MAIT Cells Come to the Rescue in Cancer Immunotherapy?
Abstract
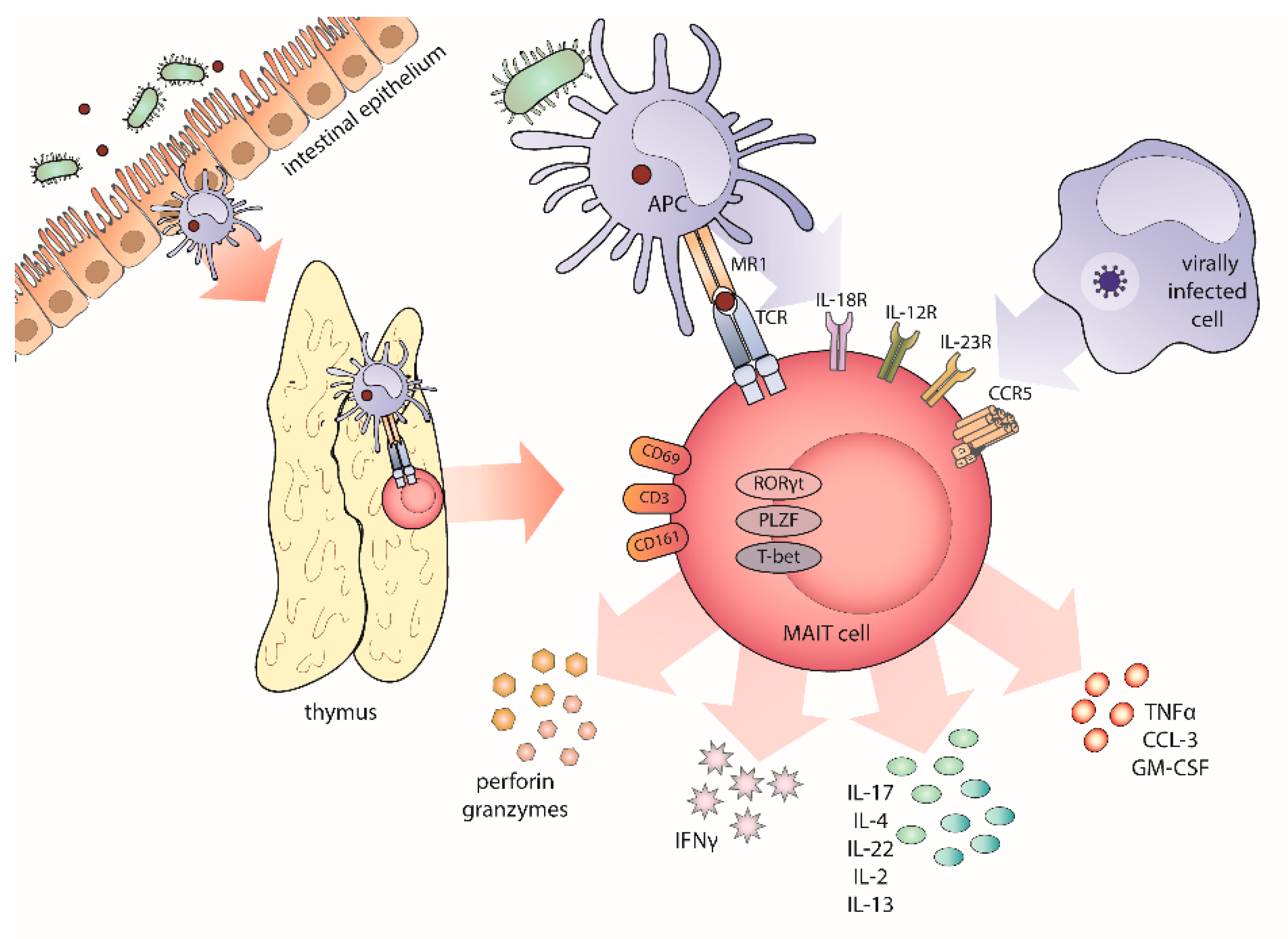
1. Untangling the Riddle of MAIT Cells’ Biology
2. The Harmony of Invariant T Cells
3. MAIT Cells in Immune-Mediated Diseases
4. MAIT Cells in Graft-Versus-Host Disease
5. MAIT Cells in Malignant Diseases
5.1. Hepatic Malignancies
5.2. Mucosal-Associated Cancers
5.3. Non Mucosal-Associated Solid Tumors
5.4. Haematological Malignancies (Multiple Myeloma)
5.5. A Role for MAIT Cells in Cancer Immunotherapy?
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef]
- Koay, H.F.; Gherardin, N.A.; Enders, A.; Loh, L.; Mackay, L.K.; Almeida, C.F.; Russ, B.E.; Nold-Petry, C.A.; Nold, M.F.; Bedoui, S.; et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 2016, 17, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Tastan, C.; Karhan, E.; Zhou, W.; Fleming, E.; Voigt, A.Y.; Yao, X.; Wang, L.; Horne, M.; Placek, L.; Kozhaya, L.; et al. Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol. 2018, 11, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Le Bourhis, L.; Martin, E.; Péguillet, I.; Guihot, A.; Froux, N.; Coré, M.; Lévy, E.; Dusseaux, M.; Meyssonnier, V.; Premel, V.; et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010, 11, 701–708. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, H.E.; Eckle, S.B.; Theodossis, A.; Liu, L.; Chen, Z.; Wubben, J.M.; Fairlie, D.P.; Strugnell, R.A.; Mintern, J.D.; McCluskey, J.; et al. The intracellular pathway for the presentation of vitamin B-related antigens by the antigen-presenting molecule MR1. Nat. Immunol. 2016, 17, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.J.; Kim, S.; Myers, N.; Huang, S.; Yu, L.; Fremont, D.H.; Diamond, M.S.; Hansen, T.H. Endogenous MHC-related protein 1 is transiently expressed on the plasma membrane in a conformation that activates mucosal-associated invariant T cells. J. Immunol. 2011, 186, 4744–4750. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, H.E.G.; Villadangos, J.A. How MR1 Presents a Pathogen Metabolic Signature to Mucosal-Associated Invariant T (MAIT) Cells. Trends Immunol. 2017, 38, 679–689. [Google Scholar] [CrossRef]
- Lepore, M.; Kalinichenko, A.; Calogero, S.; Kumar, P.; Paleja, B.; Schmaler, M.; Narang, V.; Zolezzi, F.; Poidinger, M.; Mori, L.; et al. Functionally diverse human T cells recognize non-microbial antigens presented by MR1. Elife 2017, 6, e24476. [Google Scholar] [CrossRef]
- Yan, J.; Allen, S.; McDonald, E.; Das, I.; Mak, J.Y.W.; Liu, L.; Fairlie, D.P.; Meehan, B.S.; Chen, Z.; Corbett, A.J.; et al. MAIT Cells Promote Tumor Initiation, Growth, and Metastases via Tumor MR1. Cancer Discov. 2020, 10, 124–141. [Google Scholar] [CrossRef]
- Seach, N.; Guerri, L.; Le Bourhis, L.; Mburu, Y.; Cui, Y.; Bessoles, S.; Soudais, C.; Lantz, O. Double-positive thymocytes select mucosal-associated invariant T cells. J. Immunol. 2013, 191, 6002–6009. [Google Scholar] [CrossRef]
- Salou, M.; Legoux, F.; Gilet, J.; Darbois, A.; du Halgouet, A.; Alonso, R.; Richer, W.; Goubet, A.G.; Daviaud, C.; Menger, L.; et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 2019, 216, 133–151. [Google Scholar] [CrossRef]
- Salerno-Goncalves, R.; Rezwan, T.; Sztein, M.B. B cells modulate mucosal associated invariant T cell immune responses. Front. Immunol. 2014, 4, 511. [Google Scholar] [CrossRef]
- Novak, J.; Dobrovolny, J.; Novakova, L.; Kozak, T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand. J. Immunol. 2014, 80, 271–275. [Google Scholar] [CrossRef]
- Porcelli, S.; Yockey, C.E.; Brenner, M.B.; Balk, S.P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8-alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J. Exp. Med. 1993, 178, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Toubal, A.; Nel, I.; Lotersztajn, S.; Lehuen, A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 2019, 19, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Gherardin, N.A.; Keller, A.N.; Woolley, R.E.; Le Nours, J.; Ritchie, D.S.; Neeson, P.J.; Birkinshaw, R.W.; Eckle, S.B.G.; Waddington, J.N.; Liu, L.; et al. Diversity of T Cells Restricted by the MHC Class I-Related Molecule MR1 Facilitates Differential Antigen Recognition. Immunity 2016, 44, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Boulouis, C.; Gorin, J.B.; van den Biggelaar, R.H.G.A.; Lal, K.G.; Gibbs, A.; Loh, L.; Gulam, M.Y.; Sia, W.R.; Bari, S.; et al. The CD4. Proc. Natl. Acad. Sci. USA 2018, 115, E11513–E11522. [Google Scholar] [CrossRef]
