Baseline Serum HE4 But Not Tissue HE4 Expression Predicts Response to the Levonorgestrel-Releasing Intrauterine System in Atypical Hyperplasia and Early Stage Endometrial Cancer
Abstract
1. Introduction
2. Results
2.1. Baseline HE4 and Demographics
2.2. Baseline Characteristics in Responders vs. Non-Responders to LNG-IUS
2.3. Baseline Serum HE4 in Responders vs. Non-Responders to LNG-IUS
2.4. Change in Serum HE4 from Baseline during LNG-IUS Therapy
2.5. Baseline Tissue HE4 Expression, Patient Demographics, and Serum HE4
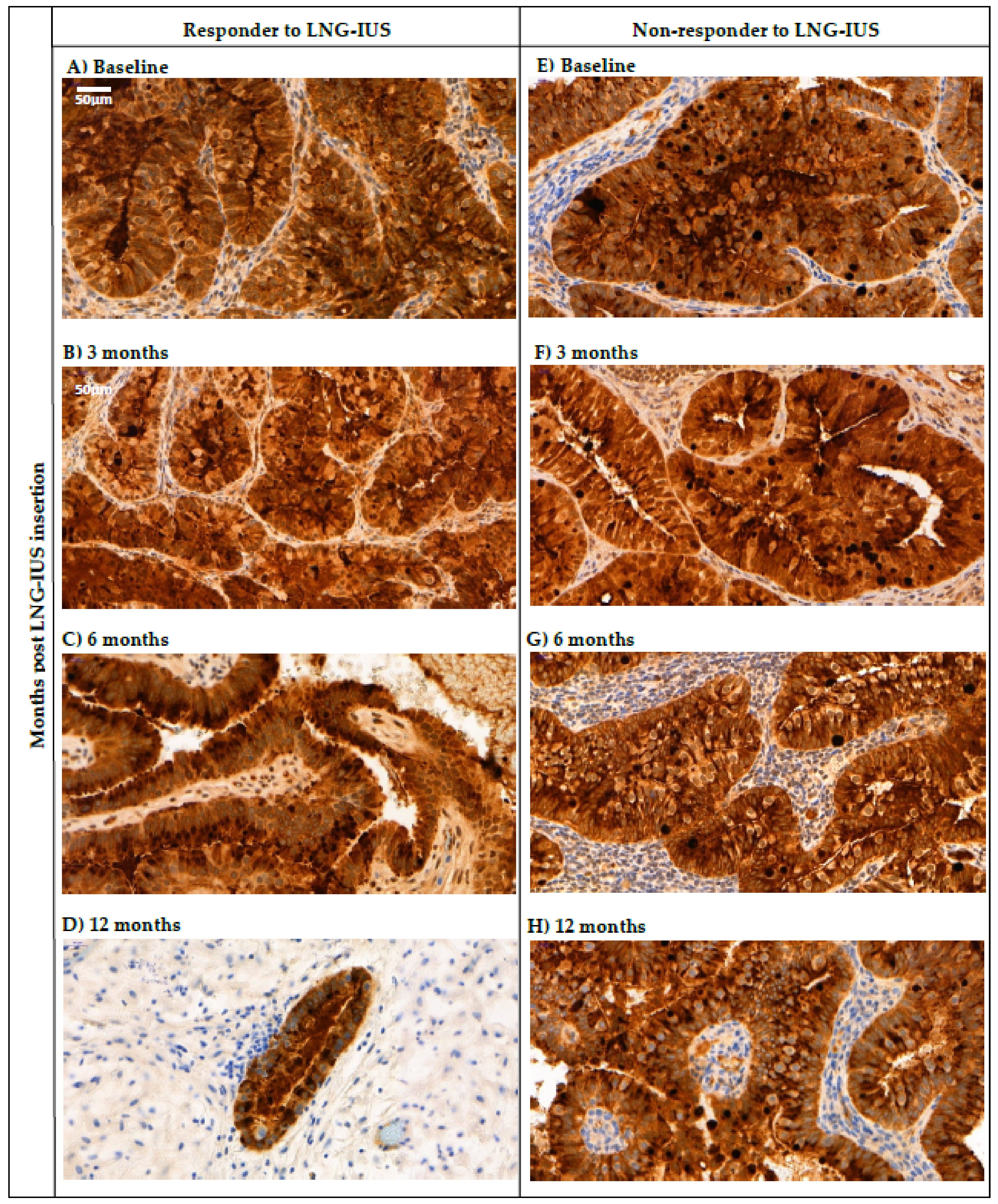
2.6. Changes in Tissue HE4 Expression after LNG-IUS Insertion in Responders vs. Non-Responders
3. Discussion
4. Materials & Methods
4.1. Patients and Sample Collection
4.2. Immunohistochemistry
4.3. Quantification of HE4 Staining
4.4. Quantification of HE4 in Human Serum
4.5. Ethical Approval
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurman, R.J.; Kaminski, P.F.; Norris, H.J. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer 1985, 56, 403–412. [Google Scholar] [CrossRef]
- Montz, F.J.; Bristow, R.E.; Bovicelli, A.; Tomacruz, R.; Kurman, R.J. Intrauterine progesterone treatment of early endometrial cancer. Am. J. Obstet. Gynecol. 2002, 186, 651–657. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Bodurka, D.C.; Sun, C.C.; Levenback, C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: A literature review. Gynecol. Oncol. 2004, 95, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Soneja, H.; Bhatia, K.; Ganesan, R.; Rollason, T.; Clark, T.J.; Gupta, J.K. The effectiveness of a levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of endometrial hyperplasia—A long-term follow-up study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 139, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.C.; Fader, A.N.; Carson, K.A.; Bristow, R.E. Oncologic and Reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 Adenocarcinoma: A systematic review. Gynecol. Oncol. 2012, 125, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Bahamondes, L.; Bahamondes, M.V.; Shulman, L.P. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum. Reprod. Update 2015, 21, 640–651. [Google Scholar] [CrossRef]
- Abu Hashim, H.; Ghayaty, E.; El Rakhawy, M. Levonorgestrel-releasing intrauterine system vs. oral progestins for non-atypical endometrial hyperplasia: A systematic review and metaanalysis of randomized trials. Am. J. Obstet. Gynecol. 2015, 213, 469–478. [Google Scholar] [CrossRef]
- Haoula, Z.J.; Walker, K.F.; Powell, M.C. Levonorgestrel intra-uterine system as a treatment option for complex endometrial hyperplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 176–179. [Google Scholar] [CrossRef]
