Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development
Abstract
Simple Summary
Abstract
1. Introduction
2. Lactoferrin in Natural and Supplemented Foods
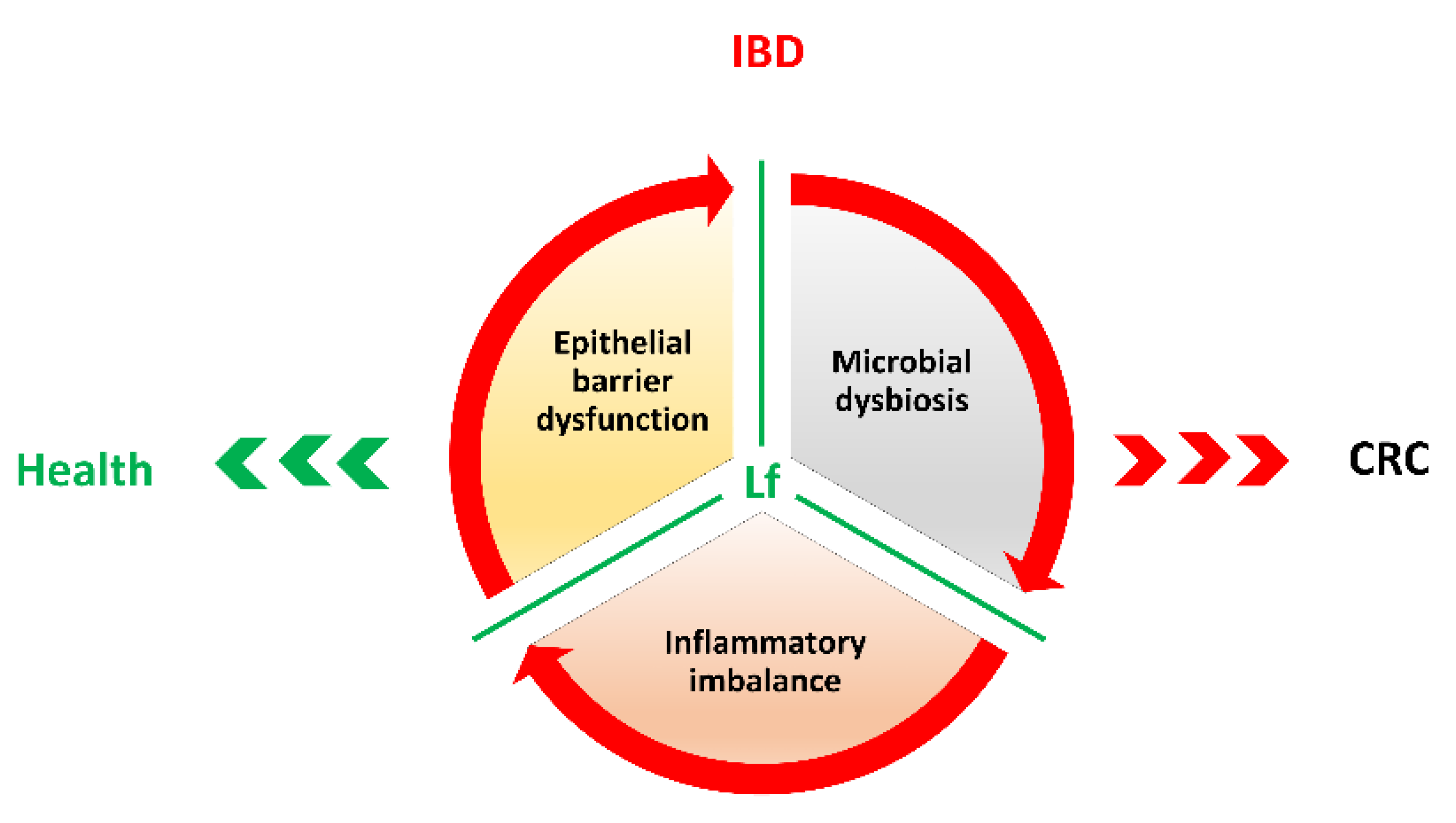
3. Inflammatory Bowel Diseases (IBDs) and Colorectal Cancer Development
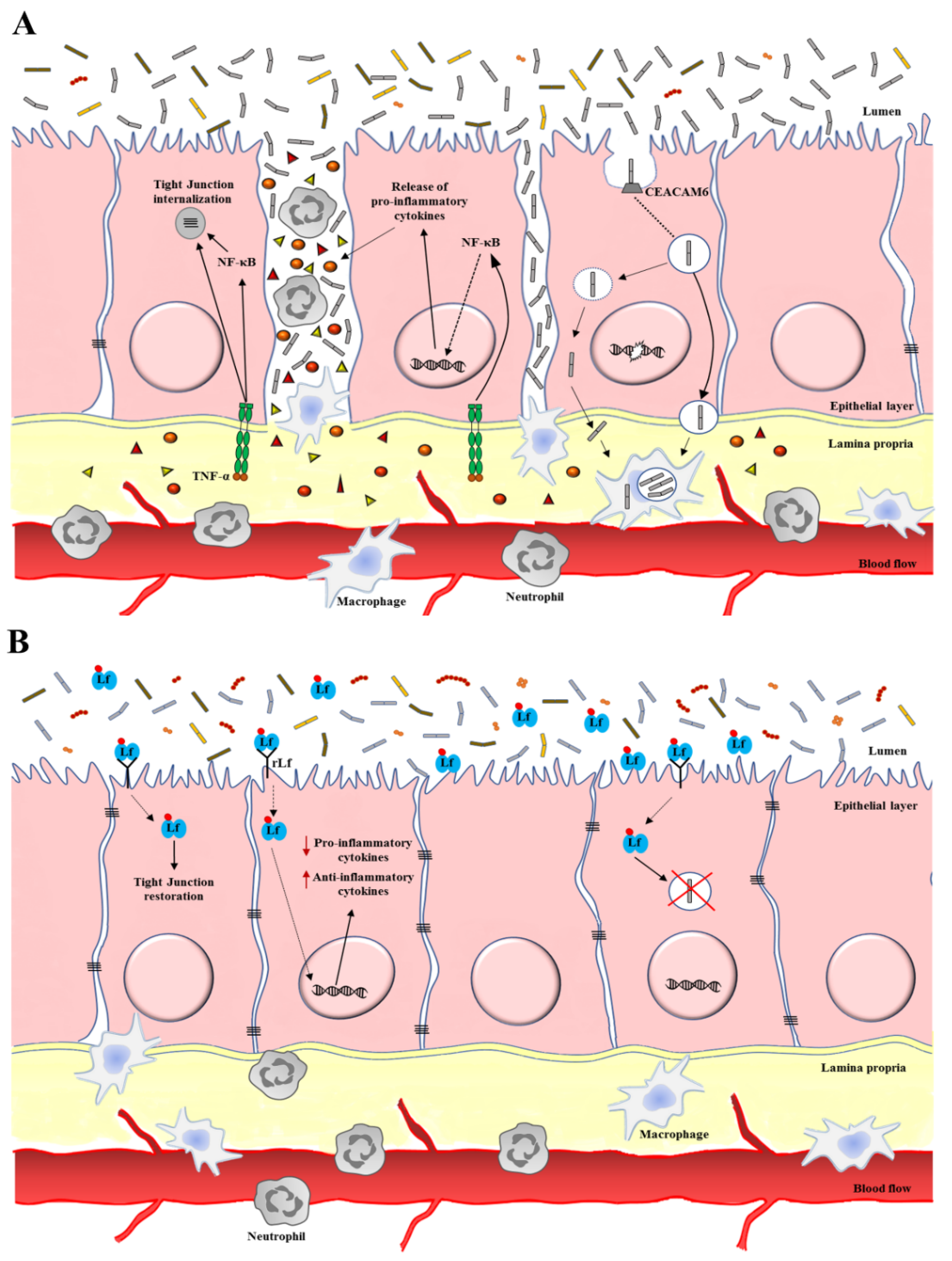
3.1. Lactoferrin Anti-Microbial Activity against IBDs
3.2. Lactoferrin Anti-Inflammatory Activity against IBDs
4. Lactoferrin in Colorectal Cancer Prevention
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sorensen, M.; Sorensen, S. The Proteins in Whey. Compte Rendu Trav. Lab. Carlsberg 1939, 23, 55–99. [Google Scholar]
- Groves, M.L. The Isolation of a Red Protein from Milk. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Johanson, B. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Orczyk-Pawiłowicz, M.; Broers, B.; Królak-Olejnik, B. Lactoferrin in Human Milk of Prolonged Lactation. Nutrients 2019, 11, 2350. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An alternative view of its role in human biological fluids. Biochem. Cell Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.L.; Heremans, J.F.; Schonne, E. Lactoferrin, an iron-binbing protein in neutrophilic leukocytes. J. Exp. Med. 1969, 130, 643–658. [Google Scholar] [CrossRef]
- Berliner, N.; Hsing, A.; Graubert, T.; Sigurdsson, F.; Zain, M.; Bruno, H.R. Granulocyte colony-stimulating factor induction of normal human bone marrow progenitors results in neutrophil-specific gene expression. Blood 1995, 85, 799–803. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef]
- Mancinelli, R.; Rosa, L.; Cutone, A.; Lepanto, M.S.; Franchitto, A.; Onori, P.; Gaudio, E.; Valenti, P. Viral Hepatitis and Iron Dysregulation: Molecular Pathways and the Role of Lactoferrin. Molecules 2020, 25, 1997. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; di Patti, M.C.B.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin Efficiently Counteracts the Inflammation-Induced Changes of the Iron Homeostasis System in Macrophages. Front. Immunol. 2017, 8, 705. [Google Scholar] [CrossRef]
- Di Patti, M.C.B.; Cutone, A.; Polticelli, F.; Rosa, L.; Lepanto, M.S.; Valenti, P.; Musci, G. The ferroportin-ceruloplasmin system and the mammalian iron homeostasis machine: Regulatory pathways and the role of lactoferrin. Biometals 2018, 31, 399–414. [Google Scholar] [CrossRef]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-Dimensional Structure of Diferric Bovine Lactoferrin at 2.8 Å Resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. A structural perspective on lactoferrin function. Biochem. Cell Biol. 2012, 90, 320–328. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Scotti, M.J.; Conte, M.P.; Paesano, R.; Valenti, P. Physico-Chemical Properties Influence the Functions and Efficacy of Commercial Bovine Lactoferrins. Biometals 2018, 31, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Apo- and Holo-Lactoferrin Stimulate Proliferation of Mouse Crypt Cells but through Different Cellular Signaling Pathways. Int. J. Biochem. Cell Biol. 2012, 44, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Colella, B.; Pagliaro, A.; Rosa, L.; Lepanto, M.S.; di Patti, M.C.B.; Valenti, P.; Di Bartolomeo, S.; Musci, G. Native and Iron-Saturated Bovine Lactoferrin Differently Hinder Migration in a Model of Human Glioblastoma by Reverting Epithelial-to-Mesenchymal Transition-like Process and Inhibiting Interleukin-6/STAT3 Axis. Cell. Signal. 2020, 65, 109461. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial Spectrum of Lactoferricin B, a Potent Bactericidal Peptide Derived from the N -Terminal Region of Bovine Lactoferrin. J. Appl. Bacteriol. 1992, 73, 472–479. [Google Scholar] [CrossRef]
- Van der Kraan, M.I.A.; Groenink, J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Nieuw Amerongen, A.V. Lactoferrampin: A Novel Antimicrobial Peptide in the N1-Domain of Bovine Lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef]
- Arias, M.; Hilchie, A.L.; Haney, E.F.; Bolscher, J.G.M.; Hyndman, M.E.; Hancock, R.E.W.; Vogel, H.J. Anticancer Activities of Bovine and Human Lactoferricin-Derived Peptides. Biochem. Cell Biol. 2017, 95, 91–98. [Google Scholar] [CrossRef]
- Epand, R.M.; Vogel, H.J. Diversity of Antimicrobial Peptides and Their Mechanisms of Action. Biochim. Biophys. Acta (BBA) Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Chen, P.W.; Liu, Z.S.; Kuo, T.C.; Hsieh, M.C.; Li, Z.W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. Biometals 2017, 30, 237–248. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Abe, F. Lactoferrin and bifidobacteria. Biometals 2014, 27, 915–922. [Google Scholar] [CrossRef]
- Haridas, M.; Anderson, B.F.; Baker, E.N. Structure of Human Diferric Lactoferrin Refined at 2.2 Å Resolution. Acta Crystallogr. D Biol. Crystallogr. 1995, 51, 629–646. [Google Scholar] [CrossRef]
- Thomassen, E.A.J.; van Veen, H.A.; van Berkel, P.H.C.; Nuijens, J.H.; Abrahams, J.P. The Protein Structure of Recombinant Human Lactoferrin Produced in the Milk of Transgenic Cows Closely Matches the Structure of Human Milk-Derived Lactoferrin. Transgenic Res. 2005, 14, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.N. A Structural Framework for Understanding the Multifunctional Character of Lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et al. Lactobacilli–Lactoferrin Interplay in Chlamydia Trachomatis Infection. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Scotti, M.J.; Conte, A.L.; Marazzato, M.; Zagaglia, C.; Longhi, C.; Berlutti, F.; Musci, G.; et al. Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes. Int. J. Mol. Sci. 2019, 20, 5666. [Google Scholar] [CrossRef]
- Valenti, P.; Frioni, A.; Rossi, A.; Ranucci, S.; De Fino, I.; Cutone, A.; Rosa, L.; Bragonzi, A.; Berlutti, F. Aerosolized Bovine Lactoferrin Reduces Neutrophils and Pro-Inflammatory Cytokines in Mouse Models of Pseudomonas Aeruginosa Lung Infections. Biochem. Cell. Biol. 2017, 95, 41–47. [Google Scholar] [CrossRef]
- Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.J.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas Aeruginosa Chronic Lung Infection. Int. J. Mol. Sci. 2019, 20, 2128. [Google Scholar] [CrossRef]
- Calvani, F.; Cutone, A.; Lepanto, M.S.; Rosa, L.; Valentini, V.; Valenti, P. Efficacy of Bovine Lactoferrin in the Post-Surgical Treatment of Patients Suffering from Bisphosphonate-Related Osteonecrosis of the Jaws: An Open-Label Study. Biometals 2018, 31, 445–455. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-Pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Derisbourg, P.; Wieruszeski, J.M.; Montreuil, J.; Spik, G. Primary Structure of Glycans Isolated from Human Leucocyte Lactotransferrin. Absence of Fucose Residues Questions the Proposed Mechanism of Hyposideraemia. Biochem. J. 1990, 269, 821–825. [Google Scholar] [CrossRef]
- Teng, C.T. Lactoferrin Gene Expression and Regulation: An Overview. Biochem. Cell Biol. 2002, 80, 7–16. [Google Scholar] [CrossRef]
- Wu, H.F.; Monroe, D.M.; Church, F.C. Characterization of the Glycosaminoglycan-Binding Region of Lactoferrin. Arch. Biochem. Biophys. 1995, 317, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E.; Bicknell, D.C.; Gillis, S.; Harris, E.L.; Pelus, L.M.; Sledge, G.W., Jr. Lactoferrin: Affinity Purification from Human Milk and Polymorphonuclear Neutrophils Using Monoclonal Antibody (II 2C) to Human Lactoferrin, Development of an Immunoradiometric Assay Using II 2C, and Myelopoietic Regulation and Receptor-Binding Characteristics. Blood Cells 1986, 11, 429–446. [Google Scholar] [PubMed]
