The Expression of CD74-Regulated Inflammatory Markers in Stage IV Melanoma: Risk of CNS Metastasis and Patient Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Study Population
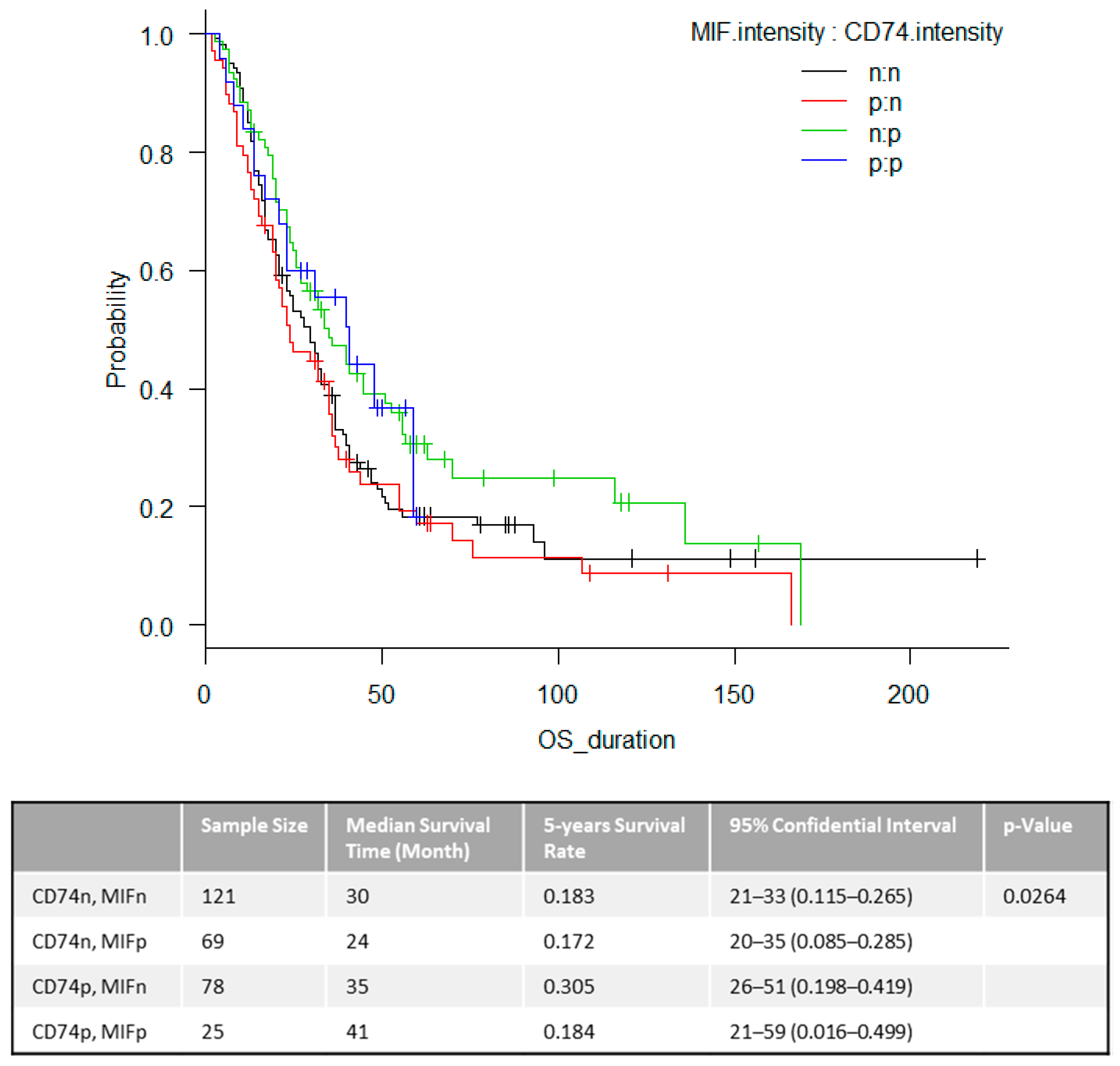
2.2. Overall Survival (OS)
2.3. The Time to First CNS Metastasis
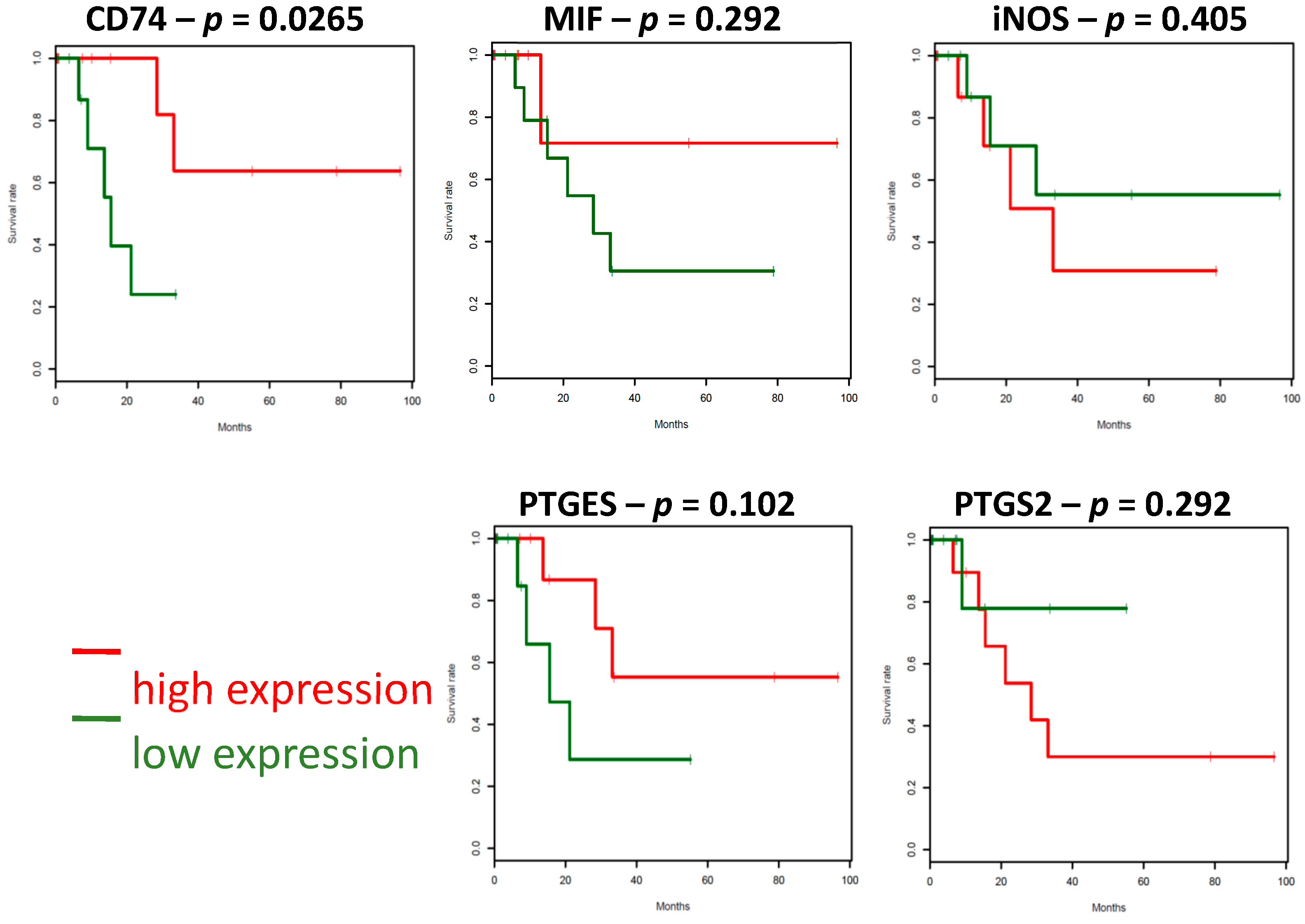
2.4. The Survival Associations in TCGA Stage IV Melanoma Dataset
2.5. Gene Expressions Associated with CD74 Expression in Stage IV Melanoma
3. Discussion
4. Materials and Methods
4.1. Melanoma Tissue Microarray (TMA)
4.2. Biomarker Assessments
4.3. TCGA and CCLE Data Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [Green Version]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA A Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [Green Version]
- Amer, M.H.; Al-Sarraf, M.; Baker, L.H.; Vaitkevicius, V.K. Malignant melanoma and central nervous system metastases.Incidence, diagnosis, treatment and survival. Cancer 1978, 42, 660–668. [Google Scholar] [CrossRef]
- Retsas, S.; Gersbuny, A.R. Central nervous system involvement in malignant melanoma. Cancer 1988, 61, 1926–1934. [Google Scholar] [CrossRef]
- Haydu, L.E.; Lo, S.N.; McQuade, J.L.; Amaria, R.N.; Wargo, J.; Ross, M.I.; Cormier, J.N.; Lucci, A.; Lee, J.E.; Ferguson, S.D.; et al. Cumulative Incidence and Predictors of CNS Metastasis for Patients With American Joint Committee on Cancer 8th Edition Stage III Melanoma. J. Clin. Oncol. 2020, 38, 1429–1441. [Google Scholar] [CrossRef]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; De Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.P.; Hamid, O.; Hodi, F.S.; Moschos, S.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Grimm, E.A.; Sikora, A.G.; Ekmekcioglu, S. Molecular Pathways: Inflammation-Associated Nitric-Oxide Production as a Cancer-Supporting Redox Mechanism and a Potential Therapeutic Target. Clin. Cancer Res. 2013, 19, 