BRAF Exon 15 Mutations in Papillary Carcinoma and Adjacent Thyroid Parenchyma: A Search for the Early Molecular Events Associated with Tumor Development
Abstract
1. Introduction
2. Results
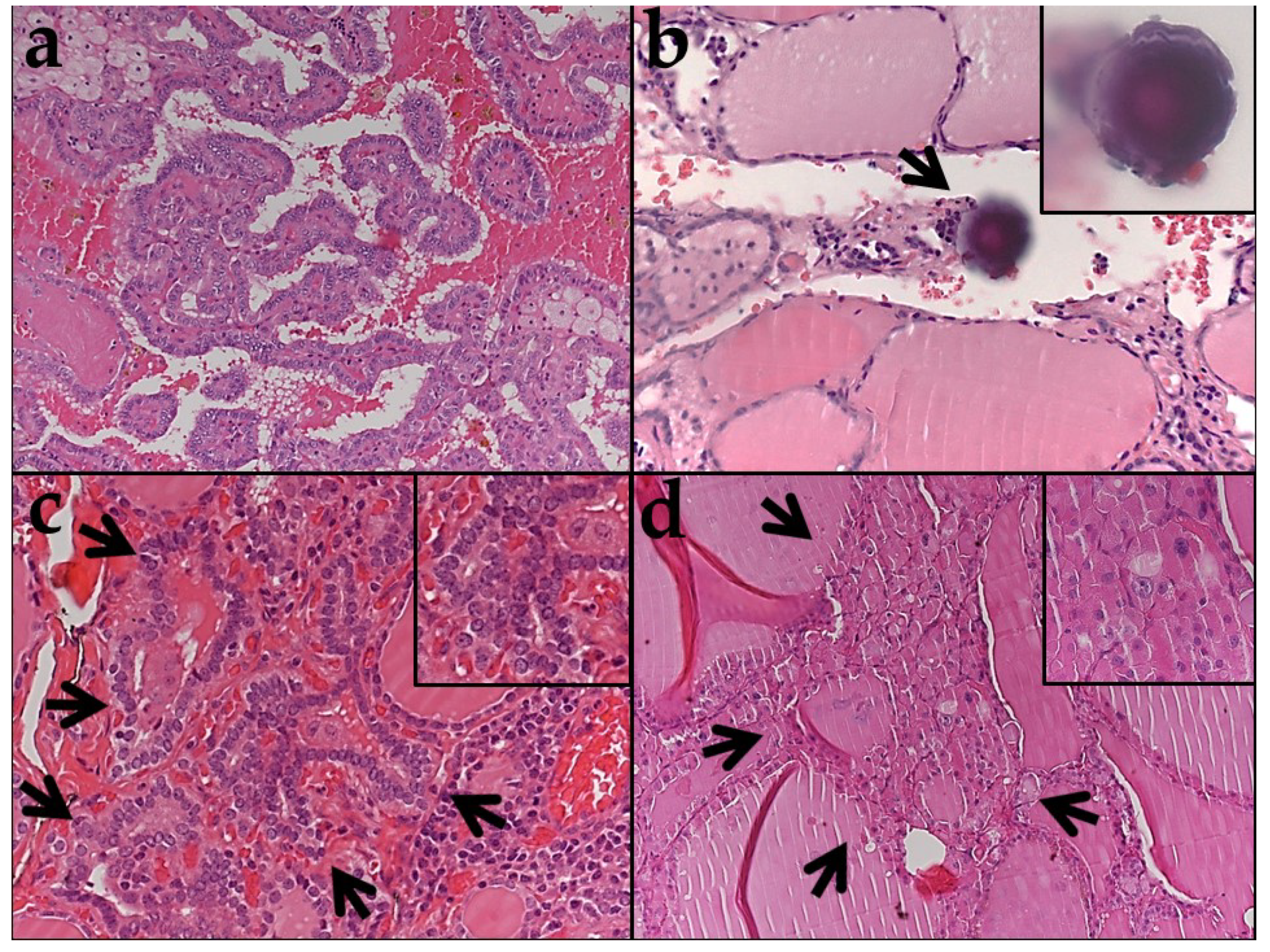
2.1. Clinicopathologic Features of Tumors and Histologic Features of Adjacent Thyroid Tissue
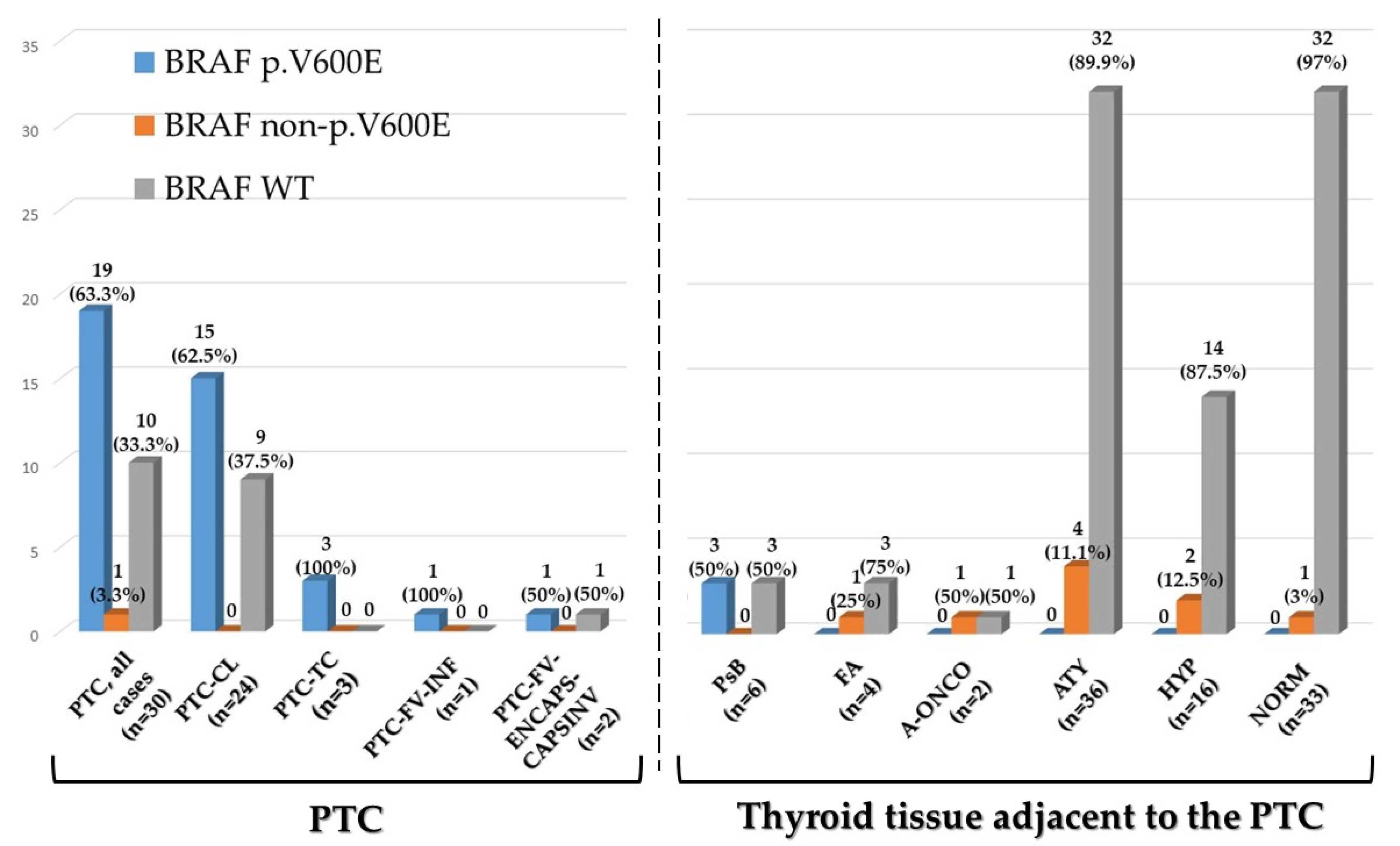
2.2. BRAF Exon 15 Mutations in Papillary Carcinoma
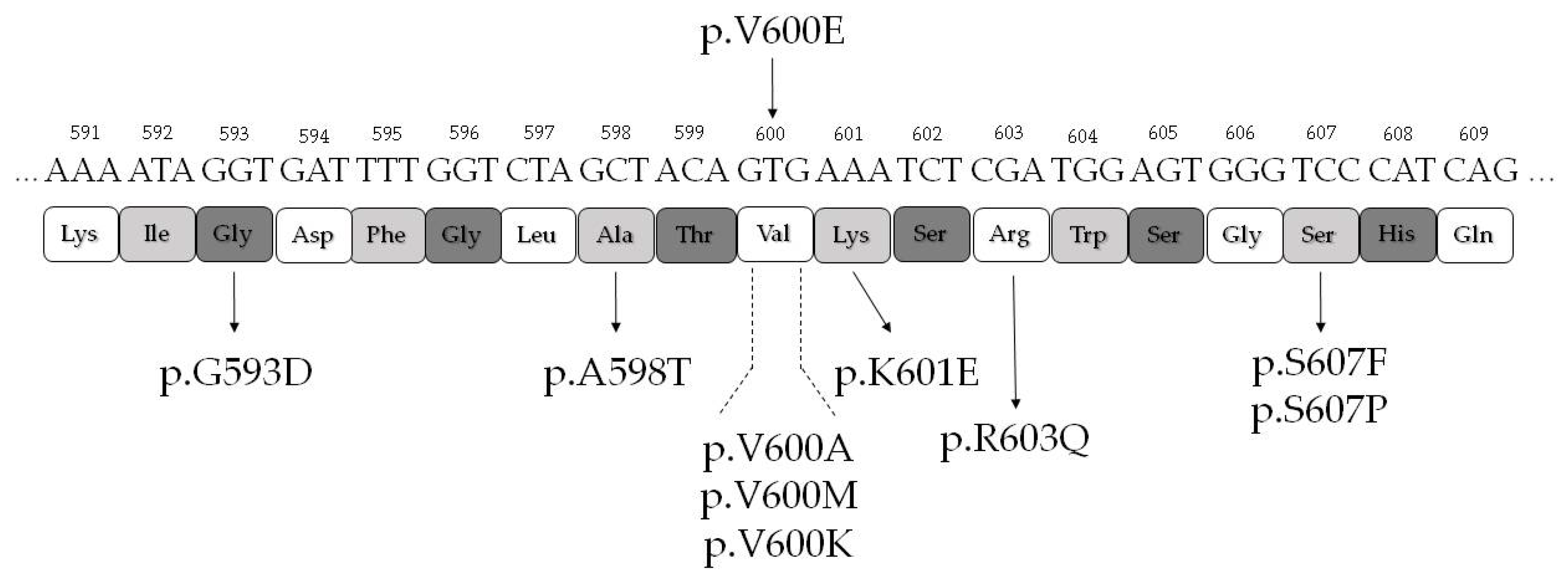
2.3. BRAF Exon 15 Mutations in Thyroid Tissue Adjacent to Papillary Carcinoma
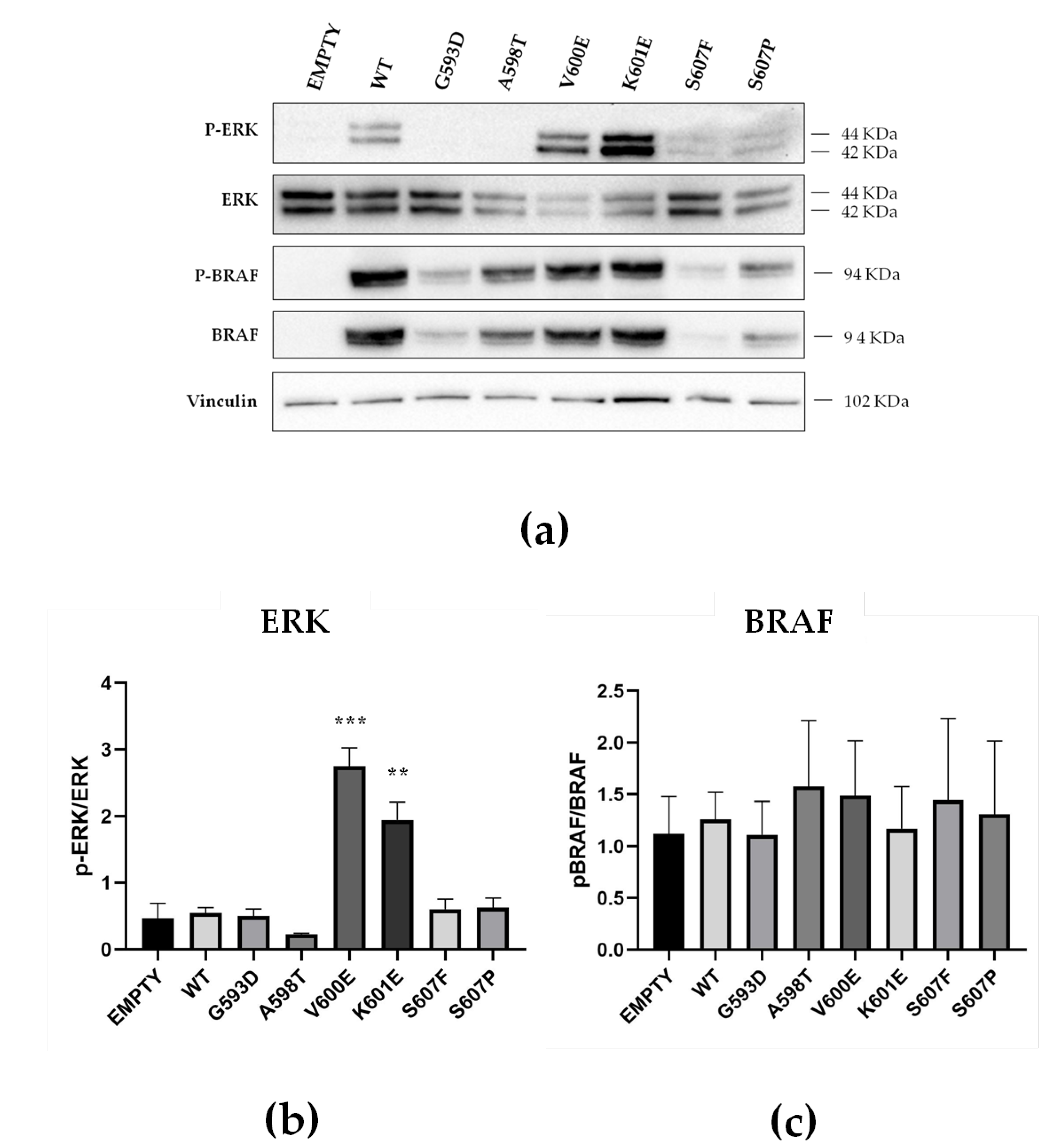
2.4. Functional Characterization of MAPK Signaling by BRAF Non p.V600E Mutant Proteins
3. Discussion
4. Materials and Methods
4.1. Case Selection
4.2. Detection of BRAF Mutations by Next Generation Sequencing
4.3. Mutant BRAF Plasmids Generation via Site-Directed Mutagenesis
4.4. Cell Lines and Transfection
4.5. Western Blots
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, G.; Visani, M.; Repaci, A.; Rhoden, K.J.; de Biase, D.; Pession, A.; Giovanni, T. Molecular pathology of thyroid tumours of follicular cells: A review of genetic alterations and their clinicopathological relevance. Histopathology 2018, 72, 6–31. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nikiforov, Y.E.; Schlumberger, M.; Vigneri, R. Increasing incidence of thyroid cancer: Controversies explored. Nat. Rev. Endocrinol. 2013, 9, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. World Health Organization Classification of Tumours of Endocrine Organs, 4th ed.; WHO/IARC: Lyon, France, 2017. [Google Scholar]