- Orbo, A.; Vereide, A.B.; Arnes, M.; Pettersen, I.; Straume, B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: A national multicentre randomised trial. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 477–486. [Google Scholar] [CrossRef]
- Orbo, A.; Arnes, M.; Lysa, L.M.; Borgfeldt, C.; Straume, B. HE4 is a novel tissue marker for therapy response and progestin resistance in medium- and low-risk endometrial hyperplasia. Br. J. Cancer 2016, 115, 725–730. [Google Scholar] [CrossRef]
- Behnamfar, F.; Ghahiri, A.; Tavakoli, M. Levonorgestrel-releasing intrauterine system (Mirena) in compare to medroxyprogesterone acetate as a therapy for endometrial hyperplasia. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 686–690. [Google Scholar]
- Baker, J.; Obermair, A.; Gebski, V.; Janda, M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: A meta-analysis and systematic review of the literature. Gynecol. Oncol. 2012, 125, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, A.E.; Ryan, N.A.J.; Crosbie, E.J. Biomarkers needed to predict progestin response in endometrial cancer. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1584. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, G.P.; Finan, M.A. Endometrial cancer in women 45 years of age or younger: A clinicopathological analysis. Am. J. Obstet. Gynecol. 2005, 193, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dowdy, S.; Tipton, T.; Podratz, K.; Lu, W.-G.; Xie, X.; Jiang, S.-W. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev. Mol. Diagn. 2009, 9, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.T.; Hampton, G.M.; Frierson, H.F. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod. Pathol. 2006, 19, 847–853. [Google Scholar] [CrossRef]
- Capriglione, S.; Plotti, F.; Miranda, A.; Ricciardi, R.; Scaletta, G.; Aloisi, A.; Guzzo, F.; Montera, R.; Angioli, R. Utility of tumor marker HE4 as prognostic factor in endometrial cancer: A single-center controlled study. Tumor Biol. 2015, 36, 4151–4156. [Google Scholar] [CrossRef]
- Angioli, R.; Plotti, F.; Capriglione, S.; Scaletta, G.; Dugo, N.; Aloisi, A.; Piccolo, C.L.; Del Vescovo, R.; Terranova, C.; Zobel, B.B. Preoperative local staging of endometrial cancer: The challenge of imaging techniques and serum biomarkers. Arch. Gynecol. Obstet. 2016, 294, 1291–1298. [Google Scholar] [CrossRef]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Badgwell, D.; Lu, Z.; Allard, W.J.; Granai, C.O.; Bast, R.C.; Lu, K. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol. Oncol. 2008, 110, 196–201. [Google Scholar] [CrossRef]