- Legoux, F.; Bellet, D.; Daviaud, C.; El Morr, Y.; Darbois, A.; Niort, K.; Procopio, E.; Salou, M.; Gilet, J.; Ryffel, B.; et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 2019, 366, 494–499. [Google Scholar] [CrossRef]
- Koay, H.F.; Su, S.; Amann-Zalcenstein, D.; Daley, S.R.; Comerford, I.; Miosge, L.; Whyte, C.E.; Konstantinov, I.E.; d’Udekem, Y.; Baldwin, T.; et al. A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Winter, S.J.; Kunze-Schumacher, H.; Imelmann, E.; Grewers, Z.; Osthues, T.; Krueger, A. MicroRNA miR-181a/b-1 controls MAIT cell development. Immunol. Cell Biol. 2019, 97, 190–202. [Google Scholar] [CrossRef]
- Patel, O.; Kjer-Nielsen, L.; Le Nours, J.; Eckle, S.B.; Birkinshaw, R.; Beddoe, T.; Corbett, A.J.; Liu, L.; Miles, J.J.; Meehan, B.; et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat. Commun. 2013, 4, 2142. [Google Scholar] [CrossRef] [PubMed]
- Mondot, S.; Boudinot, P.; Lantz, O. MAIT, MR1, microbes and riboflavin: A paradigm for the co-evolution of invariant TCRs and restricting MHCI-like molecules? Immunogenetics 2016, 68, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Merlini, E.; Cerrone, M.; van Wilgenburg, B.; Swadling, L.; Cannizzo, E.S.; d’Arminio Monforte, A.; Klenerman, P.; Marchetti, G. Association Between Impaired Vα7.2+CD161++CD8+ (MAIT) and Vα7.2+CD161-CD8+ T-Cell Populations and Gut Dysbiosis in Chronically HIV- and/or HCV-Infected Patients. Front. Microbiol. 2019, 10, 1972. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Leeansyah, E.; Sandberg, J.K. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl. Acad. Sci. USA 2017, 114, E5434–E5443. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Koay, H.F.; McCluskey, J.; Gherardin, N.A. The biology and functional importance of MAIT cells. Nat. Immunol. 2019, 20, 1110–1128. [Google Scholar] [CrossRef]
- van Wilgenburg, B.; Scherwitzl, I.; Hutchinson, E.C.; Leng, T.; Kurioka, A.; Kulicke, C.; de Lara, C.; Cole, S.; Vasanawathana, S.; Limpitikul, W.; et al. MAIT cells are activated during human viral infections. Nat. Commun. 2016, 7, 11653. [Google Scholar] [CrossRef]
- Lamichhane, R.; Galvin, H.; Hannaway, R.F.; de la Harpe, S.M.; Munro, F.; Tyndall, J.D.; Vernall, A.J.; McCall, J.L.; Husain, M.; Ussher, J.E. Type I interferons are important co-stimulatory signals during T cell receptor mediated human MAIT cell activation. Eur. J. Immunol. 2019. [Google Scholar] [CrossRef]
- Wang, H.; Kjer-Nielsen, L.; Shi, M.; D’Souza, C.; Pediongco, T.J.; Cao, H.; Kostenko, L.; Lim, X.Y.; Eckle, S.B.G.; Meehan, B.S.; et al. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Marchi, E.; Jabeen, M.; Olshansky, M.; Kurioka, A.; Pediongco, T.J.; Meehan, B.S.; Kostenko, L.; Turner, S.J.; Corbett, A.J.; et al. Activation and In Vivo Evolution of the MAIT Cell Transcriptome in Mice and Humans Reveals Tissue Repair Functionality. Cell Rep. 2019, 28, 3249–3262.e5. [Google Scholar] [CrossRef]
- Leng, T.; Akther, H.D.; Hackstein, C.P.; Powell, K.; King, T.; Friedrich, M.; Christoforidou, Z.; McCuaig, S.; Neyazi, M.; Arancibia-Cárcamo, C.V.; et al. TCR and Inflammatory Signals Tune Human MAIT Cells to Exert Specific Tissue Repair and Effector Functions. Cell Rep. 2019, 28, 3077–3091.e5. [Google Scholar] [CrossRef]
- Serriari, N.E.; Eoche, M.; Lamotte, L.; Lion, J.; Fumery, M.; Marcelo, P.; Chatelain, D.; Barre, A.; Nguyen-Khac, E.; Lantz, O.; et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin. Exp. Immunol. 2014, 176, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Minoda, Y.; Meredith, T.; Cameron, G.; Philipp, M.S.; Pellicci, D.G.; Corbett, A.J.; Kurts, C.; Gray, D.H.; Godfrey, D.I.; et al. Chronically stimulated human MAIT cells are unexpectedly potent IL-13 producers. Immunol. Cell Biol. 2019, 97, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, R.; Schneider, M.; de la Harpe, S.M.; Harrop, T.W.R.; Hannaway, R.F.; Dearden, P.K.; Kirman, J.R.; Tyndall, J.D.A.; Vernall, A.J.; Ussher, J.E. TCR- or Cytokine-Activated CD8. Cell Rep. 2019, 28, 3061–3076.e5. [Google Scholar] [CrossRef] [PubMed]