- Baintner, K. Intestinal Absorption of Macromolecules and Immune Transmission from Mother to Young, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; p. 256. ISBN 9780429275531. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Lactoferrin: Mammalian Lactoferrin Receptors: Structure and Function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, U.M.; Siimes, M.A.; Dallman, P.R. Iron Absorption in Infants: High Bioavailability of breast Milk Iron as Indicated by the Extrinsic Tag Method of Iron Absorption and by the Concentration of Serum Ferritin. J. Pediatr. 1977, 91, 36–39. [Google Scholar] [CrossRef]
- Troost, F.J.; Saris, W.H.M.; Brummer, R.-J.M. Orally Ingested Human Lactoferrin Is Digested and Secreted in the Upper Gastrointestinal Tract In Vivo in Women with Ileostomies. J. Nutr. 2002, 132, 2597–2600. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Steijns, J.; Saris, W.H.M.; Brummer, R.-J.M. Gastric Digestion of Bovine Lactoferrin In Vivo in Adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Siciliano, R.A.; Paesano, R.; Costi, R.; Musci, G.; Valenti, P. Influence of Oral Administration Mode on the Efficacy of Commercial Bovine Lactoferrin against Iron and Inflammatory Homeostasis Disorders. Biometals 2020, 33, 159–168. [Google Scholar] [CrossRef]
- Fischer, R.; Debbabi, H.; Blais, A.; Dubarry, M.; Rautureau, M.; Boyaka, P.N.; Tome, D. Uptake of Ingested Bovine Lactoferrin and Its Accumulation in Adult Mouse Tissues. Int. Immunopharmacol. 2007, 7, 1387–1393. [Google Scholar] [CrossRef]
- Peen, E.; Johansson, A.; Engquist, M.; Skogh, T. Hepatic and extrahepatic clearance of circulating human lactoferrin: An experimental study in rat. Eur. J. Haematol. 1998, 61, 151–159. [Google Scholar] [CrossRef]
- Kawakami, H.; Lonnerdal, B. Isolation and Function of a Receptor for Human Lactoferrin in Human Fetal Intestinal Brush-Border Membranes. Am. J. Physiol. Gastrointest. Liver Physiol. 1991, 261, G841–G846. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Shin, K.; Lönnerdal, B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 2001, 40, 15771–15779. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.-A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Iigo, M.; Itoh, T.; Ushida, Y.; Sekine, K.; Terada, N.; Okamura, H.; Tsuda, H. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr. Cancer 2000, 38, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Olivero, F.; Carpino, G.; Overi, D.; Rosa, L.; Lepanto, M.S.; Cutone, A.; Franchitto, A.; Alpini, G.; Onori, P.; et al. Role of Lactoferrin and Its Receptors on Biliary Epithelium. Biometals 2018, 31, 369–379. [Google Scholar] [CrossRef]
- Meilinger, M.; Haumer, M.; Szakmary, K.A.; Steinböck, F.; Scheiber, B.; Goldenberg, H.; Huettinger, M. Removal of Lactoferrin from Plasma Is Mediated by Binding to Low Density Lipoprotein Receptor-Related Protein/ α 2 -Macroglobulin Receptor and Transport to Endosomes. FEBS Lett. 1995, 360, 70–74. [Google Scholar] [CrossRef]
- Fillebeen, C.; Descamps, L.; Dehouck, M.-P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-Mediated Transcytosis of Lactoferrin through the Blood-Brain Barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef]
- Legrand, D.; Vigie, K.; Said, E.A.; Elass, E.; Masson, M.; Slomianny, M.-C.; Carpentier, M.; Briand, J.-P.; Mazurier, J.; Hovanessian, A.G. Surface Nucleolin Participates in Both the Binding and Endocytosis of Lactoferrin in Target Cells. Eur. J. Biochem. 2004, 271, 303–317. [Google Scholar] [CrossRef]
- Jiang, R.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells. J. Cell. Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef]
- Ashida, K.; Sasaki, H.; Suzuki, Y.A.; Lönnerdal, B. Cellular Internalization of Lactoferrin in Intestinal Epithelial Cells. Biometals 2004, 17, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Wong, H.; Ashida, K.; Schryvers, A.B.; Lönnerdal, B. The N1 Domain of Human Lactoferrin Is Required for Internalization by Caco-2 Cells and Targeting to the Nucleus. Biochemistry 2008, 47, 10915–10920. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Recombinant Human Intelectin Binds Bovine Lactoferrin and Its Peptides. Biol. Pharm. Bull. 2008, 31, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Pietropaoli, M.; Berlutti, F.; Valenti, P. Bovine lactoferrin in preventing preterm delivery associated with sterile inflammation. Biochem. Cell Biol. 2012, 90, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Hennet, T.; Weiss, A.; Borsig, L. Decoding Breast Milk Oligosaccharides. Swiss Med. Wkly. 2014, 144, w13927. [Google Scholar] [CrossRef] [PubMed]
- Do Rangel, A.H.N.; Sales, D.C.; Urbano, S.A.; Galvão Júnior, J.G.B.; de Andrade Neto, J.C.; de Macêdo, C.S. Lactose Intolerance and Cow’s Milk Protein Allergy. Food Sci. Technol. 2016, 36, 179–187. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Márquez-Hernández, R.I.; Hernández-Castellano, L.E. Bioactive Peptides from Milk: Animal Determinants and Their Implications in Human Health. J. Dairy Res. 2019, 86, 136–144. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Jones, O.G. Assembled Protein Nanoparticles in Food or Nutrition Applications. Adv. Food Nutr. Res. 2019, 88, 47–84. [Google Scholar] [CrossRef]
- Chatterton, D.E.W.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-Inflammatory Mechanisms of Bioactive Milk Proteins in the Intestine of Newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef]
- Brew, K.; Vanaman, T.C.; Hill, R.L. The Role of Alpha-Lactalbumin and the A Protein in Lactose Synthetase: A Unique Mechanism for the Control of a Biological Reaction. Proc. Natl. Acad. Sci. USA 1968, 59, 491–497. [Google Scholar] [CrossRef]
- Düringer, C.; Hamiche, A.; Gustafsson, L.; Kimura, H.; Svanborg, C. HAMLET Interacts with Histones and Chromatin in Tumor Cell Nuclei. J. Biol. Chem. 2003, 278, 42131–42135. [Google Scholar] [CrossRef] [PubMed]
- Brück, W.M.; Kelleher, S.L.; Gibson, G.R.; Nielsen, K.E.; Chatterton, D.E.W.; Lönnerdal, B. rRNA Probes Used to Quantify the Effects of Glycomacropeptide and α-Lactalbumin Supplementation on the Predominant Groups of Intestinal Bacteria of Infant Rhesus Monkeys Challenged with Enteropathogenic Escherichia Coli. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.E.W.; Rasmussen, J.T.; Heegaard, C.W.; Sørensen, E.S.; Petersen, T.E. In Vitro Digestion of Novel Milk Protein Ingredients for Use in Infant Formulas: Research on Biological Functions. Trends Food Sci. Technol. 2004, 15, 373–383. [Google Scholar] [CrossRef]
- Dewey, K.G.; Heinig, M.J.; Nommsen-Rivers, L.A. Differences in Morbidity between Breast-Fed and Formula-Fed Infants. J. Pediatr. 1995, 126, 696–702. [Google Scholar] [CrossRef]
- Rai, D.; Adelman, A.S.; Zhuang, W.; Rai, G.P.; Boettcher, J.; Lönnerdal, B. Longitudinal changes in lactoferrin concentrations in human milk: A global systematic review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1539–1547. [Google Scholar] [CrossRef]
- Kitagawa, H.; Yoshizawa, Y.; Yokoyama, T.; Takeuchi, T.; Talukder, M.J.; Shimizu, H.; Ando, K.; Harada, E. Persorption of bovine lactoferrin from the intestinal lumen into the systemic circulation via the portal vein and the mesenteric lymphatics in growing pigs. J. Vet. Med. Sci. 2003, 65, 567–572. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitagawa, H.; Harada, E. Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats. Exp. Physiol. 2004, 89, 263–270. [Google Scholar] [CrossRef]
- Gateway to Dairy Production and Products. Available online: http://www.fao.org/dairy-production-products/production/dairy-animals/en/ (accessed on 7 September 2020).