- Bignotti, E.; Ragnoli, M.; Zanotti, L.; Calza, S.; Falchetti, M.; Lonardi, S.; Bergamelli, S.; Bandiera, E.; Tassi, R.A.; Romani, C.; et al. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br. J. Cancer 2011, 104, 1418–1425. [Google Scholar] [CrossRef]
- Kalogera, E.; Scholler, N.; Powless, C.; Weaver, A.; Drapkin, R.; Li, J.; Jiang, S.-W.; Podratz, K.; Urban, N.; Dowdy, S. Correlation of Serum HE4 with Tumor Size and Myometrial Invasion in Endometrial Cancer. Gynecol. Oncol. 2012, 124, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Mutz-Dehbalaie, I.; Egle, D.; Fessler, S.; Hubalek, M.; Fiegl, H.; Marth, C.; Widschwendter, A. HE4 is an independent prognostic marker in endometrial cancer patients. Gynecol. Oncol. 2012, 126, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.J.; Hackethal, A.; Metcalf, A.M.; Coward, J.; Ferguson, K.; Oehler, M.K.; Quinn, M.A.; Janda, M.; Leung, Y.; Freemantle, M.; et al. Serum HE4 as a prognostic marker in endometrial cancer—A population based study. Gynecol. Oncol. 2014, 132, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, N.S.; Karlsen, M.A.; Hogdall, C.K.; Hogdall, E.V.S. HE4 Tissue Expression and Serum HE4 Levels in Healthy Individuals and Patients with Benign or Malignant Tumors: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2285–2295. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Tan, M.; Zhuang, H.; Gao, J.; Hu, Z.; Wang, H.; Zhu, L.; Liu, J.; Lin, B. Expression of HE4 in Endometrial Cancer and Its Clinical Significance. BioMed Res. Int. 2015, 6795629. [Google Scholar] [CrossRef]
- Moore, R.G.; Miller, C.M.; Brown, A.K.; Robison, K.; Steinhoff, M.; Lambert-Messerlian, G. Utility of Tumor Marker HE4 to Predict Depth of Myometrial Invasion in Endometrioid Adenocarcinoma of the Uterus. Int. J. Gynecol. Cancer 2011, 21, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Angioli, R.; Plotti, F.; Capriglione, S.; Montera, R.; Damiani, P.; Ricciardi, R.; Aloisi, A.; Luvero, D.; Cafa, E.V.; Dugo, N.; et al. The role of novel biomarker HE4 in endometrial cancer: A case control prospective study. Tumor Biol. 2013, 34, 571–576. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Mariani, A.; Chen, D.; Klatt, E.; Podratz, K.; Drapkin, R.; Broaddus, R.; Dowdy, S.; Jiang, S.-W. HE4 (WFDC2) Promotes Tumor Growth in Endometrial Cancer Cell Lines. Int. J. Mol. Sci. 2013, 14, 6026–6043. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, H.; Senkowski, C.; Wang, J.; Wang, X.; Brower, S.; Glasgow, W.; Byck, D.; Jiang, S.-W.; Li, J. Recombinant HE4 protein promotes proliferation of pancreatic and endometrial cancer cell lines. Oncol. Rep. 2016, 35, 163–170. [Google Scholar] [CrossRef]