- Rosai, J.; DeLellis, R.A.; Carcangiu, M.L.; Frabel, W.J.; Tallini, G. Tumors of the Thyroid and Parathyroid Glands, Fourth Series; AFIP Atlas of Tumor Pathology, American Registry of Pathology: Silver Spring, MA, USA, 2014. [Google Scholar]
- Fellegara, G.; Rosai, J. Multifocal fibrosing thyroiditis: Report of 55 cases of a poorly recognized entity. Am. J. Surg. Pathol. 2015, 39, 416–424. [Google Scholar] [CrossRef]
- Klinck, G.H.; Winship, T. Psammoma bodies and thyroid cancer. Cancer 1959, 12, 656–662. [Google Scholar] [CrossRef]
- Johannessen, J.V.; Sobrinho-Simoes, M. The origin and significance of thyroid psammoma bodies. Lab. Investig. 1980, 43, 287–296. [Google Scholar]
- Macerola, E.; Torregrossa, L.; Ugolini, C.; Bakkar, S.; Vitti, P.; Fadda, G.; Basolo, F. BRAF(K601E) Mutation in a Follicular Thyroid Adenoma: A Case Report. Int. J. Surg. Pathol. 2017, 25, 348–351. [Google Scholar] [CrossRef]
- Torregrossa, L.; Viola, D.; Sensi, E.; Giordano, M.; Piaggi, P.; Romei, C.; Materazzi, G.; Miccoli, P.; Elisei, R.; Basolo, F. Papillary Thyroid Carcinoma With Rare Exon 15 BRAF Mutation Has Indolent Behavior: A Single-Institution Experience. J. Clin. Endocrinol. Metab. 2016, 101, 4413–4420. [Google Scholar] [CrossRef]
- Yao, Z.; Yaeger, R.; Rodrik-Outmezguine, V.S.; Tao, A.; Torres, N.M.; Chang, M.T.; Drosten, M.; Zhao, H.; Cecchi, F.; Hembrough, T.; et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017, 548, 234–238. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. RAF protein-serine/threonine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2010, 399, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Cameselle-Teijeiro, J.; Abdulkader, I.; Perez-Becerra, R.; Vazquez-Boquete, A.; Alberte-Lista, L.; Ruiz-Ponte, C.; Forteza, J.; Sobrinho-Simoes, M. BRAF mutation in solid cell nest hyperplasia associated with papillary thyroid carcinoma. A precursor lesion? Hum. Pathol. 