5557–5563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekmekcioglu, S.; Davies, M.A.; Tanese, K.; Roszik, J.; Shin-Sim, M.; Bassett, R.L.; Milton, D.R.; Woodman, S.E.; Prieto, V.G.; Gershenwald, J.E.; et al. Inflammatory Marker Testing Identifies CD74 Expression in Melanoma Tumor Cells, and Its Expression Associates with Favorable Survival for Stage III Melanoma. Clin. Cancer Res. 2016, 22, 3016–3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velu, P.P.; Cao, C.; Yan, T.D. Current surgical management of melanoma metastases to the lung. J. Thorac. Dis. 2013, 5, S274–S276. [Google Scholar]
- Cohen, J.V.; Tawbi, H.; Margolin, K.A.; Amravadi, R.; Bosenberg, M.; Brastianos, P.K.; Chiang, V.L.; De Groot, J.; Glitza, I.C.; Herlyn, M.; et al. Melanoma central nervous system metastases: Current approaches, challenges, and opportunities. Pigment. Cell Melanoma Res. 2016, 29, 627–642. [Google Scholar] [CrossRef]
- Gutzmer, R.; Vordermark, D.; Hassel, J.C.; Krex, D.; Wendl, C.; Schadendorf, D.; Sickmann, T.; Rieken, S.; Pukrop, T.; Höller, C.; et al. Melanoma brain metastases—Interdisciplinary management recommendations 2020. Cancer Treat. Rev. 2020, 89, 102083. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Liu, P.; McIntyre, S.; Kim, K.B.; Papadopoulos, N.; Hwu, W.-J.; Hwu, P.; Bedikian, A. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2010, 117, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Milne, K.; Webb, J.R.; Watson, P.H. CD74 and intratumoral immune response in breast cancer. Oncotarget 2016, 8, 12664–12674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakikes, I.; Morrison, I.E.G.; O’Toole, P.; Metodieva, G.; Navarrete, C.V.; Gomez, J.; Miranda-Sayago, J.M.; Cherry, R.J.; Metodiev, M.; Fernández, N. Interaction of HLA-DR and CD74 at the cell surface of antigen-presenting cells by single particle image analysis. FASEB J. 2012, 26, 4886–4896. [Google Scholar] [CrossRef] [PubMed]