- Qu, W.; Li, J.; Duan, P.; Tang, Z.; Guo, F.; Chen, H.; Zhu, X.; Jiang, S.-W. Physiopathological factors affecting the diagnostic value of serum HE4-test for gynecologic malignancies. Expert Rev. Mol. Diagn. 2016, 16, 1271–1282. [Google Scholar] [CrossRef]
- Knific, T.; Osredkar, J.; Smrkolj, S.; Tonin, I.; Vouk, K.; Blejec, A.; Grazio, S.F.; Rizner, T.L. Novel algorithm including CA-125, HE4 and body mass index in the diagnosis of endometrial cancer. Gynecol. Oncol. 2017, 147, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Antonsen, S.L.; Hogdall, E.; Christensen, I.J.; Lydolph, M.; Tabor, A.; Jakobsen, A.L.; Fago-Olsen, C.L.; Andersen, E.S.; Jochumsen, K.; Hogdall, C. HE4 and CA125 levels in the preoperative assessment of endometrial cancer patients: A prospective multicenter study (ENDOMET). Acta Obstet. Gynecol. Scand. 2013, 92, 1313–1322. [Google Scholar] [CrossRef]
- Bolstad, N.; Øijordsbakken, M.; Nustad, K.; Bjerner, J. Human epididymis protein 4 reference limits and natural variation in a Nordic reference population. Tumor Biol. 2012, 33, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Eggemann, H.; Ignatov, T.; Burger, E.; Costa, S.D.; Ignatov, A. Management of elderly women with endometrial cancer. Gynecol. Oncol. 2017, 146, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Capriglione, S.; Zito, G.; Lopez, S.; Gulino, F.A.; Di Guardo, F.; Vitagliano, A.; Noventa, M.; La Rosa, V.L.; Sapia, F.; et al. Management of endometrial, ovarian and cervical cancer in the elderly: Current approach to a challenging condition. Arch. Gynecol. Obstet. 2019, 299, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Ørtoft, G.; Dueholm, M.; Mathiesen, O.; Hansen, E.S.; Lundorf, E.; Møller, C.; Marinovskij, E.; Petersen, L.K. Preoperative staging of endometrial cancer using TVS, MRI, and hysteroscopy. Acta Obstet. Gynecol. Scand. 2013, 92, 536–545. [Google Scholar] [CrossRef]
- Cignini, P.; Vitale, S.G.; Laganà, A.S.; Biondi, A.; La Rosa, V.L.; Cutillo, G. Preoperative work-up for definition of lymph node risk involvement in early stage endometrial cancer: 5-year follow-up. Updates Surg. 2017, 69, 75–82. [Google Scholar] [CrossRef]
- Chiva, L.; Lapuente, F.; González-Cortijo, L.; Carballo, N.; García, J.F.; Rojo, A.; Gonzalez-Martín, A. Sparing fertility in young patients with endometrial cancer. Gynecol. Oncol. 2008, 111, S101–S104. [Google Scholar] [CrossRef]
- Zakhour, M.; Cohen, J.G.; Gibson, A.; Walts, A.E.; Karimian, B.; Baltayan, A.; Aoyama, C.; Garcia, L.; Dhaliwal, S.K.; Elashoff, D.; et al. Abnormal mismatch repair and other clinicopathologic predictors of poor response to progestin treatment in young women with endometrial complex atypical hyperplasia and well-differentiated endometrial adenocarcinoma: A consecutive case series. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1576–1583. [Google Scholar] [CrossRef]