2009, 40, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Tschandl, P.; Berghoff, A.S.; Preusser, M.; Burgstaller-Muehlbacher, S.; Pehamberger, H.; Okamoto, I.; Kittler, H. NRAS and BRAF mutations in melanoma-associated nevi and uninvolved nevi. PLoS ONE 2013, 8, e69639. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, M.R.; Skorokhod, A.; Gransheier, D.; Weide, B.; Koch, W.; Schif, B.; Enk, A.; Garbe, C.; Bauer, J. Variables that influence BRAF mutation probability: A next-generation sequencing, non-interventional investigation of BRAFV600 mutation status in melanoma. PLoS ONE 2017, 12, e0188602. [Google Scholar] [CrossRef] [PubMed]
- Kinno, T.; Tsuta, K.; Shiraishi, K.; Mizukami, T.; Suzuki, M.; Yoshida, A.; Suzuki, K.; Asamura, H.; Furuta, K.; Kohno, T.; et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann. Oncol. 2014, 25, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Atsumi, T.; Singh, R.; Sabharwal, L.; Bando, H.; Meng, J.; Arima, Y.; Yamada, M.; Harada, M.; Jiang, J.J.; Kamimura, D.; et al. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 2014, 74, 8–14. [Google Scholar] [CrossRef]
- Lumachi, F.; Basso, S.M.; Orlando, R. Cytokines, thyroid diseases and thyroid cancer. Cytokine 2010, 50, 229–233. [Google Scholar] [CrossRef]
- Rotondi, M.; Coperchini, F.; Latrofa, F.; Chiovato, L. Role of Chemokines in Thyroid Cancer Microenvironment: Is CXCL8 the Main Player? Front Endocrinol. (Lausanne) 2018, 9, 314. [Google Scholar] [CrossRef]