- Accolla, R.S.; Lombardo, L.; Abdallah, R.; Raval, G.; Forlani, G.; Tosi, G. Boosting the MHC Class II-Restricted Tumor Antigen Presentation to CD4+ T Helper Cells: A Critical Issue for Triggering Protective Immunity and Re-Orienting the Tumor Microenvironment Toward an Anti-Tumor State. Front. Oncol. 2014, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Accolla, R.S.; Tosi, G. Optimal MHC-II-restricted tumor antigen presentation to CD4+ T helper cells: The key issue for development of anti-tumor vaccines. J. Transl. Med. 2012, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Thibodeau, J.; Bourgeois-Daigneault, M.-C.; Lapointe, R. Targeting the MHC Class II antigen presentation pathway in cancer immunotherapy. OncoImmunology 2012, 1, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Hussain, F.; Freissmuth, M.; Völkel, D.; Thiele, M.; Douillard, P.; Antoine, G.; Thurner, P.; Ehrlich, H.; Schwarz, H.-P.; Scheiflinger, F.; et al. Human Anti-Macrophage Migration Inhibitory Factor Antibodies Inhibit Growth of Human Prostate Cancer Cells In Vitro and In Vivo. Mol. Cancer Ther. 2013, 12, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Al-Abed, Y.; Dabideen, D.; Aljabari, B.; Valster, A.; Messmer, D.; Ochani, M.; Tanovic, M.; Ochani, K.; Bacher, M.; Nicoletti, F.; et al. ISO-1 Binding to the Tautomerase Active Site of MIF Inhibits Its Pro-inflammatory Activity and Increases Survival in Severe Sepsis. J. Biol. Chem. 2005, 280, 36541–36544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkova, Z.; Tao, R.-H.; Samaniego, F. Milatuzumab—A promising new immunotherapeutic agent. Expert Opin. Investig. Drugs 2010, 19, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Tanese, K.; Hashimoto, Y.; Berkova, Z.; Wang, Y.; Samaniego, F.; Lee, J.E.; Ekmekcioglu, S.; Grimm, E.A. Cell Surface CD74–MIF Interactions Drive Melanoma Survival in Response to Interferon-γ. J. Investig. Dermatol. 2015, 135, 2775–2784. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Cheng, X.; Zhang, L.; Lu, X.; Chaudhary, S.; Teng, R.; Frederickson, C.; Champion, M.M.; Zhao, R.; Cheng, L.; et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl. Acad. Sci. USA 2018, 115, 10094–10099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnboim, H.C.; Lemay, A.