- Wallington, J.C.; Williams, A.P.; Staples, K.J.; Wilkinson, T.M.A. IL-12 and IL-7 synergize to control mucosal-associated invariant T-cell cytotoxic responses to bacterial infection. J. Allergy Clin. Immunol. 2018, 141, 2182–2195.e6. [Google Scholar] [CrossRef]
- Ussher, J.E.; van Wilgenburg, B.; Hannaway, R.F.; Ruustal, K.; Phalora, P.; Kurioka, A.; Hansen, T.H.; Willberg, C.B.; Phillips, R.E.; Klenerman, P. TLR signaling in human antigen-presenting cells regulates MR1-dependent activation of MAIT cells. Eur. J. Immunol. 2016, 46, 1600–1614. [Google Scholar] [CrossRef]
- Hassane, M.; Jouan, Y.; Creusat, F.; Soulard, D.; Boisseau, C.; Gonzalez, L.; Patin, E.C.; Heuzé-Vourc’h, N.; Sirard, J.C.; Faveeuw, C.; et al. Interleukin-7 protects against bacterial respiratory infection by promoting IL-17A-producing innate T-cell response. Mucosal Immunol. 2019, 13, 128–139. [Google Scholar] [CrossRef]
- Kurioka, A.; Ussher, J.E.; Cosgrove, C.; Clough, C.; Fergusson, J.R.; Smith, K.; Kang, Y.H.; Walker, L.J.; Hansen, T.H.; Willberg, C.B.; et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015, 8, 429–440. [Google Scholar] [CrossRef]
- Salio, M.; Gasser, O.; Gonzalez-Lopez, C.; Martens, A.; Veerapen, N.; Gileadi, U.; Verter, J.G.; Napolitani, G.; Anderson, R.; Painter, G.; et al. Activation of Human Mucosal-Associated Invariant T Cells Induces CD40L-Dependent Maturation of Monocyte-Derived and Primary Dendritic Cells. J. Immunol. 2017, 199, 2631–2638. [Google Scholar] [CrossRef]
- Rouxel, O.; Da Silva, J.; Beaudoin, L.; Nel, I.; Tard, C.; Cagninacci, L.; Kiaf, B.; Oshima, M.; Diedisheim, M.; Salou, M.; et al. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat. Immunol. 2017, 18, 1321–1331. [Google Scholar] [CrossRef]
- Constantinides, M.G.; Link, V.M.; Tamoutounour, S.; Wong, A.C.; Perez-Chaparro, P.J.; Han, S.J.; Chen, Y.E.; Li, K.; Farhat, S.; Weckel, A.; et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 2019, 366, eaax6624. [Google Scholar] [CrossRef]
- Mortier, C.; Govindarajan, S.; Venken, K.; Elewaut, D. It Takes “Guts” to Cause Joint Inflammation: Role of Innate-Like T Cells. Front. Immunol. 2018, 9, 1489. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lynch, L.; Brennan, P.J.; Cohen, N.R.; Brenner, M.B. The transcriptional programs of iNKT cells. Semin Immunol. 2015, 27, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.K.; Constantinides, M.G.; Han, J.; Picard, D.; Martin, E.; Li, B.; Lantz, O.; Bendelac, A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 2008, 29, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Gherardin, N.A.; Souter, M.N.; Koay, H.F.; Mangas, K.M.; Seemann, T.; Stinear, T.P.; Eckle, S.B.; Berzins, S.P.; d’Udekem, Y.; Konstantinov, I.E.; et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 2018, 96, 507–525. [Google Scholar] [CrossRef]
- Favreau, M.; Venken, K.; Faict, S.; Maes, K.; De Veirman, K.; De Bruyne, E.; Leleu, X.; Boon, L.; Elewaut, D.; Vanderkerken, K.; et al. Both mucosal-associated invariant and natural killer T-cell deficiency in multiple myeloma can be countered by PD-1 inhibition. Haematologica 2017, 102, e266–e270. [Google Scholar] [CrossRef]
- Martin, E.; Treiner, E.; Duban, L.; Guerri, L.; Laude, H.; Toly, C.; Premel, V.; Devys, A.; Moura, I.C.; Tilloy, F.; et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009, 7, e54. [Google Scholar] [CrossRef]
- Jing, Y.; Gravenstein, S.; Chaganty, N.R.; Chen, N.; Lyerly, K.H.; Joyce, S.; Deng, Y. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp. Gerontol. 2007, 42, 719–732. [Google Scholar] [CrossRef]
- Crosby, C.M.; Kronenberg, M. Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 2018, 18, 559–574. [Google Scholar] [CrossRef]