- Gillespie, E.F.; Papageorgiou, K.I.; Fernando, R.; Raychaudhuri, N.; Cockerham, K.P.; Charara, L.K.; Goncalves, A.C.; Zhao, S.X.; Ginter, A.; Lu, Y.; et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J. Clin. Endocrinol. Metab. 2012, 97, E740–E746. [Google Scholar] [CrossRef]
- Visciano, C.; Liotti, F.; Prevete, N.; Cali, G.; Franco, R.; Collina, F.; de Paulis, A.; Marone, G.; Santoro, M.; Melillo, R.M. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene 2015, 34, 5175–5186. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Pignatti, P.; Leporati, P.; Carbone, A.; Croce, L.; Magri, F.; Chiovato, L.; Rotondi, M. Normal human thyroid cells, BCPAP, and TPC-1 thyroid tumor cell lines display different profile in both basal and TNF-alpha-induced CXCL8 secretion. Endocrine 2016, 54, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhou, L.; Wang, J.; Li, F.; Xie, L. Cytokine production of papillary thyroid carcinoma coexisting with Hashimoto’s thyroiditis. Int. J. Clin. Exp. Pathol. 2017, 10, 9567–9574. [Google Scholar] [PubMed]
- Buitrago, D.; Keutgen, X.M.; Crowley, M.; Filicori, F.; Aldailami, H.; Hoda, R.; Liu, Y.F.; Hoda, R.S.; Scognamiglio, T.; Jin, M.; et al. Intercellular adhesion molecule-1 (ICAM-1) is upregulated in aggressive papillary thyroid carcinoma. Ann. Surg. Oncol. 2012, 19, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Invest. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Cesari, V.; Visani, M.; Casadei, G.P.; Cremonini, N.; Gandolfi, G.; Sancisi, V.; Ragazzi, M.; Pession, A.; Ciarrocchi, A.; et al. High-sensitivity BRAF mutation analysis: BRAF V600E is acquired early during tumor development but is heterogeneously distributed in a subset of papillary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E1530–E1538. [Google Scholar] [CrossRef]
| Clinicopathologic Features | Number (%) |
|---|---|
| Age (years) | 47.6 ± 13.6 a |
| Female sex | 20 (66.7%) |
| Papillary carcinoma | |
| Classic | 24 (80.0%) |
| Tall cell variant | 3 (10.0%) |
| Follicular variant-infiltrative | 1 (3.3%) |
| Follicular variant-encapsulated with capsular invasion | 2 (6.7%) |
| Tumor size (cm) | 1.5 ± 1 a |
| BRAF p.V600E mutation | 20 (66.7%) |
| Lymph node metastasis | 12 (40.0%) |
| ATA (2015) recurrence risk groups | |
| Low risk | 12 (40.0%) |
| Intermediate risk | 18 (60.0%) |
| AJCC stage (8th ed.) | |
| I | 29 (96.7%) |
| II | 1 (3.3%) |
| Other thyroid pathology | |
| Follicular adenoma | 3 (10%) |
| Follicular adenoma, oncocytic | 1 (3.3%) |
| Chronic thyroiditis | 4 (13.3%) |
| Graves-Basedow disease | 1 (3.3%) |
| Samples Analyzed (n = 130) | BRAF p.V600E | BRAF non p.V600E | BRAF WT | NA | Total (Samples) |
|---|---|---|---|---|---|
| Papillary carcinoma, all cases (n = 30) | 19/130 (14.6%) | 1/130 (0.8%) | 10/130 (7.7%) | - | 30/130 (23.1%) |
| Papillary carcinoma Classic (n = 24) | 15/130 (11.5%) | - | 9/130 (6.9%) | - | 24/130 (18.5%) |
| Papillary carcinoma Tall cell variant (n = 3) | 3/130 (2.3%) | - | - | - | 3/130 (2.3%) |
| Papillary carcinoma Follicular variant—infiltrative (n = 1) | 1/130 (0.8%) | - | - | - | 1/130 (0.8%) |