- Leone Roberti Maggiore, U.; Martinelli, F.; Dondi, G.; Bogani, G.; Chiappa, V.; Evangelista, M.T.; Liberale, V.; Ditto, A.; Ferrero, S.; Raspagliesi, F. Efficacy and fertility outcomes of levonorgestrel-releasing intra-uterine system treatment for patients with atypical complex hyperplasia or endometrial cancer: A retrospective study. J. Gynecol. Oncol. 2019, 30, e57. [Google Scholar] [CrossRef]
- Wan, Y.L.; Beverley-Stevenson, R.; Carlisle, D.; Clarke, S.; Edmondson, R.J.; Glover, S.; Holland, J.; Hughes, C.; Kitchener, H.C.; Kitson, S.; et al. Working together to shape the endometrial cancer research agenda: The top ten unanswered research questions. Gynecol. Oncol. 2016, 143, 287–293. [Google Scholar] [CrossRef] [PubMed]
- McCarty, K.S.; Szabo, E.; Flowers, J.L.; Cox, E.B.; Leight, G.S.; Miller, L.; Konrath, J.; Soper, J.T.; Budwit, D.A.; Creasman, W.T.; et al. Use of a Monoclonal Anti-Estrogen Receptor Antibody in the Immunohistochemical Evaluation of Human Tumors. Cancer Res. 1986, 46, 4244s–4248s. [Google Scholar] [PubMed]



| Age (Years) | |
|---|---|
| Mean | 54.4 ± 2.0 |
| Range | 25–89 |
| Menopausal Status | |
| Pre-menopausal | 28 (38%) |
| Post-menopausal | 46 (62%) |
| Body Mass Index (kg/m2) | |
| Mean | 44.8 ± 1.3 |
| Range | 16.4–70.1 |
| Diagnosis | |
| AH | 34 (46%) |
| G1EEC | 35 (47%) |
| G2EEC | 5 (7%) |
| Smoking Status | |
| Smoker | 7 (9%) |
| Non-smoker | 67 (91%) |
| Baseline Characteristics | Baseline Serum HE4 (pM) | 95% CI | N | p-Value |
|---|---|---|---|---|
| AH | 58.7 ± 1.1 * | 49.0–70.2 | 32 | * AH vs. G1EEC p = 0.003 AH vs. G2EEC p = 0.110 G1EEC vs. G2EEC p = 0.521 |
| G1EEC | 105.8 ± 1.1 * | 75.6–147.9 | 31 | |
| G2EEC | 90.0 ± 1.2 | 42.7–189.7 | 3 | |
| Pre-Menopausal | 46.9 ± 1.1 | 36.2–60.6 | 25 | p < 0.001 |
| Post-Menopausal | 108.4 ± 1.1 | 87.5–134.1 | 41 | |
| Smoker | 106.3 ± 1.3 | 51.1–221.1 | 6 | p = 0.317 |
| Non-Smoker | 76.6 ± 1.1 | 62.7–93.5 | 60 |
| Response to LNG-IUS | Baseline Serum HE4 (pM) | 95% CI | N | p-Value |
|---|---|---|---|---|
| Responders | 62.1 ± 1.1 | 52.7–73.2 | 35 | 0.014 |
| Non-Responders | 125.6 ± 1.3 | 74.5–211.7 | 14 |
| Baseline Characteristics and Response to LNG-IUS | Baseline HE4 Expression | ||||
|---|---|---|---|---|---|
| Low | Medium | High | N | p-Value | |
| Age (years) | 51.8 ± 3.3 (45.0–58.6) n = 30 | 57.1 ± 3.1 (50.8–63.4) n = 26 | 51.5 ± 5.7 (38.8–64.1) n = 11 | 67 | 0.466 |
| BMI (m/kg2) | 41.3 ± 2.5 (36.2–46.3) n = 30 | 45.7 ± 1.7 (42.1–49.3) n = 26 | 48.0 ± 3.4 (40.3–55.6) n = 11 | 67 | 0.181 |