-M.; Lam, D.K.Y.; Goldstein, R.; Webb, J.R. Cutting Edge: MHC Class II-Restricted Peptides Containing the Inflammation-Associated Marker 3-Nitrotyrosine Evade Central Tolerance and Elicit a Robust Cell-Mediated Immune Response. J. Immunol. 2003, 171, 528–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, L.L.; Wick, D.A.; Webb, J.R. Conversion of Tyrosine to the Inflammation-Associated Analog 3′-Nitrotyrosine at Either TCR- or MHC-Contact Positions Can Profoundly Affect Recognition of the MHC Class I-Restricted Epitope of Lymphocytic Choriomeningitis Virus Glycoprotein 33 by CD8 T Cells. J. Immunol. 2008, 180, 5956–5962. [Google Scholar] [CrossRef] [Green Version]
- Madhurantakam, C.; Duru, A.D.; Sandalova, T.; Webb, J.R.; Achour, A. Inflammation-Associated Nitrotyrosination Affects TCR Recognition through Reduced Stability and Alteration of the Molecular Surface of the MHC Complex. PLoS ONE 2012, 7, e32805. [Google Scholar] [CrossRef] [Green Version]
- Brito, C.; Naviliat, M.; Tiscornia, A.C.; Vuillier, F.; Gualco, G.; Dighiero, G.; Radi, R.; Cayota, A.M. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J. Immunol. 1999, 162, 3356–3366. [Google Scholar] [PubMed]
- Nagaraj, S.; Schrum, A.G.; Cho, H.-I.; Celis, E.; Gabrilovich, D.I. Mechanism of T Cell Tolerance Induced by Myeloid-Derived Suppressor Cells. J. Immunol. 2010, 184, 3106–3116. [Google Scholar] [CrossRef]
- Bronte, V.; Kasic, T.; Gri, G.; Gallana, K.; Borsellino, G.; Marigo, I.; Battistini, L.; Iafrate, M.; Prayer-Galetti, T.; Pagano, F.; et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 2005, 201, 1257–1268. [Google Scholar] [CrossRef]
- Aulak, K.S.; Miyagi, M.; Yan, L.; West, K.A.; Massillon, D.; Crabb, J.W.; Stuehr, D.J. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc. Natl. Acad. Sci. USA 2001, 98, 12056–12061. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Patient Characteristics | n = 315 |