| Papillary carcinoma Follicular variant—encapsulated with capsular invasion (n = 2) | - | 1/130 (0.8%) a | 1/130 (0.8%) | - | 2/130 (1.5%) |
| Thyroid tissue adjacent the papillary carcinoma (n = 100) | |||||
| Psammoma Body (n = 6) | 3/130 (2.3%) | - | 3/130 (2.3%) | - | 6/130 (4.6%) |
| Follicular Adenoma (n = 4) | - | 1/130 (0.8%) | 3/130 (2.3%) | - | 4/130 (3.1%) |
| Oncocytic Follicular Adenoma (n = 2) | - | 1/130 (0.8%) | 1/130 (0.8%) | - | 2/130 (%) |
| Follicular cell atypia (n = 38) | - | 4/130 (3.1%) | 32/130 (24.6%) | 2/130 (1.5%) | 38/130 (29.2%) |
| Follicular cell hyperplasia (n = 16) | - | 2/130 (1.5%) | 14/130 (10.8) | - | 16/130 (12.3%) |
| Histologically normal (n = 34) | - | 1/130 (0.8%) | 32/130 (24.6%) | 1/130 (0.8%) | 34/130 (26.2%) |
| Total (Mutation status) | 22/130 (16.9%) | 10/130 (7.7%) | 95/130 (73.1%) | 3/130 (2.3%) | 130/130 (100%) |
| Thyroid Tissue Samples Adjacent the PTC (n = 12) | Mutation | Reference (PMID) | PolyPhen-2 (Score) |
|---|---|---|---|
| BRAF p.V600E | |||
| PsB (n = 3) | p.V600E | PMID: 15035987 | PD (0.971) |
| BRAF non p.V600E | |||
| ATY (n = 2), HYP (n = 1) | p.G593D | PMID: 19269016 | PD (1.000) |
| ATY (n = 1) | p.A598T | PMID: 22926515 | PD (0.871) |
| FA-ONC (n = 1) | p.V600K | PMID: 15035987 | PD (1.000) |
| ATY (n = 1) | p.V600M | PMID: 28783719 | PD (0.904) |
| FA (n = 1) | p.K601E | PMID: 15035987 | PoD (0.784) |
| HYP (n = 1) | p.R603Q | PMID: 23861977 | PoD (0.786) |
| NORM (n = 1) | p.S607F | PMID: 23861977 | PD (0.998) |
| FA-ONC (n = 1), HYP (n = 1) | p.S607P | PMID: 29176861 | B (0.186) |
| BRAF Exon 15 Mutations in Thyroid Tissue Adjacent to PTC with the BRAF p.V600E Mutation (n = 19) | ||||