- Dusseaux, M.; Martin, E.; Serriari, N.; Péguillet, I.; Premel, V.; Louis, D.; Milder, M.; Le Bourhis, L.; Soudais, C.; Treiner, E.; et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 2011, 117, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Debusschere, K.; Lories, R.J.; Elewaut, D. MAIT cells: Not just another brick in the wall. Ann. Rheum. Dis. 2016, 75, 2057–2059. [Google Scholar] [CrossRef] [PubMed]
- Meierovics, A.; Yankelevich, W.J.; Cowley, S.C. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl. Acad. Sci. USA 2013, 110, E3119–E3128. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Yamamura, M.; Nabeshima, T.; Kitano, N.; van den Elzen, P.; Yesilkaya, H.; Andrew, P.; Illarionov, P. Activation of invariant natural killer T cells stimulated with microbial α-mannosyl glycolipids. Sci. Rep. 2017, 7, 9703. [Google Scholar] [CrossRef]
- Schneiders, F.L.; Huijts, C.M.; Mantici, A.; Menks, M.A.; Scotet, E.; Veerhuis, R.; Verheul, H.M.; de Gruijl, T.D.; van der Vliet, H.J. Aminobisphosphonates inhibit dendritic cell-mediated antigen-specific activation of CD1d-restricted iNKT cells. Clin. Immunol. 2015, 158, 92–99. [Google Scholar] [CrossRef]
- Pereira, C.S.; Ribeiro, H.; Pérez-Cabezas, B.; Cardoso, M.T.; Alegrete, N.; Gaspar, A.; Leão-Teles, E.; Macedo, M.F. The GM2 ganglioside inhibits iNKT cell responses in a CD1d-dependent manner. Mol. Genet. Metab. 2018, 125, 161–167. [Google Scholar] [CrossRef]
- Garner, L.C.; Klenerman, P.; Provine, N.M. Insights Into Mucosal-Associated Invariant T Cell Biology From Studies of Invariant Natural Killer T Cells. Front. Immunol. 2018, 9, 1478. [Google Scholar] [CrossRef]
- Ling, L.; Lin, Y.; Zheng, W.; Hong, S.; Tang, X.; Zhao, P.; Li, M.; Ni, J.; Li, C.; Wang, L.; et al. Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci. Rep. 2016, 6, 20358. [Google Scholar] [CrossRef]
- Won, E.J.; Ju, J.K.; Cho, Y.N.; Jin, H.M.; Park, K.J.; Kim, T.J.; Kwon, Y.S.; Kee, H.J.; Kim, J.C.; Kee, S.J.; et al. Clinical relevance of circulating mucosal-associated invariant T cell levels and their anti-cancer activity in patients with mucosal-associated cancer. Oncotarget 2016, 7, 76274–76290. [Google Scholar] [CrossRef]
- Fujii, S.I.; Shimizu, K. Immune Networks and Therapeutic Targeting of iNKT Cells in Cancer. Trends Immunol. 2019, 40, 984–997. [Google Scholar] [CrossRef]
- Pichavant, M.; Rémy, G.; Bekaert, S.; Le Rouzic, O.; Kervoaze, G.; Vilain, E.; Just, N.; Tillie-Leblond, I.; Trottein, F.; Cataldo, D.; et al. Oxidative stress-mediated iNKT-cell activation is involved in COPD pathogenesis. Mucosal Immunol. 2014, 7, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Gherardin, N.A.; Loh, L.; Admojo, L.; Davenport, A.J.; Richardson, K.; Rogers, A.; Darcy, P.K.; Jenkins, M.R.; Prince, H.M.; Harrison, S.J.; et al. Enumeration, functional responses and cytotoxic capacity of MAIT cells in newly diagnosed and relapsed multiple myeloma. Sci. Rep. 2018, 8, 4159. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Neparidze, N.; Zhang, L.; Nair, S.; Monesmith, T.; Sundaram, R.; Miesowicz, F.; Dhodapkar, K.M.; Dhodapkar, M.V. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood 2013, 121, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, A.; Caputo, V.S.; Holubova, M.; Baxan, N.; Dubois, O.; Chaudhry, M.S.; Xiao, X.; Goudevenou, K.; Pitcher, D.S.; Petevi, K.; et al. Enhanced Anti-lymphoma Activity of CAR19-iNKT Cells Underpinned by Dual CD19 and CD1d Targeting. Cancer Cell. 2018, 34, 596–610.e11. [Google Scholar] [CrossRef] [PubMed]
- Leeansyah, E.; Loh, L.; Nixon, D.F.; Sandberg, J.K. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat. Commun. 2014, 5, 3143. [Google Scholar] [CrossRef] [PubMed]
- Hapil, F.Z.; Wingender, G. The interaction between invariant Natural Killer T cells and the mucosal microbiota. Immunology 2018, 155, 164–175. [Google Scholar] [CrossRef]
- Cho, Y.N.; Kee, S.J.; Kim, T.J.; Jin, H.M.; Kim, M.J.; Jung, H.J.; Park, K.J.; Lee, S.J.; Lee, S.S.; Kwon, Y.S.; et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J. Immunol. 2014, 193, 3891–3901. [Google Scholar] [CrossRef]
- Gracey, E.; Qaiyum, Z.; Almaghlouth, I.; Lawson, D.; Karki, S.; Avvaru, N.; Zhang, Z.; Yao, Y.; Ranganathan, V.; Baglaenko, Y.; et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann. Rheum. Dis. 2016, 75, 2124–2132. [Google Scholar] [CrossRef]