|---|---|
| Gender, n (%) | |
| Male | 223 (70.8%) |
| Female | 92 (29.2%) |
| Age at stage IV (years), median(range) | 56 (13–84) |
| Ulceration | |
| Yes | 41 |
| No | 67 |
| Missing | 207 |
| Breslow thickness (mm) | |
| N | 131 |
| Median (range) | 2.05 (0.3–23.0) |
| Brain met at first distant met | |
| Exist | 26 |
| None | 289 |
| OS, n (%) | |
| Alive | 81 (25.7%) |
| Dead | 234 (74.3%) |
| Tissue | Lung | Intestine | Liver | Adrenal | Spleen | Gall | Others | Total |
|---|---|---|---|---|---|---|---|---|
| N | 163 | 85 | 20 | 13 | 12 | 5 | 17 | 315 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, D.; Roszik, J.; Oba, J.; Kim, S.-H.; Bassett, R.L., Jr.; Haydu, L.E.; Tanese, K.; Grimm, E.A.; Ekmekcioglu, S. The Expression of CD74-Regulated Inflammatory Markers in Stage IV Melanoma: Risk of CNS Metastasis and Patient Survival. Cancers 2020, 12, 3754. https://doi.org/10.3390/cancers12123754
Ogata D, Roszik J, Oba J, Kim S-H, Bassett RL Jr., Haydu LE, Tanese K, Grimm EA, Ekmekcioglu S. The Expression of CD74-Regulated Inflammatory Markers in Stage IV Melanoma: Risk of CNS Metastasis and Patient Survival. Cancers. 2020; 12(12):3754. https://doi.org/10.3390/cancers12123754
Chicago/Turabian StyleOgata, Dai, Jason Roszik, Junna Oba, Sun-Hee Kim, Roland L. Bassett, Jr., Lauren E. Haydu, Keiji Tanese, Elizabeth A. Grimm, and Suhendan Ekmekcioglu. 2020. "The Expression of CD74-Regulated Inflammatory Markers in Stage IV Melanoma: Risk of CNS Metastasis and Patient Survival" Cancers 12, no. 12: 3754. https://doi.org/10.3390/cancers12123754
APA StyleOgata, D., Roszik, J., Oba, J., Kim, S.-H., Bassett, R. L., Jr., Haydu, L. E., Tanese, K., Grimm, E. A., & Ekmekcioglu, S. (2020). The Expression of CD74-Regulated Inflammatory Markers in Stage IV Melanoma: Risk of CNS Metastasis and Patient Survival. Cancers, 12(12), 3754. https://doi.org/10.3390/cancers12123754