| Total samples (n = 71) | BRAF p.V600E | BRAF non p.V600E | BRAF WT | NA |
| Psammoma Body (n = 3) | 3/71 (4.2%) | - | - | - |
| Follicular Adenoma (n = 1) | - | - | 1/71 (1.4%) | - |
| Oncocytic Follicular Adenoma (n = 2) | - | 1/71 (1.4%) | 1/71 (1.4%) | - |
| Atypical tissue (n = 30) | - | 4/71 (5.6%) | 25/71 (35.2%) | 1/71 (1.4%) |
| Hyperplasia (n = 12) | - | 2/71 (2.8%) | 10/71 (14.1%) | - |
| Normal tissue (n = 23) | - | 1/71 (1.4%) | 21/71 (29.6%) | 1/71 (1.4%) |
| BRAF Exon 15 Mutations in Thyroid Tissue Adjacent to PTC BRAF Exon 15 Wild Type (n = 10) or with BRAF non p.V600E Mutation (n = 1) | ||||
| Total samples (n = 29) | BRAF p.V600E | BRAF non p.V600E | BRAF WT | NA |
| Psammoma Body (n = 3) | - | - | 3/29 (10.3%) | - |
| Follicular Adenoma (n = 3) | - | 1/29 (3.5%) | 2/29 (6.9%) | - |
| Atypical tissue (n = 8) | - | - | 7/29 (24.1%) | 1/29 (3.5%) |
| Hyperplasia (n = 4) | - | - | 4/29 (13.8%) | - |
| Normal tissue (n = 11) | - | - | 11/29 (37.9%) | - |
| BRAF (Exon 15) Primers for Next Generation Sequencing | ||
| BRAF (Exon 15) | FW: | TGCTTGCTCTGATAGGAAAATGA |
| RV: | TGGATCCAGACAACTGTTCAAA | |
| BRAF (Exon 15) Primers for Mutagenesis | ||
| p.G593D | FW: | GTAAAAATAGATGATTTTGGTCTAGC |
| RV: | TGTGAGGTCTTCATGAAG | |
| p.A598T | FW: | TTTTGGTCTAACTACAGTGAAATC |
| RV: | TCACCTATTTTTACTGTGAG | |
| p.V600E | FW: | CTAGCTACAGAGAAATCTCGATG |
| RV: | ACCAAAATCACCTATTTTTAC | |
| p.K601E | FW: | AGCTACAGTGGAATCTCGATG |
| RV: | AGACCAAAATCACCTATTTTTAC | |
| p.S607F | FW: | TGGAGTGGGTTCCATCAGTTT |
| RV: | TCGAGATTTCACTGTAGCTAG | |
| p.S607P | FW: | ATGGAGTGGGCCCCATCAGTT |
| RV: | CGAGATTTCACTGTAGCTAG | |