- Koppejan, H.; Jansen, D.T.S.L.; Hameetman, M.; Thomas, R.; Toes, R.E.M.; van Gaalen, F.A. Altered composition and phenotype of mucosal-associated invariant T cells in early untreated rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 3. [Google Scholar] [CrossRef]
- Braudeau, C.; Amouriaux, K.; Néel, A.; Herbreteau, G.; Salabert, N.; Rimbert, M.; Martin, J.C.; Hémont, C.; Hamidou, M.; Josien, R. Persistent deficiency of circulating mucosal-associated invariant T (MAIT) cells in ANCA-associated vasculitis. J. Autoimmun. 2016, 70, 73–79. [Google Scholar] [CrossRef]
- Mekinian, A.; Mahevas, T.; Mohty, M.; Jachiet, V.; Rivière, S.; Fain, O.; Gaugler, B. Mucosal-associated Invariant Cells are Deficient in Systemic Sclerosis. Scand. J. Immunol. 2017, 86, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Macardle, C.; Weedon, H.; Beroukas, D.; Banovic, T. Mucosal-associated invariant T cells are reduced and functionally immature in the peripheral blood of primary Sjögren’s syndrome patients. Eur. J. Immunol. 2016, 46, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- von Seth, E.; Zimmer, C.L.; Reuterwall-Hansson, M.; Barakat, A.; Arnelo, U.; Bergquist, A.; Ivarsson, M.A.; Björkström, N.K. Primary sclerosing cholangitis leads to dysfunction and loss of MAIT cells. Eur. J. Immunol. 2018, 48, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Chiba, A.; Shibuya, T.; Osada, T.; Ishikawa, D.; Kodani, T.; Nomura, O.; Watanabe, S.; Miyake, S. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J. Gastroenterol. Hepatol. 2016, 31, 965–972. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Hanafi, L.A.; Sheih, A.; Golob, J.L.; Srinivasan, S.; Boeckh, M.J.; Pergam, S.A.; Mahmood, S.; Baker, K.K.; Gooley, T.A.; et al. Graft-Derived Reconstitution of Mucosal-Associated Invariant T Cells after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 242–251. [Google Scholar] [CrossRef]
- Solders, M.; Erkers, T.; Gorchs, L.; Poiret, T.; Remberger, M.; Magalhaes, I.; Kaipe, H. Mucosal-Associated Invariant T Cells Display a Poor Reconstitution and Altered Phenotype after Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2017, 8, 1861. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Umeda, K.; Hiejima, E.; Iwai, A.; Mikami, M.; Nodomi, S.; Saida, S.; Kato, I.; Hiramatsu, H.; Yasumi, T.; et al. Influence of post-transplant mucosal-associated invariant T cell recovery on the development of acute graft-versus-host disease in allogeneic bone marrow transplantation. Int. J. Hematol. 2018, 108, 66–75. [Google Scholar] [CrossRef]
- Varelias, A.; Bunting, M.D.; Ormerod, K.L.; Koyama, M.; Olver, S.D.; Straube, J.; Kuns, R.D.; Robb, R.J.; Henden, A.S.; Cooper, L.; et al. Recipient mucosal-associated invariant T cells control GVHD within the colon. J. Clin. Investig. 2018, 128, 1919–1936. [Google Scholar] [CrossRef]
- van der Waart, A.B.; van der Velden, W.J.; van Halteren, A.G.; Leenders, M.J.; Feuth, T.; Blijlevens, N.M.; van der Voort, R.; Dolstra, H. Decreased levels of circulating IL17-producing CD161+CCR6+ T cells are associated with graft-versus-host disease after allogeneic stem cell transplantation. PLoS ONE 2012, 7, e50896. [Google Scholar] [CrossRef]
- Varelias, A.; Gartlan, K.H.; Wilkinson, A.N.; Olver, S.D.; Samson, L.D.; Tey, S.K.; MacDonald, K.P.A.; Hill, G.R. Expansion of IL-17A-secreting CD8. Blood Adv. 2019, 3, 718–723. [Google Scholar] [CrossRef]
- Omenetti, S.; Bussi, C.; Metidji, A.; Iseppon, A.; Lee, S.; Tolaini, M.; Li, Y.; Kelly, G.; Chakravarty, P.; Shoaie, S.; et al. The Intestine Harbors Functionally Distinct Homeostatic Tissue-Resident and Inflammatory Th17 Cells. Immunity 2019, 51, 77–89.e6. [Google Scholar] [CrossRef] [PubMed]
- Vandeven, N.; Nghiem, P. Pathogen-driven cancers and emerging immune therapeutic strategies. Cancer Immunol. Res. 2014, 2, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.N.; Eckle, S.B.; Xu, W.; Liu, L.; Hughes, V.A.; Mak, J.Y.; Meehan, B.S.; Pediongco, T.; Birkinshaw, R.W.; Chen, Z.; et al. Drugs and drug-like molecules can modulate the function of mucosal-associated invariant T cells. Nat. Immunol. 2017, 18, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Cameron, T.O.; Sidobre, S.; Manlongat, N.; Kronenberg, M.; Briskin, M.J.; Dustin, M.L.; Littman, D.R. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005, 3, e113. [Google Scholar] [CrossRef] [PubMed]