- Cheng, J.B.; Wang, J.Q.; Bu, D.P.; Liu, G.L.; Zhang, C.G.; Wei, H.Y.; Zhou, L.Y.; Wang, J.Z. Factors Affecting the Lactoferrin Concentration in Bovine Milk. J. Dairy Sci. 2008, 91, 970–976. [Google Scholar] [CrossRef]
- Franco, I.; Pérez, M.D.; Conesa, C.; Calvo, M.; Sánchez, L. Effect of Technological Treatments on Bovine Lactoferrin: An Overview. Food Res. Int. 2018, 106, 173–182. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F. Lactoferrin in Milk from Different Species. Comp. Biochem. Physiol. B 1971, 39, 119–129. [Google Scholar] [CrossRef]
- Dupont, D.; Arnould, C.; Rolet-Repecaud, O.; Duboz, G.; Faurie, F.; Martin, B.; Beuvier, E. Determination of Bovine Lactoferrin Concentrations in Cheese with Specific Monoclonal Antibodies. Int. Dairy J. 2006, 16, 1081–1087. [Google Scholar] [CrossRef]
- Campanella, L.; Martini, E.; Pintore, M.; Tomassetti, M. Determination of Lactoferrin and Immunoglobulin G in Animal Milks by New Immunosensors. Sensors 2009, 9, 2202–2221. [Google Scholar] [CrossRef] [PubMed]
- Tsakali, E.; Petrotos, K.; Chatzilazarou, A.; Stamatopoulos, K.; D’Alessandro, A.G.; Goulas, P.; Massouras, T.; Van Impe, J.F.M. Short Communication: Determination of Lactoferrin in Feta Cheese Whey with Reversed-Phase High-Performance Liquid Chromatography. J. Dairy Sci. 2014, 97, 4832–4837. [Google Scholar] [CrossRef] [PubMed]
- Dračková, M.; Borkovcová, I.; Janštová, B.; Naiserová, M.; Přidalová, H.; Navrátilová, P.; Vorlová, L. Determination of Lactoferrin in Goat Milk by HPLC Method. Czech. J. Food Sci. 2009, 27, S102–S104. [Google Scholar] [CrossRef]
- Wang, J.; Shi, H.; Zhang, T.; Huang, J.; Li, Z.; Ma, G.; Zhang, X. The Lactoferrin Content Variation and Its Related Factors in Milk of Xinong Saanen Goats. J. Appl. Anim. Res. 2018, 46, 1032–1035. [Google Scholar] [CrossRef]
- Navarro, F.; Galan-Malo, P.; Pérez, M.D.; Abecia, J.-A.; Mata, L.; Calvo, M.; Sánchez, L. Lactoferrin and IgG Levels in Ovine Milk throughout Lactation: Correlation with Milk Quality Parameters. Small Rumin. Res. 2018, 168, 12–18. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Loiseau, G.; Levieux, D. Lactoferrin and Immunoglobulin Contents in Camel’s Milk (Camelus Bactrianus, Camelus Dromedarius, and Hybrids) from Kazakhstan. J. Dairy Sci. 2007, 90, 38–46. [Google Scholar] [CrossRef]
- Conesa, C.; Pocovi, C.; Perez, M.-D.; Calvo, M.; Sanchez, L. Transport of iron bound to recombinant human lactoferrin from rice and iron citrate across Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2009, 73, 2515–2620. [Google Scholar] [CrossRef]
- Chen, H.L.; Lai, Y.W.; Yen, C.C.; Lin, Y.Y.; Lu, C.Y.; Yang, S.H.; Tsai, T.C.; Lin, Y.J.; Lin, C.W.; Chen, C.M. Production of recombinant porcine lactoferrin exhibiting antibacterial activity in methylotrophic yeast, Pichia pastoris. J. Mol. Microbiol. Biotechnol. 2004, 8, 141–149. [Google Scholar] [CrossRef]
- Sun, X.-L.; Baker, H.M.; Shewry, S.C.; Jameson, G.B.; Baker, E.N. Structure of Recombinant Human Lactoferrin Expressed in Aspergillus Awamori. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, P.H.C.; Welling, M.M.; Geerts, M.; van Veen, H.A.; Ravensbergen, B.; Salaheddine, M.; Pauwels, E.K.J.; Pieper, F.; Nuijens, J.H.; Nibbering, P.H. Large Scale Production of Recombinant Human Lactoferrin in the Milk of Transgenic Cows. Nat. Biotechnol. 2002, 20, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Lactoferrin Market Size, Share & Trends Analysis Report by Function (Iron Absorption, Anti-inflammatory, Intestinal Flora Protection, Antibacterial), by Application, by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/lactoferrin-market (accessed on 7 September 2020).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on bovine lactoferrin. EFSA J. 2012, 10, 2701–2727. [Google Scholar] [CrossRef]
- U.S. FDA. GRN 000465 [Cow’s Milk-Derived Lactoferrin, Tokyo, Japan: Morinaga Milk Industry Co., Ltd.]. Silver Spring (MD): U.S. Food and Drug Administration (U.S. FDA), Center for Food Safety & Applied Nutrition (CFSAN), Office of Food Additive Safety. 2014. Available online: http://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=465 (accessed on 7 September 2020).