| BRAF (Exon 15) Primers for Sanger Sequencing | ||
| BRAF (Exon 15) | FW: | ACACGCCAAGTCAATCATCC |
| RV: | TCTGACTGAAAGCTGTATGGATT | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquaviva, G.; de Biase, D.; Diquigiovanni, C.; Argento, C.M.; De Leo, A.; Bonora, E.; Rhoden, K.J.; Pession, A.; Tallini, G. BRAF Exon 15 Mutations in Papillary Carcinoma and Adjacent Thyroid Parenchyma: A Search for the Early Molecular Events Associated with Tumor Development. Cancers 2020, 12, 430. https://doi.org/10.3390/cancers12020430
Acquaviva G, de Biase D, Diquigiovanni C, Argento CM, De Leo A, Bonora E, Rhoden KJ, Pession A, Tallini G. BRAF Exon 15 Mutations in Papillary Carcinoma and Adjacent Thyroid Parenchyma: A Search for the Early Molecular Events Associated with Tumor Development. Cancers. 2020; 12(2):430. https://doi.org/10.3390/cancers12020430
Chicago/Turabian StyleAcquaviva, Giorgia, Dario de Biase, Chiara Diquigiovanni, Chiara Maria Argento, Antonio De Leo, Elena Bonora, Kerry Jane Rhoden, Annalisa Pession, and Giovanni Tallini. 2020. "BRAF Exon 15 Mutations in Papillary Carcinoma and Adjacent Thyroid Parenchyma: A Search for the Early Molecular Events Associated with Tumor Development" Cancers 12, no. 2: 430. https://doi.org/10.3390/cancers12020430
APA StyleAcquaviva, G., de Biase, D., Diquigiovanni, C., Argento, C. M., De Leo, A., Bonora, E., Rhoden, K. J., Pession, A., & Tallini, G. (2020). BRAF Exon 15 Mutations in Papillary Carcinoma and Adjacent Thyroid Parenchyma: A Search for the Early Molecular Events Associated with Tumor Development. Cancers, 12(2), 430. https://doi.org/10.3390/cancers12020430