- Kurioka, A.; Walker, L.J.; Klenerman, P.; Willberg, C.B. MAIT cells: New guardians of the liver. Clin. Transl. Immunol. 2016, 5, e98. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Goswami, S.; Shi, J.Y.; Wu, L.J.; Wang, X.Y.; Ma, J.Q.; Zhang, Z.; Shi, Y.; Ma, L.J.; Zhang, S.; et al. Activated and Exhausted MAIT Cells Foster Disease Progression and Indicate Poor Outcome in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 3304–3316. [Google Scholar] [CrossRef] [PubMed]
- Beudeker, B.J.B.; van Oord, G.W.; Arends, J.E.; Schulze Zur Wiesch, J.; van der Heide, M.S.; de Knegt, R.J.; Verbon, A.; Boonstra, A.; Claassen, M.A.A. Mucosal-associated invariant T-cell frequency and function in blood and liver of HCV mono- and HCV/HIV co-infected patients with advanced fibrosis. Liver Int. 2018, 38, 458–468. [Google Scholar] [CrossRef]
- Setsu, T.; Yamagiwa, S.; Tominaga, K.; Kimura, N.; Honda, H.; Kamimura, H.; Tsuchiya, A.; Takamura, M.; Terai, S. Persistent reduction of mucosal-associated invariant T cells in primary biliary cholangitis. J. Gastroenterol. Hepatol. 2018, 33, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, B.; Jiang, X.; Chen, W.; Zhang, J.; Wei, Y.; Chen, Y.; Lian, M.; Bian, Z.; Miao, Q.; et al. Mucosal-Associated Invariant T Cells Improve Nonalcoholic Fatty Liver Disease Through Regulating Macrophage Polarization. Front. Immunol. 2018, 9, 1994. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, K.; Rombouts, K.; Saffioti, F.; Roccarina, D.; Rosselli, M.; Hall, A.; Luong, T.; Tsochatzis, E.A.; Thorburn, D.; Pinzani, M. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 2018, 68, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Yan, J.; Xu, J.; Pang, X.H.; Chen, M.S.; Li, L.; Wu, C.; Li, S.P.; Zheng, L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J. Hepatol. 2009, 50, 980–989. [Google Scholar] [CrossRef]
- Fabre, J.; Giustiniani, J.; Garbar, C.; Antonicelli, F.; Merrouche, Y.; Bensussan, A.; Bagot, M.; Al-Dacak, R. Targeting the Tumor Microenvironment: The Protumor Effects of IL-17 Related to Cancer Type. Int. J. Mol. Sci. 2016, 17, 1433. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.; Weiss, E.; Paradis, V.; Wan, J.; Mabire, M.; Sukriti, S.; Rautou, P.E.; Albuquerque, M.; Picq, O.; Gupta, A.C.; et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat. Commun. 2018, 9, 2146. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, L.; Yoo, J.K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Shaler, C.R.; Tun-Abraham, M.E.; Skaro, A.I.; Khazaie, K.; Corbett, A.J.; Mele, T.; Hernandez-Alejandro, R.; Haeryfar, S.M.M. Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment. Cancer Immunol. Immunother. 2017, 66, 1563–1575. [Google Scholar] [CrossRef]
- Yang, J.D.; Roberts, L.R. Hepatocellular carcinoma: A global view. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 448–458. [Google Scholar] [CrossRef]
- Yong, Y.K.; Tan, H.Y.; Saeidi, A.; Rosmawati, M.; Atiya, N.; Ansari, A.W.; Rajarajeswaran, J.; Vadivelu, J.; Velu, V.; Larsson, M.; et al. Decrease of CD69 levels on TCR Vα7.2. Innate Immun. 2017, 23, 459–467. [Google Scholar] [CrossRef]
- Barathan, M.; Mohamed, R.; Vadivelu, J.; Chang, L.Y.; Saeidi, A.; Yong, Y.K.; Ravishankar Ram, M.; Gopal, K.; Velu, V.; Larsson, M.; et al. Peripheral loss of CD8(+) CD161(++) TCRVα7·2(+) mucosal-associated invariant T cells in chronic hepatitis C virus-infected patients. Eur. J. Clin. Investig. 2016, 46, 170–180. [Google Scholar] [CrossRef]
- Yong, Y.K.; Saeidi, A.; Tan, H.Y.; Rosmawati, M.; Enström, P.F.; Batran, R.A.; Vasuki, V.; Chattopadhyay, I.; Murugesan, A.; Vignesh, R.; et al. Hyper-Expression of PD-1 Is Associated with the Levels of Exhausted and Dysfunctional Phenotypes of Circulating CD161. Front. Immunol. 2018, 9, 472. [Google Scholar] [CrossRef]