- Kawaguchi, S.; Hayashi, T.; Masano, J.; Okuyama, K.; Suzuki, T.; Kawase, K. A study concerning the effect of lactoferrin-enriched infant formula on low birth weight infants. Perinat. Med. 1989, 19, 557–562. (In Japanese) [Google Scholar]
- Roberts, A.; Chierici, R.; Sawatzki, G.; Hill, M.; Volpato, S.; Vigi, V. Supplementation of an Adapted Formula with Bovine Lactoferrin: 1. Effect on the Infant Faecal Flora. Acta Paediatr. 1992, 81, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chierici, R.; Sawatzki, G.; Tamisari, L.; Volpato, S.; Vigi, V. Supplementation of an Adapted Formula with Bovine Lactoferrin. 2. Effects on Serum Iron, Ferritin and Zinc Levels. Acta Paediatr. 1992, 81, 475–479. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; de Waard, R. A Double-Blind, Placebo-Controlled, Pilot Study of Bovine Lactoferrin Supplementation in Bottle-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Egashira, M.; Takayanagi, T.; Moriuchi, M.; Moriuchi, H. Does Daily Intake of Bovine Lactoferrin-Containing Products Ameliorate Rotaviral Gastroenteritis? Acta Paediatr. 2007, 96, 1242–1244. [Google Scholar] [CrossRef]
- Ishii, K. Long-Term Follow-up of Chronic Hepatitis C Patients Treated with Oral Lactoferrin for 12 Months. Hepatol. Res. 2003, 25, 226–233. [Google Scholar] [CrossRef]
- Okuda, M.; Miyashiro, E.; Nakazawa, T.; Yamauchi, K.; Koizumi, R.; Teraguchi, S.; Tamura, Y.; Booka, M.; Yoshikawa, N.; Adachi, Y.; et al. Bovine Lactoferrin Is Effective to Suppress Helicobacter Pylori Colonization in the Human Stomach: A Randomized, Double-Blind, Placebo-Controlled Study. J. Infect. Chemother. 2005, 11, 265–269. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef] [PubMed]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and Peptide-Derivatives: Antimicrobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr. Pharm. Des. 2018, 24, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Pleiotropic Effect of Lactoferrin in the Prevention and Treatment of COVID-19 Infection: Randomized Clinical Trial, in Vitro and in Silico Preliminary Evidences. BioRxiv 2020. [Google Scholar] [CrossRef]
- Tsuda, H.; Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Saito, D.; Akasu, T.; Alexander, D.B.; Futakuchi, M.; Fukamachi, K.; et al. Cancer prevention by bovine lactoferrin: From animal studies to human trial. Biometals 2010, 23, 399–409. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Hanauer, S.B. Inflammatory Bowel Disease: Epidemiology, Pathogenesis, and Therapeutic Opportunities. Inflamm. Bowel Dis. 2006, 12, S3–S9. [Google Scholar] [CrossRef]
- Hruz, P.; Juillerat, P.; Kullak-Ublick, G.-A.; Schoepfer, A.M.; Mantzaris, G.J.; Rogler, G. Management of the Elderly Inflammatory Bowel Disease Patient. Digestion 2020, 1–15. [Google Scholar] [CrossRef]
- Yue, B.; Luo, X.; Yu, Z.; Mani, S.; Wang, Z.; Dou, W. Inflammatory Bowel Disease: A Potential Result from the Collusion between Gut Microbiota and Mucosal Immune System. Microorganisms 2019, 7, 440. [Google Scholar] [CrossRef]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef]
- Arnott, I.D.R.; Kingstone, K.; Ghosh, S. Abnormal Intestinal Permeability Predicts Relapse in Inactive Crohn Disease. Scand. J. Gastroenterol. 2000, 35, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.G.; Huang, Q. Recent Progress on the Role of Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. J. Dig. Dis. 2013, 14, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.F. Adherent-invasive Escherichia Coli in Inflammatory Bowel Disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in gut microbiota in patients with vs without Inflammatory Bowel Diseases: A systematic review. Gastroenterology 2020, 158, 930–946. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium Prausnitzii is an anti-Inflammatory commensal bacterium Identified by gut microbiota analysis of Crohn Disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Wagner, J.; Short, K.; Catto-Smith, A.G.; Cameron, D.J.S.; Bishop, R.F.; Kirkwood, C.D. Identification and characterization of Pseudomonas 16S ribosomal DNA from ileal biopsies of children with Crohn’s Disease. PLoS ONE 2008, 3, e3578. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339. [Google Scholar] [CrossRef]
- Sam, Q.H.; Chang, M.W.; Chai, L.Y.A. The Fungal Mycobiome and Its Interaction with Gut Bacteria in the Host. Int. J. Mol. Sci. 2017, 18, 330. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.T.; Shao, T.-Y.; Ang, W.G.; Kinder, J.M.; Turner, L.H.; Pham, G.; Whitt, J.; Alenghat, T.; Way, S.S. Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell Host Microbe 2017, 22, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, F.; Yang, X.; Wu, N.; Jiang, W.; Li, X.; Li, X.; Liu, Y. Changes in the composition of intestinal fungi and their role in mice with dextran sulfate sodium-induced colitis. Sci. Rep. 2015, 5, 10416. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.L.; Erb Downward, J.R.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2017, 18, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Li, N.; Li, J. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in crohn’s disease. J. Clin. Gastroenterol. 2014, 48, 513–523. [Google Scholar] [CrossRef]
- Minot, S.; Bryson, A.; Chehoud, C.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Rapid evolution of the human gut virome. Proc. Natl. Acad. Sci. USA 2013, 110, 12450–12455. [Google Scholar] [CrossRef]
- Perez-Brocal, V.; Garcia-Lopez, R.; Nos, P.; Beltran, B.; Moret, I.; Moya, A. Metagenomic Analysis of Crohn’s Disease Patients Identifies Changes in the Virome and Microbiome Related to Disease Status and Therapy, and Detects Potential Interactions and Biomarkers. Inflamm. Bowel Dis. 2015, 21, 2515–2532. [Google Scholar] [CrossRef]
- Zuo, T.; Lu, X.-J.; Zhang, Y.; Cheung, C.P.; Lam, S.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Zhao, R.; Chan, P.K.S.; et al. Gut mucosal virome alterations in ulcerative colitis. Gut 2019, 68, 1169–1179. [Google Scholar] [CrossRef]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Garrett Brown, D.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 2019, 25, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Galtier, M.; De Sordi, L.; Sivignon, A.; de Vallee, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages Targeting Adherent Invasive Escherichia coli Strains as a Promising New Treatment for Crohn’s Disease. J. Crohns Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef]
- Lim, E.S.; Zhou, Y.; Zhao, G.; Bauer, I.K.; Droit, L.; Ndao, I.M.; Warner, B.B.; Tarr, P.I.; Wang, D.; Holtz, L.R. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 2015, 21, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jovel, J.; Halloran, B.; Wine, E.; Patterson, J.; Ford, G.; O’Keefe, S.; Meng, B.; Song, D.; Zhang, Y.; et al. Metagenomic Analysis of Microbiome in Colon Tissue from Subjects with Inflammatory Bowel Diseases Reveals Interplay of Viruses and Bacteria. Inflamm. Bowel Dis. 2015, 21, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Human virome and disease: High-throughput sequencing for virus discovery, identification of phage-bacteria dysbiosis and development of therapeutic approaches with emphasis on the human gut. Viruses 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K.; Patel, K.K.; Maloney, N.S.; Liu, T.C.; Ng, A.C.; Storer, C.E.; Head, R.D.; Xavier, R.; Stappenbeck, T.S.; Virgin, H.W. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 2010, 141, 1135–1145. [Google Scholar] [CrossRef]
- Basic, M.; Keubler, L.M.; Buettner, M.; Achard, M.; Breves, G.; Schroder, B.; Smoczek, A.; Jorns, A.; Wedekind, D.; Zschemisch, N.H.; et al. Norovirus Triggered Microbiota-driven Mucosal Inflammation in Interleukin 10-deficient Mice. Inflamm. Bowel Dis. 2014, 20, 431–443. [Google Scholar] [CrossRef]
- Audebert, C.; Even, G.; Cian, A.; Blastocystis Investigation Group; Loywick, A.; Merlin, S.; Viscogliosi, E.; Chabe, M. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci. Rep. 2016, 6, 25255. [Google Scholar] [CrossRef]
- Kok, M.; Cekin, Y.; Cekin, A.H.; Uyar, S.; Harmandar, F.; Sahinturk, Y. The role of Blastocystis hominis in the activation of ulcerative colitis. Turk. J. Gastroenterol. 2019, 30, 40–46. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K.; Torijano-Carrera, E. Intestinal protozoa infections among patients with ulcerative colitis: Prevalence and impact on clinical disease course. Digestion 2010, 82, 18–23. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Targan, S.R. Role of cytokines in the pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Med. 2000, 51, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Goethel, A.; Croitoru, K.; Philpott, D.J. The Interplay between Microbes and the Immune Response in Inflammatory Bowel Disease: Interplay between NOD2, Microbiota and Immune Response in IBD. J. Physiol. 2018, 596, 3869–3882. [Google Scholar] [CrossRef] [PubMed]