| Serum HE4 (pM) | 69.1 ± 1.2 (50.0–96.7) n = 24 | 88.5 ± 1.9 (62.0–126.4) n = 24 | 85.2 ± 1.3 (48.9–148.6) n = 11 | 59 | 0.567 |
| AH | 12 (39%) | 13 (42%) | 6 (19%) | 31 | 0.822 |
| G1EEC | 15 (47%) | 12 (38%) | 5 (16%) | 32 | |
| G2EEC | 3 (75%) | 1 (25%) | 0 (0%) | 4 | |
| Pre-Menopausal | 7 (27%) | 15 (58%) | 4 (15%) | 26 | 0.666 |
| Post-Menopausal | 15 (37%) | 19 (46%) | 7 (17%) | 41 | |
| Responder to LNG-IUS | 15 (44%) | 15 (44%) | 4 (12%) | 34 | 0.999 |
| Non-Responder to LNG-IUS | 8 (47%) | 7 (41%) | 2 (12%) | 17 | |
| Time Post-LNG-IUS Insertion | Response to LNG-IUS | Reduced H Score | Same/Increased H Score | N | p-Value |
|---|---|---|---|---|---|
| 3 Months | Responder | 7 (30%) | 16 (70%) | 23 | 0.280 |
| Non-responder | 2 (15%) | 11 (85%) | 13 | ||
| 6 Months | Responder | 7 (33%) | 14 (67%) | 21 | 0.710 |
| Non-responder | 3 (25%) | 9 (75%) | 12 | ||
| 12 Months | Responder | 4 (20%) | 16 (80%) | 20 | 0.545 |
| Non-responder | 0 (0%) | 7 (100%) | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behrouzi, R.; Ryan, N.A.J.; Barr, C.E.; Derbyshire, A.E.; Wan, Y.L.; Maskell, Z.; Stocking, K.; Pemberton, P.W.; Bolton, J.; McVey, R.J.; et al. Baseline Serum HE4 But Not Tissue HE4 Expression Predicts Response to the Levonorgestrel-Releasing Intrauterine System in Atypical Hyperplasia and Early Stage Endometrial Cancer. Cancers 2020, 12, 276. https://doi.org/10.3390/cancers12020276
Behrouzi R, Ryan NAJ, Barr CE, Derbyshire AE, Wan YL, Maskell Z, Stocking K, Pemberton PW, Bolton J, McVey RJ, et al. Baseline Serum HE4 But Not Tissue HE4 Expression Predicts Response to the Levonorgestrel-Releasing Intrauterine System in Atypical Hyperplasia and Early Stage Endometrial Cancer. Cancers. 2020; 12(2):276. https://doi.org/10.3390/cancers12020276
Chicago/Turabian StyleBehrouzi, Roya, Neil A. J. Ryan, Chloe E. Barr, Abigail E. Derbyshire, Y. Louise Wan, Zoe Maskell, Katie Stocking, Philip W. Pemberton, James Bolton, Rhona J. McVey, and et al. 2020. "Baseline Serum HE4 But Not Tissue HE4 Expression Predicts Response to the Levonorgestrel-Releasing Intrauterine System in Atypical Hyperplasia and Early Stage Endometrial Cancer" Cancers 12, no. 2: 276. https://doi.org/10.3390/cancers12020276
APA StyleBehrouzi, R., Ryan, N. A. J., Barr, C. E., Derbyshire, A. E., Wan, Y. L., Maskell, Z., Stocking, K., Pemberton, P. W., Bolton, J., McVey, R. J., & Crosbie, E. J. (2020). Baseline Serum HE4 But Not Tissue HE4 Expression Predicts Response to the Levonorgestrel-Releasing Intrauterine System in Atypical Hyperplasia and Early Stage Endometrial Cancer. Cancers, 12(2), 276. https://doi.org/10.3390/cancers12020276