- Focosi, D.; Bestagno, M.; Burrone, O.; Petrini, M. CD57+ T lymphocytes and functional immune deficiency. J. Leukoc. Biol. 2010, 87, 107–116. [Google Scholar] [CrossRef]
- Negro, F. Hepatitis D virus coinfection and superinfection. Cold Spring Harb. Perspect. Med. 2014, 4, a021550. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Hengst, J.; Parrot, T.; Leeansyah, E.; Lunemann, S.; Malone, D.F.G.; Hardtke, S.; Strauss, O.; Zimmer, C.L.; Berglin, L.; et al. Chronic hepatitis delta virus infection leads to functional impairment and severe loss of MAIT cells. J. Hepatol. 2019, 71, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zabijak, L.; Attencourt, C.; Guignant, C.; Chatelain, D.; Marcelo, P.; Marolleau, J.P.; Treiner, E. Increased tumor infiltration by mucosal-associated invariant T cells correlates with poor survival in colorectal cancer patients. Cancer Immunol. Immunother. 2015, 64, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Sundström, P.; Ahlmanner, F.; Akéus, P.; Sundquist, M.; Alsén, S.; Yrlid, U.; Börjesson, L.; Sjöling, Å.; Gustavsson, B.; Wong, S.B.; et al. Human Mucosa-Associated Invariant T Cells Accumulate in Colon Adenocarcinomas but Produce Reduced Amounts of IFN-γ. J. Immunol. 2015, 195, 3472–3481. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.M.; O’Brien, A.M.; Phelan, J.J.; Kennedy, S.A.; Wood, N.A.W.; Veerapen, N.; Besra, G.S.; Clarke, N.E.; Foley, E.K.; Ravi, A.; et al. Mucosal-Associated Invariant T Cells Display Diminished Effector Capacity in Oesophageal Adenocarcinoma. Front. Immunol. 2019, 10, 1580. [Google Scholar] [CrossRef]
- Peterfalvi, A.; Gomori, E.; Magyarlaki, T.; Pal, J.; Banati, M.; Javorhazy, A.; Szekeres-Bartho, J.; Szereday, L.; Illes, Z. Invariant Valpha7.2-Jalpha33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int. Immunol. 2008, 20, 1517–1525. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Le Nours, J.; Andrews, D.M.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473. [Google Scholar] [CrossRef]
- Crowther, M.D.; Dolton, G.; Legut, M.; Caillaud, M.E.; Lloyd, A.; Attaf, M.; Galloway, S.A.E.; Rius, C.; Farrell, C.P.; Szomolay, B.; et al. Genome-wide CRISPR-Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat. Immunol. 2020, 21, 178–185. [Google Scholar] [CrossRef]
- Meermeier, E.W.; Laugel, B.F.; Sewell, A.K.; Corbett, A.J.; Rossjohn, J.; McCluskey, J.; Harriff, M.J.; Franks, T.; Gold, M.C.; Lewinsohn, D.M. Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat. Commun. 2016, 7, 12506. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Kostov, B.; Fraile, G.; Caravia-Durán, D.; Maure, B.; Rascón, F.J.; Zamora, M.; Casanovas, A.; Lopez-Dupla, M.; Ripoll, M.; et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J. Hematol. Oncol. 2017, 10, 90. [Google Scholar] [CrossRef]
- Heczey, A.; Liu, D.; Tian, G.; Courtney, A.N.; Wei, J.; Marinova, E.; Gao, X.; Guo, L.; Yvon, E.; Hicks, J.; et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 2014, 124, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Courtney, A.N.; Jena, B.; Heczey, A.; Liu, D.; Marinova, E.; Guo, L.; Xu, X.; Torikai, H.; Mo, Q.; et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J. Clin. Investig. 2016, 126, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Cappione, J.E.; Nixon, D.F.; Chi, J.C.; Nguyen, M.L.; Kirby, C.K.; Milush, J.M.; Koth, L.L. Invariant natural killer T (iNKT) cell exhaustion in sarcoidosis. Eur. J. Immunol. 2013, 43, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Favreau, M.; Menu, E.; Gaublomme, D.; Vanderkerken, K.; Faict, S.; Maes, K.; De Bruyne, E.; Govindarajan, S.; Drennan, M.; Van Calenbergh, S.; et al. Leptin receptor antagonism of iNKT cell function: A novel strategy to combat multiple myeloma. Leukemia 2017, 31, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.V.; Lalani, S.; Kim, S.; Halder, R.; Azuma, M.; Yagita, H.; Kumar, V.; Wu, L.; Kaer, L.V. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J. Immunol. 2009, 182, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Fung, B.M.; Lindor, K.D.; Tabibian, J.H. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J. Gastroenterol. 2019, 25, 659–671. [Google Scholar] [CrossRef]