- Mitselou, A.; Grammeniatis, V.; Varouktsi, A.; Papadatos, S.S.; Katsanos, K.; Galani, V. Proinflammatory Cytokines in Irritable Bowel Syndrome: A Comparison with Inflammatory Bowel Disease. Intest. Res. 2020, 18, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Muñoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in Inflammatory Bowel Disease. World J. Gastroenterol. 2008, 14, 4280. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Wang, L.; Walia, B.; Evans, J.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. IL-6 induces NF-ΚB activation in the intestinal epithelia. J. Immunol. 2003, 171, 3194–3201. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and Colitis-Associated Cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef]
- Rose-John, S.; Scheller, J.; Elson, G.; Jones, S.A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: Role in inflammation and cancer. J. Leukoc. Biol. 2006, 80, 227–236. [Google Scholar] [CrossRef]
- Brown, S.J.; Mayer, L. The immune response in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2007, 102, 2058–2069. [Google Scholar] [CrossRef]
- Yongvanit, P.; Pinlaor, S.; Bartsch, H. Oxidative and Nitrative DNA Damage: Key Events in Opisthorchiasis-Induced Carcinogenesis. Parasitol. Int. 2012, 61, 130–135. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Baklien, K.; Fausa, O.; Hoel, P.S. Immunohistochemical characterization of local immunoglobulin formation in ulcerative colitis. Gastroenterology 1974, 66, 1123–1136. [Google Scholar] [CrossRef]
- Baklien, K.; Brandtzaeg, P. Comparative mapping of the local distribution of immunoglobulin-containing cells in ulcerative colitis and Crohn’s disease of the colon. Clin. Exp. Immunol. 1975, 22, 197–209. [Google Scholar] [PubMed]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, H.J.M.; Pouwels, S.D.; Funke, A.; Bos, N.A.; Dijkstra, G. Crohn’s disease patients have more IgG-binding fecal bacteria than controls. Clin. Vaccine Immunol. 2012, 19, 515–521. [Google Scholar] [CrossRef]
- Lin, R.; Chen, H.; Shu, W.; Sun, M.; Fang, L.; Shi, Y.; Pang, Z.; Wu, W.; Liu, Z. Clinical significance of soluble immunoglobulins A and G and their coated bacteria in feces of patients with inflammatory bowel disease. J. Transl. Med. 2018, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Castro-Dopico, T.; Dennison, T.W.; Ferdinand, J.R.; Mathews, R.J.; Fleming, A.; Clift, D.; Stewart, B.J.; Jing, C.; Strongili, K.; Labzin, L.I.; et al. Anti-commensal IgG drives intestinal inflammation and type 17 immunity in ulcerative colitis. Immunity 2019, 50, 1099–1114. [Google Scholar] [CrossRef] [PubMed]
- Mattar, M.C.; Lough, D.; Pishvaian, M.J.; Charabaty, A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest. Cancer Res. 2011, 4, 53–61. [Google Scholar]
- Taylor, C.C.; Millien, V.O.; Hou, J.K.; Massarweh, N.N. Association Between Inflammatory Bowel Disease and Colorectal Cancer Stage of Disease and Survival. J. Surg. Res. 2020, 247, 77–85. [Google Scholar] [CrossRef]
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal Cancer in Inflammatory Bowel Disease: Review of the Evidence. Technol. Coloproctol. 2019, 23, 3–13. [Google Scholar] [CrossRef]
- Lakatos, L.; Mester, G.; Erdelyi, Z.; David, G.; Pandur, T.; Balogh, M.; Fischer, S.; Vargha, P.; Lakatos, P.L. Risk Factors for Ulcerative Colitis-Associated Colorectal Cancer in a Hungarian Cohort of Patients with Ulcerative Colitis: Results of a Population-Based Study. Inflamm. Bowel Dis. 2006, 12, 205–211. [Google Scholar] [CrossRef]
- Choi, P.M.; Zelig, M.P. Similarity of Colorectal Cancer in Crohn’s Disease and Ulcerative Colitis: Implications for Carcinogenesis and Prevention. Gut 1994, 35, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Gillen, C.D.; Walmsley, R.S.; Prior, P.; Andrews, H.A.; Allan, R.N. Ulcerative Colitis and Crohn’s Disease: A Comparison of the Colorectal Cancer Risk in Extensive Colitis. Gut 1994, 35, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.E.; Rickert, R.R.; Nance, F.C. Crohn’s Disease-Associated Carcinoma: A Poorly Recognized Complication of Inflammatory Bowel Disease. Ann. Surg. 1989, 209, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Itzkowitz, S.H. Cancer in Inflammatory Bowel Disease. World J. Gastroenterol. 2008, 14, 378. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P.; Loe, R.H. Sclerosing cholangitis; review of recent case reports and associated diseases and four new cases. Am. J. Surg. 1965, 110, 239–246. [Google Scholar] [CrossRef]
- Moss, A.C. The meaning of low-grade inflammation in clinically quiescent inflammatory bowel disease. Curr. Opin. Gastroenterol. 2014, 30, 365–369. [Google Scholar] [CrossRef]
- Herbeuval, J.-P.; Lelievre, E.; Lambert, C.; Dy, M.; Genin, C. Recruitment of STAT3 for Production of IL-10 by Colon Carcinoma Cells Induced by Macrophage-Derived IL-6. J. Immunol. 2004, 172, 4630–4636. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Brighenti, E.; Calabrese, C.; Liguori, G.; Giannone, F.A.; Trerè, D.; Montanaro, L.; Derenzini, M. Interleukin 6 Downregulates P53 Expression and Activity by Stimulating Ribosome Biogenesis: A New Pathway Connecting Inflammation to Cancer. Oncogene 2014, 33, 4396–4406. [Google Scholar] [CrossRef]
- Kawanishi, S.; Hiraku, Y.; Pinlaor, S.; Ma, N. Oxidative and Nitrative DNA Damage in Animals and Patients with Inflammatory Diseases in Relation to Inflammation-Related Carcinogenesis. Biol. Chem. 2006, 387, 365–372. [Google Scholar] [CrossRef]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal Inflammation and Cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef]
- Cooks, T.; Pateras, I.S.; Tarcic, O.; Solomon, H.; Schetter, A.J.; Wilder, S.; Lozano, G.; Pikarsky, E.; Forshew, T.; Rozenfeld, N.; et al. Mutant P53 Prolongs NF-ΚB Activation and Promotes Chronic Inflammation and Inflammation-Associated Colorectal Cancer. Cancer Cell 2013, 23, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Sandborn, W.J.; Gupta, S. Colorectal Cancer and Dysplasia in Inflammatory Bowel Disease: A Review of Disease Epidemiology, Pathophysiology, and Management. Cancer Prev. Res. 2016, 9, 887–894. [Google Scholar] [CrossRef]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal Cancer in Ulcerative Colitis: A Scandinavian Population-Based Cohort Study. Lancet 2020, 395, 123–131. [Google Scholar] [CrossRef]
- Hvas, C.L.; Dahl Jørgensen, S.M.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium Difficile Infection. Gastroenterology 2019, 156, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.B.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin Differently Modulates the Inflammatory Response in Epithelial Models Mimicking Human Inflammatory and Infectious Diseases. Biometals 2014, 27, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Rosa, L.; Cutone, A.; Frioni, A.; Berlutti, F.; Paesano, R.; Valenti, P. Effect of Bovine Lactoferrin on Chlamydia Trachomatis Infection and Inflammation. Biochem. Cell Biol. 2017, 95, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Enhanced Antifungal Activity of Bovine Lactoferrin-Producing Probiotic Lactobacillus Casei in the Murine Model of Vulvovaginal Candidiasis. BMC Microbiol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Kieckens, E.; Rybarczyk, J.; De Zutter, L.; Duchateau, L.; Vanrompay, D.; Cox, E. Clearance of Escherichia Coli O157:H7 Infection in Calves by Rectal Administration of Bovine Lactoferrin. Appl. Environ. Microbiol. 2015, 81, 1644–1651. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin: An Important Host Defence against Microbial and Viral Attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef]
- Appelmelk, B.J.; An, Y.Q.; Geerts, M.; Thijs, B.G.; de Boer, H.A.; MacLaren, D.M.; de Graaff, J.; Nuijens, J.H. Lactoferrin Is a Lipid A-Binding Protein. Infect. Immun. 1994, 62, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Jürgens, G.; Müller, M.; Fukuoka, S.; Koch, M.H.J. Biophysical Characterization of Lipopolysaccharide and Lipid a Inactivation by Lactoferrin. Biol. Chem. 2001, 382, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodríguez, R. Lactoferrin-Lipopolysaccharide (LPS) Binding as Key to Antibacterial and Antiendotoxic Effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Giansanti, F.; Boffi, A.; Ajello, M.; Valenti, P.; Chiancone, E.; Antonini, G. Ca 2+ Binding to Bovine Lactoferrin Enhances Protein Stability and Influences the Release of Bacterial Lipopolysaccharide. Biochem. Cell Biol. 2002, 80, 41–48. [Google Scholar] [CrossRef]
- Longhi, C.; Conte, M.; Seganti, L.; Polidoro, M.; Alfsen, A.; Valenti, P. Influence of Lactoferrin on the Entry Process of Escherichia Coli HB101(PRI203) in HeLa Cells. Med. Microbiol. Immunol. 1993, 182. [Google Scholar] [CrossRef]
- Alugupalli, K.R.; Kalfas, S. Characterization of the Lactoferrin-Dependent Inhibition of the Adhesion of Actinobacilllus Actinomycetemcomitans, Prevotella Intermedia and Prevotella Nigrescens to Fibroblasts and to a Reconstituted Basement Membrane. APMIS 1997, 105, 680–688. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Tazume, S.; Shimizu, K.; Matsuzawa, H.; Dosako, S.; Isoda, H.; Tsukiji, M.; Fujimura, R.; Muranaka, Y.; Isihida, H. Inhibitory Effects of Bovine Lactoferrin on the Adherence of Enterotoxigenic Escherichia Coli to Host Cells. Biosci. Biotech. Biochem. 2000, 64, 348–354. [Google Scholar] [CrossRef]
