | 4.5–11.5 | 7 | 5–9.5 | 0.63 | 6 | 4–9 | 0.22 | 6 | 4–8.5 | 0.33 | 6.5 | 4–9 | 0.28 |
| Reduced activity score | 4–20 | 9 | 4.5–13 | 11 | 9.5–13.5 | 0.07 | 10 | 8–13 | 0.30 | 9 | 6–12 | 0.82 | 9 | 5.5–10.5 | 0.73 |
| Reduced motivation score | 4–20 | 6.5 | 4–11.5 | 7 | 4.5–10 | 0.83 | 7 | 5–10 | 0.59 | 6 | 4.5–10.5 | 0.78 | 5 | 4–9 | 0.52 |
| Method | Before Surgery | 1 Month after Surgery | 3 Months after Surgery | 1 Year after Surgery | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | p | Mean | 95% CI | p | Mean | 95% CI | p | Mean | 95% CI | p | |
| Isokinetic dynamometer 1 | ||||||||||||
| Healthy side | 80.27 | 73.46–87.09 | 0.60 | 83.06 | 75.16–90.96 | <0.01 * | 81.59 | 71.96–91.21 | 0.04 * | 85.04 | 73.14–96.95 | 0.05 * |
| Disease-affected side | 79.17 | 71.80–86.53 | 69.44 | 59.02–79.87 | 75.05 | 66.86–83.25 | 78.56 | 68.80–88.33 | ||||
| Total average strength | 79.72 | 72.96–86.48 | 76.25 | 67.78–84.72 | 78.32 | 69.94–86.70 | 81.80 | 71.42–92.19 | ||||
| 10-MWT (m/s) | 2.12 | 1.91–2.34 | Baseline | 1.68 | 1.29–2.07 | 0.02 * | 1.95 | 1.75–2.16 | 0.24 | 2.08 | 1.66–2.49 | 0.80 |
| 9-HPT (seconds) | ||||||||||||
| Healthy side | 20.85 | 16.98–24.73 | 0.08 | 22.46 | 17.03–27.89 | 0.74 | 21.37 | 15.96–26.78 | 0.81 | 21.70 | 12.97–30.43 | 0.86 |
| Disease-affected side | 22.06 | 18.88–25.25 | 21.91 | 19.12–24.70 | 21.05 | 18.42–23.68 | 21.55 | 9.52–33.57 | ||||
| Method | Before Surgery(Baseline) | 1 Month after Surgery | 3 Months after Surgery | 1 Year after Surgery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p | Median | IQR | p | Median | IQR | p | |
| 6-MWT: | 88.70 | 80.53–94.21 | 81.25 | 56.25–92.52 | 0.20 | 84.31 | 76.41–92.25 | 0.26 | 91.70 | 82.76–98.26 | 0.43 |
| Method | Isokinetic Dynamometer | 10-MWT (LE) | 9-HPT (UE) | 6-MWT | ||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| TESS | 0.24 | <0.01 * | 13.09 | <0.01 * | 0.68 | 0.39 | 0.05 | 0.44 |
| MSTS | 0.24 | 0.17 | 28.80 | <0.01 * | 1.11 | 0.03 * | 0.14 | 0.22 |
| WHO-5 well-being | 0.31 | 0.07 | 6.58 | 0.34 | 2.39 | 0.20 | 0.17 | 0.16 |
| HADS | ||||||||
| Anxiety score | −0.01 | 0.89 | 0.75 | 0.43 | −0.60 | 0.02 * | 0.01 | 0.80 |
| Depression score | −0.04 | 0.06 | −0.08 | 0.91 | 0.03 | 0.91 | −0.03 | 0.02 * |
| ISI | −0.04 | 0.35 | −0.03 | 0.99 | −0.73 | 0.08 | 0.01 | 0.72 |
| MFI-20 | ||||||||
| General fatigue score | −0.07 | 0.01 * | −2.16 | 0.05 * | −0.54 | 0.09 | −0.05 | 0.01 * |
| Physical fatigue score | −0.13 | <0.01 * | −3.00 | 0.01 * | 0.01 | 0.99 | −0.07 | <0.01 * |
| Mental fatigue score | −0.03 | 0.18 | 0.51 | 0.55 | −0.37 | 0.19 | −0.02 | 0.28 |
| Reduced activity score | −0.10 | <0.01 * | −2.72 | 0.02 * | −0.32 | 0.27 | −0.04 | 0.06 |
| Reduced motivation score | −0.08 | <0.01 * | −0.83 | 0.36 | −0.32 | 0.23 | −0.04 | 0.01 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saebye, C.; Amidi, A.; Keller, J.; Andersen, H.; Baad-Hansen, T. Changes in Functional Outcome and Quality of Life in Soft Tissue Sarcoma Patients within the First Year after Surgery: A Prospective Observational Study. Cancers 2020, 12, 463. https://doi.org/10.3390/cancers12020463
Saebye C, Amidi A, Keller J, Andersen H, Baad-Hansen T. Changes in Functional Outcome and Quality of Life in Soft Tissue Sarcoma Patients within the First Year after Surgery: A Prospective Observational Study. Cancers. 2020; 12(2):463. https://doi.org/10.3390/cancers12020463
Chicago/Turabian StyleSaebye, Casper, Ali Amidi, Johnny Keller, Henning Andersen, and Thomas Baad-Hansen. 2020. "Changes in Functional Outcome and Quality of Life in Soft Tissue Sarcoma Patients within the First Year after Surgery: A Prospective Observational Study" Cancers 12, no. 2: 463. https://doi.org/10.3390/cancers12020463
APA StyleSaebye, C., Amidi, A., Keller, J., Andersen, H., & Baad-Hansen, T. (2020). Changes in Functional Outcome and Quality of Life in Soft Tissue Sarcoma Patients within the First Year after Surgery: A Prospective Observational Study. Cancers, 12(2), 463. https://doi.org/10.3390/cancers12020463