- Theander, E.; Henriksson, G.; Ljungberg, O.; Mandl, T.; Manthorpe, R.; Jacobsson, L.T. Lymphoma and other malignancies in primary Sjögren’s syndrome: A cohort study on cancer incidence and lymphoma predictors. Ann. Rheum. Dis. 2006, 65, 796–803. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Sværke, C.; Thomsen, R.W.; Nørgaard, M. Sarcoidosis and subsequent cancer risk: A Danish nationwide cohort study. Eur. Respir. J. 2015, 45, 269–272. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Matsuyama, H.; Isshiki, T.; Chiba, A.; Yamaguchi, T.; Murayama, G.; Akasaka, Y.; Eishi, Y.; Sakamoto, S.; Homma, S.; Miyake, S. Activation of mucosal-associated invariant T cells in the lungs of sarcoidosis patients. Sci. Rep. 2019, 9, 13181. [Google Scholar] [CrossRef]

| Feature | MAIT Cells | iNKT Cells | Ref. |
|---|---|---|---|
| TCR combinations | Human: TRAV1-2–TRAJ33 (pairs predominantly with TRBV20-1 or TRBV6 (polyclonal); TRAJ12 and TRAJ20 have also been demonstrated Mice: Vα19 combined with Vβ6 or Vβ8 | Human: TRAV10–TRAJ18 (invariant) pairs predominantly with TRBV25-1 (polyclonal) Mice: Vα14–Jα18 combined with Vβ2, Vβ7, or Vβ8.2 | [25,41] |
| Prevalence in peripheral blood | 1–10% of T lymphocytes in peripheral blood of a healthy adult (mouse 0.1%); increases until the 3rd decade of life and then drops | 0.01–1% of T lymphocytes in peripheral blood of a healthy adult; decreases with age | [13,48,49] |
| Tissue frequency | 40–50% of intrahepatic T lymphocytes (in contrast, 0.6% in mice) 1–5% of lymphoid cells in the intestinal mucosa 2% of skin T cells, mostly residing at the interface of dermis and epidermis | 1% of intrahepatic lymphocytes (in contrast 10–30% in mice) 5% of lung-resident lymphocytes >15% of adipose tissue T cells | [40,50,51,52,53] |
| MHC restriction | MR1 | CD1d | [48] |
| Activating ligands | Riboflavin intermediate metabolites (e.g., 5-OP-RU and 5-OE-RU) Drug metabolites (e.g., diclofenac metabolites). | Bacterial and synthetic glycol(sphingo)lipids and phospholipids (e.g., α- GalCer, β-GlcCer, diacylglycerols, cholesteryl α-glucosides and ether-bonded mono-alkyl glycerophosphates) | [7,54] |
| Inhibiting antigens/ligands | Folate derivatives (e.g., 6-FP, Ac-6-FP), | GM2 ganglioside, aminobisphosphonates | [55,56] |
| Cell markers and phenotype | PLZF, RORγt, T-bet; IL-7R, IL-12R, IL-15R, IL-18R; CD3, CD4, CD8, CD161, CD44, CD69, CD25; display an effector-memory phenotype prior to antigen exposure, TCR-independent activation | T-bet, GATA3, PLZF, RORγt; IL-12R, IL-18R, IL-27R; CD3, CD4, CD8, CD161, CD69, CD103, CRTAM; display an effector-memory phenotype prior to antigen exposure, TCR-independent activation | [25,39,50,57] |
| Subsets | MAIT1, MAIT17 | NKT1, NKT2, NKT10 and NKT17; ability to further differentiate functionally under inflammatory conditions | [19,50] |
| Role in solid tumors |
|
| [15,32,47,58,59,60,61] |
| Role in hematological malignancies |
| - effective coaddition of iNKT cell stimulation to standard therapies in multiple myeloma and lymphomas | [47,62,63,64] |
| Dependence on microbiome | Commensal microbiota required for MAIT cell maturation, activation of MAIT cells by microbial antigens | (immature) iNKT cell numbers increase in germ-free mice, microbial antigens induce proinflammatory responses in iNKT cells | [65,66] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukasik, Z.; Elewaut, D.; Venken, K. MAIT Cells Come to the Rescue in Cancer Immunotherapy? Cancers 2020, 12, 413. https://doi.org/10.3390/cancers12020413
Lukasik Z, Elewaut D, Venken K. MAIT Cells Come to the Rescue in Cancer Immunotherapy? Cancers. 2020; 12(2):413. https://doi.org/10.3390/cancers12020413
Chicago/Turabian StyleLukasik, Zuzanna, Dirk Elewaut, and Koen Venken. 2020. "MAIT Cells Come to the Rescue in Cancer Immunotherapy?" Cancers 12, no. 2: 413. https://doi.org/10.3390/cancers12020413
APA StyleLukasik, Z., Elewaut, D., & Venken, K. (2020). MAIT Cells Come to the Rescue in Cancer Immunotherapy? Cancers, 12(2), 413. https://doi.org/10.3390/cancers12020413