- Berlutti, F.; Morea, C.; Battistoni, A.; Sarli, S.; Cipriani, P.; Superti, F.; Ammendolia, M.G.; Valenti, P. Iron Availability Influences Aggregation, Biofilm, Adhesion and Invasion of Pseudomonas Aeruginosa and Burkholderia Cenocepacia. Int. J. Immunopathol. Pharmacol. 2005, 18, 661–670. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Brown, E.L.; Guion, C.E.; Chen, J.Z.; McMahon, R.J.; Cleary, T.G. Effect of lactoferrin on enteroaggregative E. coli (EAEC). Biochem. Cell Biol. 2006, 84, 369–376. [Google Scholar] [CrossRef]
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M.E. Correlation between Lactoferrin and Beneficial Microbiota in Breast Milk and Infant’s Feces. Biometals 2014, 27, 1077–1086. [Google Scholar] [CrossRef]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Finlay, B.B. The Role of the Intestinal Microbiota in Enteric Infection: Intestinal Microbiota and Enteric Infections. J. Physiol. 2009, 587, 4159–4167. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. The microbiome and its potential as a cancer preventive intervention. Semin Oncol. 2016, 43, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An Ecological and Evolutionary Perspective on Human–Microbe Mutualism and Disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Schippa, S.; Iebba, V.; Totino, V.; Santangelo, F.; Lepanto, M.; Alessandri, C.; Nuti, F.; Viola, F.; Di Nardo, G.; Cucchiara, S.; et al. A Potential Role of Escherichia Coli Pathobionts in the Pathogenesis of Pediatric Inflammatory Bowel Disease. Can. J. Microbiol. 2012, 58, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille-Michaud, A.; Neut, C.; Barnich, N.; Lederman, E.; Di Martino, P.; Desreumaux, P.; Gambiez, L.; Joly, B.; Cortot, A.; Colombel, J.-F. Presence of Adherent Escherichia Coli Strains in Ileal Mucosa of Patients with Crohn’s Disease. Gastroenterology 1998, 115, 1405–1413. [Google Scholar] [CrossRef]
- Nash, J.H.; Villegas, A.; Kropinski, A.M.; Aguilar-Valenzuela, R.; Konczy, P.; Mascarenhas, M.; Ziebell, K.; Torres, A.G.; Karmali, M.A.; Coombes, B.K. Genome Sequence of Adherent-Invasive Escherichia Coli and Comparative Genomic Analysis with Other E. Coli Pathotypes. BMC Genom. 2010, 11, 667. [Google Scholar] [CrossRef]
- Miquel, S.; Peyretaillade, E.; Claret, L.; de Vallée, A.; Dossat, C.; Vacherie, B.; Zineb, E.H.; Segurens, B.; Barbe, V.; Sauvanet, P.; et al. Complete Genome Sequence of Crohn’s Disease-Associated Adherent-Invasive E. Coli Strain LF82. PLoS ONE 2010, 5, e12714. [Google Scholar] [CrossRef]
- Conte, M.P.; Longhi, C.; Marazzato, M.; Conte, A.L.; Aleandri, M.; Lepanto, M.S.; Zagaglia, C.; Nicoletti, M.; Aloi, M.; Totino, V.; et al. Adherent-Invasive Escherichia Coli (AIEC) in Pediatric Crohn’s Disease Patients: Phenotypic and Genetic Pathogenic Features. BMC Res. Notes 2014, 7, 748. [Google Scholar] [CrossRef]
- Hornef, M. Pathogens, Commensal Symbionts, and Pathobionts: Discovery and Functional Effects on the Host. ILAR J. 2015, 56, 159–162. [Google Scholar] [CrossRef]
- Glasser, A.-L.; Boudeau, J.; Barnich, N.; Perruchot, M.-H.; Colombel, J.-F.; Darfeuille-Michaud, A. Adherent Invasive Escherichia Coli Strains from Patients with Crohn’s Disease Survive and Replicate within Macrophages without Inducing Host Cell Death. Infect. Immun. 2001, 69, 5529–5537. [Google Scholar] [CrossRef] [PubMed]
- Shawki, A.; McCole, D.F. Mechanisms of Intestinal Epithelial Barrier Dysfunction by Adherent-Invasive Escherichia Coli. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Perna, A.; Marano, A.; Lucariello, A.; Rotondi Aufiero, V.; Sorrentino, A.; Melina, R.; Guerra, G.; Taccone, F.S.; Iaquinto, G.; et al. Pathogenic Role of Associated Adherent-Invasive Escherichia Coli in Crohn’s Disease: Role of Adherent-Invasive E.Coli in Crohn Disease. J. Cell. Physiol. 2017, 232, 2860–2868. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Hay, E.; Contieri, M.; De Luca, A.; Guerra, G.; Lucariello, A. Adherent-invasive Escherichia Coli (AIEC): Cause or Consequence of Inflammation, Dysbiosis, and Rupture of Cellular Joints in Patients with IBD? J. Cell. Physiol. 2020, 235, 5041–5049. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Manso, M.A.; López-Fandiño, R.; Recio, I. Activity against Listeria Monocytogenes of Human Milk during Lactation. A Preliminary Study. J. Dairy Res. 2008, 75, 24–29. [Google Scholar] [CrossRef]
- Ammons, M.C.; Copié, V. Mini-Review: Lactoferrin: A Bioinspired, Anti-Biofilm Therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef]
- Rastogi, N.; Nagpal, N.; Alam, H.; Pandey, S.; Gautam, L.; Sinha, M.; Shin, K.; Manzoor, N.; Virdi, J.S.; Kaur, P.; et al. Preparation and Antimicrobial Action of Three Tryptic Digested Functional Molecules of Bovine Lactoferrin. PLoS ONE 2014, 9, e90011. [Google Scholar] [CrossRef]
- Sánchez-Gómez, S.; Ferrer-Espada, R.; Stewart, P.S.; Pitts, B.; Lohner, K.; de Tejada, G.M. Antimicrobial Activity of Synthetic Cationic Peptides and Lipopeptides Derived from Human Lactoferricin against Pseudomonas Aeruginosa Planktonic Cultures and Biofilms. BMC Microbiol. 2015, 15, 137. [Google Scholar] [CrossRef]
- Sinha, M.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Antimicrobial Lactoferrin Peptides: The Hidden Players in the Protective Function of a Multifunctional Protein. Int. J. Pept. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, A.M.; Tinari, A.; Pietrantoni, A.; Antonini, G.; Valenti, P.; Conte, M.P.; Superti, F. Effect of Bovine Lactoferricin on Enteropathogenic Yersinia Adhesion and Invasion in HEp-2 Cells. J. Med. Microbiol. 2004, 53, 407–412. [Google Scholar] [CrossRef]
- Nikawa, H.; Samaranayake, L.P.; Hamada, T. Modulation of the anti-Candida activity of apo-lactoferrin by dietary sucrose and tunicamycin in vitro. Arch. Oral Biol. 1995, 40, 581–584. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Samaranayake, Y.H.; Samaranayake, L.P.; Nikawa, H. In vitro susceptibility of Candida species to lactoferrin. Med. Mycol. 1998, 37, 35–41. [Google Scholar] [CrossRef]
- Kirkpatrick, C.H.; Green, I.; Rich, R.R.; Schade, A.L. Inhibition of growth of Candida albicans by iron unsaturated lactoferrin: Relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 1971, 124, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, H. Effect of lactoferrin and iron on the growth of human pathogenic Candida species. Pak. J. Biol. Sci. 2009, 12, 91–94. [Google Scholar] [CrossRef]
- Lai, Y.W.; Campbell, L.T.; Wilkins, M.R.; Pang, C.N.I.; Chen, S.; Carter, D.A. Synergy and antagonism between iron chelators and antifungal drugs in Cryptococcus. Int. J. Antimicrob. Agents 2016, 48, 388–394. [Google Scholar] [CrossRef]
- Zarember, K.A.; Sugui, J.A.; Chang, Y.C.; Kwon-Chung, K.J.; Gallin, J.I. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J. Immunol. 2007, 178, 6367–6373. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Abe, S.; Okutomi, T.; Tansho, S.; Kawase, K.; Yamaguchi, H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 1996, 40, 821–825. [Google Scholar] [CrossRef]
- Kondori, N.; Baltzer, L.; Dolphin, G.T.; Mattsby-Baltzer, I. Fungicidal activity of human lactoferrin-derived peptides based on the antimicrobial αβ region. Int. J. Antimicrob. Agents 2011, 37, 51–57. [Google Scholar] [CrossRef]
- Viejo-Diaz, M.; Andrés, M.T.; Fierro, J.F. Effects of human lactoferrin on the cytoplasmic membrane of Candida albicans cells related with its candidacidal activity. FEMS Immunol. Med. Microbiol. 2004, 42, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.T.; Viejo-Díaz, M.; Fierro, J.F. Human lactoferrin induces apoptosis-like cell death in Candida albicans: Critical role of K+-channel-mediated K+ efflux. Antimicrob. Agents Chemother. 2008, 52, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Zaldívar, M.; Andrés, M.T.; Rego, A.; Pereira, C.S.; Fierro, J.F.; Côrte-Real, M. Human lactoferrin triggers a mitochondrial- and caspase-dependent regulated cell death in Saccharomyces cerevisiae. Apoptosis 2016, 21, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Velliyagounder, K.; Rozario, S.D.; Fine, D.H. The effects of human lactoferrin in experimentally induced systemic candidiasis. J. Med. Microbiol. 2019, 68, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Awano, N.; Fukui, T.; Sasaki, H.; Kyuwa, S. The protective effects of lactoferrin against murine norovirus infection through inhibition of both viral attachment and replication. Biochem. Biophys. Res. Commun. 2013, 434, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Kolawole, A.O.; Mirabelli, C.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Wobus, C.E. Antiviral Effects of Bovine Lactoferrin on Human Norovirus. Biochem. Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Klems, M.; Untersmayr, E. The role of gastrointestinal permeability in food allergy. Ann. Allergy Asthma Immunol. 2018, 121, 168–173. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef]
- Weber, C.R. Dynamic properties of the tight junction barrier. Ann. N. Y. Acad. Sci. 2012, 1257, 77–84. [Google Scholar] [CrossRef]
- Reinhold, A.K.; Rittner, H.L. Barrier function in the peripheral and central nervous system—A review. Eur. J. Physiol. 2017, 469, 123–134. [Google Scholar] [CrossRef]
- Rubio, M.G.; Amo-Mensah, K.; Gray, J.M.; Nguyen, V.Q.; Nakat, S.; Grider, D.; Love, K.; Boone, J.H.; Sorrentino, D. Fecal Lactoferrin Accurately Reflects Mucosal Inflammation in Inflammatory Bowel Disease. World J. Gastrointest. Pathophysiol. 2019, 10, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hering, N.A.; Luettig, J.; Krug, S.M.; Wiegand, S.; Gross, G.; van Tol, E.A.; Schulzke, J.D.; Rosenthal, R. Lactoferrin Protects against Intestinal Inflammation and Bacteria-Induced Barrier Dysfunction in Vitro: Barrier-Protective Properties of Lactoferrin. Ann. N. Y. Acad. Sci. 2017, 1405, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, X.-X.; Liu, Y.; Xi, E.-Z.; An, J.-J.; Tabys, D.; Liu, N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules 2019, 24, 148. [Google Scholar] [CrossRef] [PubMed]
- Togawa, J.; Nagase, H.; Tanaka, K.; Inamori, M.; Nakajima, A.; Ueno, N.; Saito, T.; Sekihara, H. Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. J. Gastroenterol. Hepatol. 2002, 17, 1291–1298. [Google Scholar] [CrossRef]
- Li, L.; Ren, F.; Yun, Z.; An, Y.; Wang, C.; Yan, X. Determination of the effects of lactoferrin in a preclinical mouse model of experimental colitis. Mol. Med. Rep. 2013, 8, 1125–1129. [Google Scholar] [CrossRef]
- Haversen, L.A.; Baltzer, L.; Dolphin, G.; Hanson, L.A.; Mattsby-Baltzer, I. Anti-Inflammatory Activities of Human Lactoferrin in Acute Dextran Sulphate-Induced Colitis in Mice. Scand. J. Immunol. 2003, 57, 2–10. [Google Scholar] [CrossRef]
- Alexander, D.; Iigo, M.; Abdelgied, M.; Ozeki, K.; Tanida, S.; Takashi, J.; Tsuda, H. Bovine lactoferrin and Crohn’s disease: A case study. Biochem. Cell Biol. 2017, 95, 133–141. [Google Scholar] [CrossRef]
- Cutone, A.; Frioni, A.; Berlutti, F.; Valenti, P.; Musci, G.; Bonaccorsi di Patti, M.C. Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals 2014, 27, 807–813. [Google Scholar] [CrossRef]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and Efficacy of Lactoferrin versus Ferrous Sulphate in Curing Iron Deficiency and Iron Deficiency Anaemia in Hereditary Thrombophilia Pregnant Women: An Interventional Study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef]
- Chea, C.; Miyauchi, M.; Inubushi, T.; FebriyantiAyuningtyas, N.; Subarnbhesaj, A.; Nguyen, P.T.; Shrestha, M.; Haing, S.; Ohta, K.; Takata, T. Molecular mechanism of inhibitory effects of bovine lactoferrin on the growth of oral squamous cell carcinoma. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Bojesen, R.D.; Riis, L.B.; Høgdall, E.; Nielsen, O.H.; Jess, T. Inflammatory Bowel Disease and Small Bowel Cancer Risk, Clinical Characteristics, and Histopathology: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.A.; Speizer, F.E. Relation of Meat, Fat, and Fiber Intake to the Risk of Colon Cancer in a Prospective Study among Women. N. Engl. J. Med. 1990, 323, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Giovannucci, E.L. Primary Prevention of Colorectal Cancer. Gastroenterology 2010, 138, 2029–2043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer effects of lactoferrin: Underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef]
- Shimamura, M.; Yamamoto, Y.; Ashino, H.; Oikawa, T.; Hazato, T.; Tsuda, H.; Iigo, M. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer. 2004, 111, 111–116. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, R.K.; Kanwar, J.R. Lactoferrin and cancer in different cancer models. Front. Biosci. 2011, 3, 1080–1088. [Google Scholar] [CrossRef]
- Varadhachary, A.; Wolf, J.S.; Petrak, K.; O’Malley, B.W.; Spadaro, M.; Curcio, C.; Forni, G.; Pericle, F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int. J. Cancer 2004, 111, 398–403. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, R.; Przepiorski, A.; Reddy, S.; Palmano, K.P.; Krissansen, G.W. “Iron-saturated” bovine lactoferrin improves the chemotherapeutic effects of tamoxifen in the treatment of basal-like breast cancer in mice. BMC Cancer 2012, 12, 591. [Google Scholar] [CrossRef]
- Ushida, Y.; Sekine, K.; Kuhara, T.; Takasuka, N.; Iigo, M.; Maeda, M.; Tsuda, H. Possible chemopreventive effects of bovine lactoferrin on esophagus and lung carcinogenesis in the rat. Jpn. J. Cancer Res. 1999, 90, 262–267. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawabata, K.; Kohno, H.; Honjo, S.; Murakami, M.; Ota, T.; Tsuda, H. Chemopreventive effect of bovine lactoferrin on 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male F344 rats. Jpn. J. Cancer Res. 2000, 91, 25–33. [Google Scholar] [CrossRef]
- Sugihara, Y.; Zuo, X.; Takata, T.; Jin, S.; Miyauti, M.; Isikado, A.; Imanaka, H.; Tatsuka, M.; Qi, G.; Shimamoto, F. Inhibition of DMH-DSS-induced colorectal cancer by liposomal bovine lactoferrin in rats. Oncol. Lett. 2017, 14, 5688–5694. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, R.R.; Mansour, D.F.; Salama, A.A.; Abdel-Rahman, R.F.; Hassan, A.M. Regulation of PKB/Akt-pathway in the chemopreventive effect of lactoferrin against diethylnitrosamine-induced hepatocarcinogenesis in rats. Pharmacol. Rep. 2019, 71, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sakashita, S.; Morishita, Y.; Kano, J.; Shiba, A.; Sato, T.; Noguchi, M. Binding of lactoferrin to IGBP1 triggers apoptosis in a lung adenocarcinoma cell line. Anticancer Res. 2011, 31, 529–534. [Google Scholar] [PubMed]
- Rodrigues, L.; Teixeira, J.; Schmitt, F.; Paulsson, M.; Månsson, H.L. Lactoferrin and cancer disease prevention. Crit. Rev. Food Sci. Nutr. 2009, 49, 203–217. [Google Scholar] [CrossRef]
- Fischer, R.; Debbabi, H.; Dubarry, M.; Boyaka, P.; Tomé, D. Regulation of Physiological and Pathological Th1 and Th2 Responses by Lactoferrin. Biochem. Cell Biol. 2006, 84, 303–311. [Google Scholar] [CrossRef]
- Damiens, E.; El Yazidi, I.; Mazurier, J.; Elass-Rochard, E.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Role of heparan sulphate proteoglycans in the regulation of human lactoferrin binding and activity in the MDA-MB-231 breast cancer cell line. Eur. J. Cell Biol. 1998, 77, 344–351. [Google Scholar] [CrossRef]
- Legrand, D.; van Berkel, P.H.; Salmon, V.; van Veen, H.A.; Slomianny, M.C.; Nuijens, J.H.; Spik, G. The N-terminal Arg2, Arg3 and Arg4 of human lactoferrin interact with sulphated molecules but not with the receptor present on Jurkat human lymphoblastic T-cells. Biochem. J. 1997, 327, 841–846. [Google Scholar] [CrossRef]
- Kühnle, A.; Veelken, R.; Galuska, C.E.; Saftenberger, M.; Verleih, M.; Schuppe, H.C.; Rudlo, S.; Kunz, C.; Galuska, S.P. Polysialic acid interacts with lactoferrin and supports its activity to inhibit the release of neutrophil extracellular traps. Carbohydr. Polym. 2019, 208, 32–41. [Google Scholar] [CrossRef]
- Sekine, K.; Watanabe, E.; Nakamura, J.; Takasuka, N.; Kim, D.J.; Asamoto, M.; Krutovskikh, V.; Baba-Toriyama, H.; Ota, T.; Moore, M.A.; et al. Inhibition of azoxymethane-initiated colon tumor by bovine lactoferrin administration in F344 rats. Jpn. J. Cancer Res. 1997, 88, 523–526. [Google Scholar] [CrossRef]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Takakura, N.; Wakabayashi, H.; Yamauchi, K.; Takase, M. Influences of orally administered lactoferrin on IFN-gamma and IL-10 production by intestinal intraepithelial lymphocytes and mesenteric lymph-node cells. Biochem. Cell Biol. 2006, 84, 363–368. [Google Scholar] [CrossRef]
- Shau, H.; Kim, A.; Golub, S.H. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. J. Leukoc. Biol. 1992, 51, 343–349. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin Acts as an Alarmin to Promote the Recruitment and Activation of APCs and Antigen-Specific Immune Responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.H.; Regester, G.O.; Le Leu, R.K.; Royle, P.J.; Smithers, G.W. Dairy proteins protect against dimethylhydrazine-induced intestinal cancers in rats. J. Nutr. 1995, 125, 809–816. [Google Scholar] [CrossRef]
- Tsuda, H.; Sekine, K.; Nakamura, J.; Ushida, Y.; Kuhara, T.; Takasuka, N.; Kim, D.J.; Asamoto, M.; Baba-Toriyama, H.; Moore, M.A.; et al. Inhibition of Azoxymethane Initiated Colon Tumor and Aberrant Crypt Foci Development by Bovine Lactoferrin Administration in F344 Rats. Adv. Exp. Med. Biol. 1998, 443, 273–284. [Google Scholar] [CrossRef]
- Ushida, Y.; Sekine, K.; Kyhara, T.; Takasuka, N.; Iigo, M.; Tsuda, H. Inhibitory effects of bovine lactoferrin on intestinal polyposis in the Apc (Min) mouse. Cancer Lett. 1998, 134, 141–145. [Google Scholar] [CrossRef]
- Fujita, K.; Matsuda, E.; Sekine, K.; Iigo, M.; Tsuda, H. Lactoferrin enhances Fas expression and apoptosis in the colon mucosa of azoxymethane-treated rats. Carcinogenesis 2004, 25, 1961–1966. [Google Scholar] [CrossRef]
- Iigo, M.; Kuhara, T.; Ushida, Y.; Sekine, K.; Moore, M.A.; Tsuda, H. Inhibitory effects of bovine lactoferrin on colon carcinoma 26 lung metastasis in mice. Clin. Exp. Metastasis 1999, 17, 35–40. [Google Scholar] [CrossRef]
- Wang, W.-P.; Iigo, M.; Sato, J.; Sekine, K.; Adachi, I.; Tsuda, H. Activation of Intestinal Mucosal Immunity in Tumor-Bearing Mice by Lactoferrin. Jpn. J. Cancer Res. 2000, 91, 1022–1027. [Google Scholar] [CrossRef]
- Iigo, M.; Shimamura, M.; Matsuda, E.; Fujita, K.; Nomoto, H.; Satoh, J.; Kojima, S.; Alexander, D.B.; Moore, M.A.; Tsuda, H. Orally Administered Bovine Lactoferrin Induces Caspase-1 and Interleukin-18 in the Mouse Intestinal Mucosa: A Possible Explanation for Inhibition of Carcinogenesis and Metastasis. Cytokine 2004, 25, 36–44. [Google Scholar] [CrossRef]
- Coughlin, C.M.; Salhany, K.E.; Wysocka, M.; Aruga, E.; Kurzawa, H.; Chang, A.E.; Hunter, C.A.; Fox, J.C.; Trinchieri, G.; Lee, W.M. Interleukin-12 and Interleukin-18 Synergistically Induce Murine Tumor Regression Which Involves Inhibition of Angiogenesis. J. Clin. Investig. 1998, 101, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Yamauchi, K.; Tamura, Y.; Okamura, H. Oral Administration of Lactoferrin Increases NK Cell Activity in Mice via Increased Production of IL-18 and Type I IFN in the Small Intestine. J. Interferon Cytokine Res. 2006, 26, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-A.; Wilk, K.M.; Bangale, Y.A.; Kruzel, M.L.; Actor, J.K. Lactoferrin Modulation of IL-12 and IL-10 Response from Activated Murine Leukocytes. Med. Microbiol. Immunol. 2007, 196, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Alexander, D.; Long, N.; Xu, J.; Fukamachi, K.; Futakuchi, M.; Takase, M.; Tsuda, H. Anticarcinogenesis Pathways Activated by Bovine Lactoferrin in the Murine Small Intestine. Biochimie 2009, 91, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of Orally Administered Bovine Lactoferrin on the Growth of Adenomatous Colorectal Polyps in a Randomized, Placebo-Controlled Clinical Trial. Cancer Prev. Res. 2009, 2, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Iigo, M.; Alexander, D.B.; Xu, J.; Futakuchi, M.; Suzui, M.; Kozu, T.; Akasu, T.; Saito, D.; Kakizoe, T.; Yamauchi, K.; et al. Inhibition of Intestinal Polyp Growth by Oral Ingestion of Bovine Lactoferrin and Immune Cells in the Large Intestine. Biometals 2014, 27, 1017–1029. [Google Scholar] [CrossRef]
- Curran, C.S.; Demick, K.P.; Mansfield, J.M. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell. Immunol. 2006, 242, 23–30. [Google Scholar] [CrossRef]
- Puddu, P.; Carollo, M.G.; Belardelli, F.; Valenti, P.; Gessani, S. Role of endogenous interferon and LPS in the immunomodulatory effects of bovine lactoferrin in murine peritoneal macrophages. J. Leukoc. Biol. 2007, 82, 347–353. [Google Scholar] [CrossRef]
- Puddu, P.; Latorre, D.; Carollo, M.; Catizone, A.; Ricci, G.; Valenti, P.; Gessani, S. Bovine lactoferrin counteracts Toll-like receptor mediated activation signals in antigen presenting cells. PLoS ONE 2011, 6, e22504. [Google Scholar] [CrossRef]
- Sabra, S.; Agwa, M.M. Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int. J. Biol. Macromol. 2020, 164, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Toida, T.; Nishimura, S.; Nagano, E.; Kusuoka, O.; Teraguchi, S.; Hayasawa, H.; Shimamura, S.; Tomita, M. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem. Toxicol. 2000, 38, 503–512. [Google Scholar] [CrossRef]
- Hayes, T.G.; Falchook, G.F.; Varadhachary, G.R.; Smith, D.P.; Davis, L.D.; Dhingra, H.M.; Hayes, B.P.; Varadhachary, A. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Investig. New Drugs. 2006, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Jiang, P.; Stensballe, A.; Bendixen, E.; Sangild, P.T.; Chatterton, D.E.W. Bovine lactoferrin regulates cell survival, apoptosis and inflammation in intestinal epithelial cells and preterm pig intestine. J. Proteom. 2016, 139, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Miehlke, S.; Reddy, R.; Osato, M.S.; Ward, P.P.; Conneely, O.M.; Graham, D.Y. Direct activity of recombinant human lactoferrin against Helicobacter pylori. J. Clin. Microbiol. 1996, 34, 2593–2594. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.Q.; Campbell, M.A.F.; Couper, R.T.L.; Tran, C.D.; Lawrence, A.; Butler, R.N. Lactoferrin and desferrioxamine are ineffective in the treatment of Helicobacter pylori infection and may enhance H. pylori growth and gastric inflammation in mice. Lett. Appl. Microbiol. 2009, 48, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, F.; Aragona, G.; Dal Bò, N.; Cavestro, G.M.; Cavallaro, L.; Iori, V.; Comparato, G.; Leandro, G.; Pilotto, A.; Franzè, A. Use of bovine lactoferrin for Helicobacter pylori eradication. Dig. Liver Dis. 2003, 35, 706–710. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Q.; Cheng, G.; Liu, X.; Liu, S.; Luo, J.; Zhang, A.; Bian, L.; Chen, J.; Lv, J.; et al. Recombinant human lactoferrin enhances the efficacy of triple therapy in mice infected with Helicobacter pylori. Int. J. Mol. Med. 2015, 36, 363–368. [Google Scholar] [CrossRef]
- Zullo, A.; De Francesco, V.; Scaccianoce, G.; Hassan, C.; Panarese, A.; Piglionica, D.; Panella, C.; Morini, S.; Ierardi, E. Quadruple therapy with lactoferrin for Helicobacter pylori eradication: A randomised, multicentre study. Dig. Liver Dis. 2005, 37, 496–500. [Google Scholar] [CrossRef]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Linsell, L.; Murray, D.; Partlett, C.; Patel, M.; et al. Enteral lactoferrin supplementation for very preterm infants: A randomised placebo-controlled trial. Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, Italian Society of Neonatology. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Meyer, M.; Stolfi, I.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Decembrino, L.; Laforgia, N.; et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial. Early Hum. Dev. 2014, 90 (Suppl. 1), S60–S65. [Google Scholar] [CrossRef]
- Akin, I.M.; Atasay, B.; Dogu, F.; Okulu, E.; Arsan, S.; Karatas, H.D.; Ikinciogullari, A.; Turmen, T. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am. J. Perinatol. 2014, 31, 1111–1120. [Google Scholar] [PubMed]
- Kaur, G.; Gathwala, G. Efficacy of Bovine Lactoferrin Supplementation in Preventing Late-onset Sepsis in lowBirth Weight Neonates: A Randomized Placebo-Controlled Clinical Trial. J. Trop. Pediatr. 2015, 61, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Shastri, S.; Sharma, P. Role of lactoferrin in neonatal care: A systematic review. J. Matern. Fetal Neonatal Med. 2017, 30, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
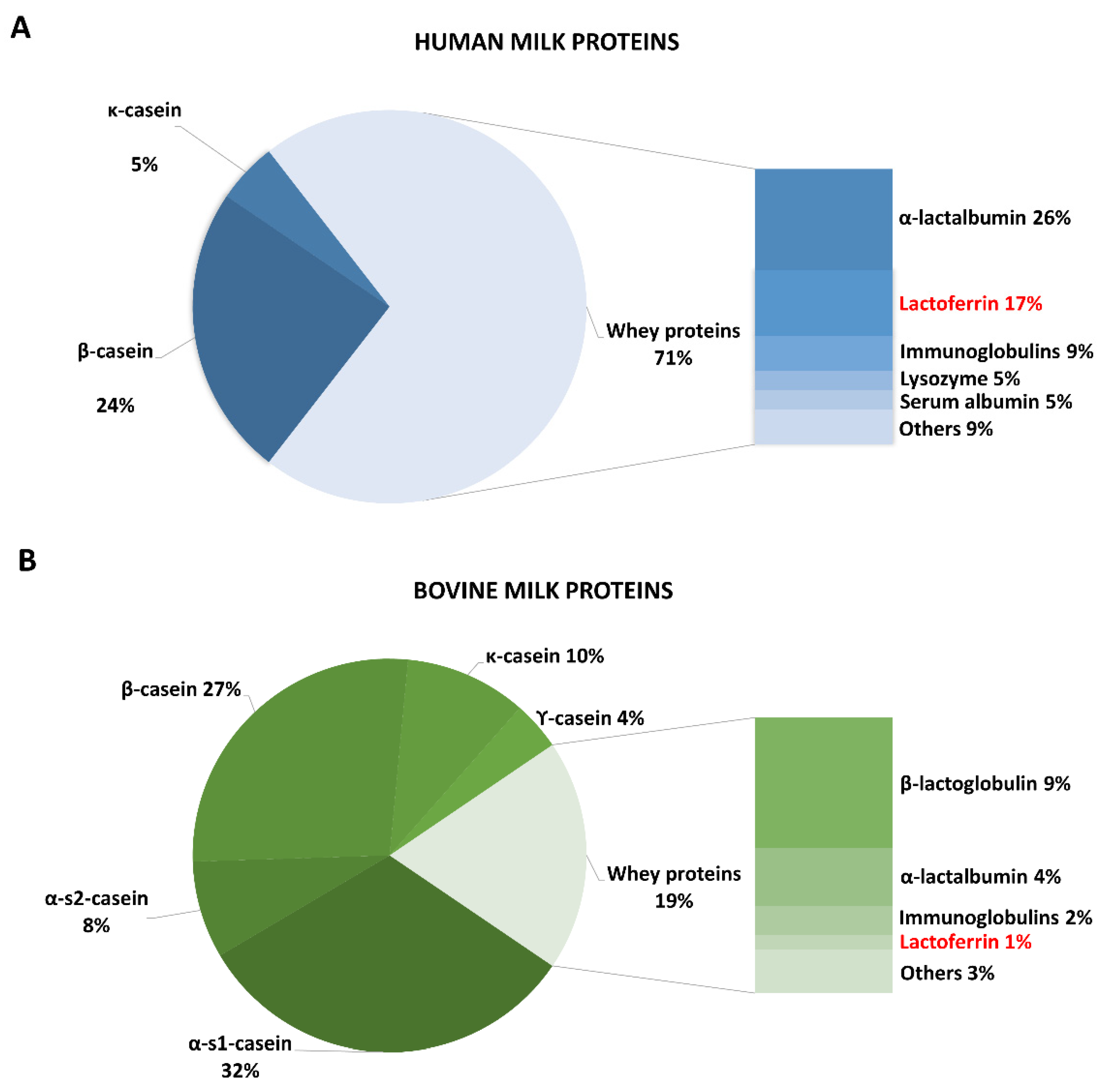
| Milk or Dairy Product | Lactoferrin mg/mL | Detection Assay | Reference | |
|---|---|---|---|---|
| Cow Milk | Raw | 0.020–0.200 | Radial Immunodiffusion | [83] |
| 0.157 ± 0.007 | ELISA | [84] | ||
| 0.182 (RSD% ≤ 5.5) | Immunosensors | [85] | ||
| 188.4 ± 13.2 | Reversed-phase HPLC | [86] | ||
| Pasteurized | 0.174 ± 0.017 | ELISA | [84] | |
| UHT | 0.018 (RSD% ≤ 5.5) | Immunosensors | [85] | |
| Buffalo milk | Raw | 0.232 (RSD% ≤ 5.5) | Immunosensors | [85] |
| Goat Milk | Raw | 20–200 | Radial Immunodiffusion | [83] |
| 0.125 ± 0.020 | Reversed-phase HPLC | [87] | ||
| 0.042 ± 0.008 | ELISA | [88] | ||
| 927.3 ± 52.1 | Reversed-phase HPLC | [86] | ||
| Pasteurized | 0.105 ± 0.015 | Reversed-phase HPLC | [87] | |
| Partially skimmed | 0.017 (RSD% ≤ 5.5) | Immunosensors | [85] | |
| Sheep Milk | Raw | 0.260 ± 0.050 | Radial Immunodiffusion | [89] |
| 0.466 ± 0.023 | Reversed-phase HPLC | [86] | ||
| Camel Milk | Raw | 0.229 ± 0.135 | Radial Immunodiffusion | [90] |
| Cheese | Swiss type | 1.112 ± 0.111 mg/g | ELISA | [84] |
| semi-hard | 1.143 ± 0.118 mg/g | ELISA | [84] | |
| soft | 0.680 ± 0.015 mg/g | ELISA | [84] | |
| Feta | 0.272 ± 0.024 | Reversed-phase HPLC | [86] | |
| Food | BLf Maximum Level (EU) | BLf Maximum Level (USA) |
|---|---|---|
| Infant formulae—powder | 30–770 mg/100 g | 100 mg/100 g |
| Infant formulae—ready-to-feed | 4–100 mg/100 mL | 13 mg/100 mL |
| Infant formulae—liquid concentrate | 26 mg/100 mL | - |
| Milk beverages | 50–200 mg/100 g | 100 mg/100 g |
| Powdered milk | 300 mg/100 g | 400 mg/100 g |
| Ice creams | 130 mg/100 g | 200 mg/100 g |
| Sherbets | - | 200 mg/100 g |
| Yogurt | 80 mg/100 g | 100 mg/100 g |
| Chewing gum | 30 mg/g | 30 mg/g |
| Processed cereal food | 670 mg/100 g | - |
| Product based on cheese | 2000 mg/100 g | - |
| Non alcoholic drinks | 120 mg/100 g | - |
| Cakes and pastries | 1000 mg/100 g | - |
| Candies | 7 mg/g | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutone, A.; Ianiro, G.; Lepanto, M.S.; Rosa, L.; Valenti, P.; Bonaccorsi di Patti, M.C.; Musci, G. Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development. Cancers 2020, 12, 3806. https://doi.org/10.3390/cancers12123806
Cutone A, Ianiro G, Lepanto MS, Rosa L, Valenti P, Bonaccorsi di Patti MC, Musci G. Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development. Cancers. 2020; 12(12):3806. https://doi.org/10.3390/cancers12123806
Chicago/Turabian StyleCutone, Antimo, Giusi Ianiro, Maria Stefania Lepanto, Luigi Rosa, Piera Valenti, Maria Carmela Bonaccorsi di Patti, and Giovanni Musci. 2020. "Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development" Cancers 12, no. 12: 3806. https://doi.org/10.3390/cancers12123806
APA StyleCutone, A., Ianiro, G., Lepanto, M. S., Rosa, L., Valenti, P., Bonaccorsi di Patti, M. C., & Musci, G. (2020). Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development. Cancers, 12(12), 3806. https://doi.org/10.3390/cancers12123806











