Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular Strategies for Viral Gene Therapy of the GBM
2.1. Viral Vector Types Proposed for Gene Therapy of GBM
2.2. Adenovirus-Based Vectors
2.3. Herpes Simplex Virus-Based Vectors
2.4. Vectors Based on other Viral Backgrounds
2.5. Evaluating Vector Efficacy
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, Y.; Uhrbom, L. On the origin of glioma. Upsala J. Med. Sci. 2012, 117, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.; Wirsching, H.-G.; Knobbe-Thomsen, G.R.C.B.; Weller, H.-G.W.M. Advances in the molecular genetics of gliomas—Implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Bent, M.J.V.D.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Schaich, M.; Kestel, L.; Pfirrmann, M.; Robel, K.; Illmer, T.; Kramer, M.; Dill, C.; Ehninger, G.; Schackert, G.; Krex, D. A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Ann. Oncol. 2009, 20, 175–181. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Chowdhary, S.A.; Ryken, T.; Newton, H.B. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: A meta-analysis. J. Neuro Oncol. 2015, 122, 367–382. [Google Scholar] [CrossRef]
- Vasilev, A.; Sofi, R.; Tong, L.; Teschemacher, A.G.; Kasparov, S. In Search of a Breakthrough Therapy for Glioblastoma Multiforme. Neuroglia 2018, 1, 292–310. [Google Scholar] [CrossRef]
- Liu, T.-C.; Galanis, E.; Kirn, D.H. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007, 4, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; O’Leary, M.P.; Fong, Y.; Chen, N.G. From Benchtop to Bedside: A Review of Oncolytic Virotherapy. Biomedicines 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Darwiche, K.; Sakkas, A.; Yarmus, L.; Huang, H.; Li, Q.; Freitag, L.; Zarogoulidis, K.; Malecki, M. Suicide Gene Therapy for Cancer—Current Strategies. J. Genet. Syndr. Gene Ther. 2013, 4, 16849. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses (ICTV). Available online: http://www.ictvonline.org/ (accessed on 15 October 2020).
- Harrach, B. Adenoviruses: General Features. In Encyclopedia of Virology; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 1–9. [Google Scholar]
- Knipe, D.M.; Howley, P.M. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Pelka, P.; Miller, M.S.; Cecchini, M.; Yousef, A.F.; Bowdish, D.M.E.; Dick, F.; Whyte, P.; Mymryk, J.S. Adenovirus E1A Directly Targets the E2F/DP-1 Complex. J. Virol. 2011, 85, 8841–8851. [Google Scholar] [CrossRef] [PubMed]
- Wold, W.S.M.; Toth, K. Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr. Gene Ther. 2014, 13, 421–433. [Google Scholar] [CrossRef]
- Ganly, I.; Kirn, D.; Eckhardt, G.; I Rodriguez, G.; Soutar, D.S.; Otto, R.; Robertson, A.G.; Park, O.; Gulley, M.L.; Heise, C.; et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000, 6, 798–806. [Google Scholar]
- Rothmann, T.; Hengstermann, A.; Whitaker, N.J.; Scheffner, M.; Hausen, H.Z. Replication of ONYX-015, a Potential Anticancer Adenovirus, Is Independent of p53 Status in Tumor Cells. J. Virol. 1998, 72, 9470–9478. [Google Scholar] [CrossRef]
- Edwards, S.; Dix, B.R.; Myers, C.J.; Dobson-Le, D.; Huschtscha, L.; Hibma, M.; Royds, J.; Braithwaite, A.W. Evidence that Replication of the Antitumor Adenovirus ONYX-015 Is Not Controlled by the p53 and p14ARF Tumor Suppressor Genes. J. Virol. 2002, 76, 12483–12490. [Google Scholar] [CrossRef]
- Marina, N.; Christie, I.N.; Korsak, A.; Doronin, M.; Brazhe, A.; Hosford, P.S.; Wells, J.A.; Sheikhbahaei, S.; Humoud, I.; Paton, J.F.R.; et al. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Wong, L.; Polson, J.W.; Murphy, D.; Paton, J.F.R.; Kasparov, S. Genetic and pharmacological dissection of pathways involved in the angiotensin II-mediated depression of baroreflex function. FASEB J. 2002, 16, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.V.; Kasymov, V.; Marina, N.; Tang, F.; Figueiredo, M.F.; Lane, S.; Teschemacher, A.G.; Spyer, K.M.; Deisseroth, K.; Kasparov, S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science 2010, 329, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Fueyo-margareto, J.; Manzano-gomez, C.; Conrad, C.; Lang, F.; Yung, W.A.; Tufaro, F. Treatment of Brain Cancer with Oncolytic Adenovirus. WIPO Patent No. WO 2014/204814 A1, 24 December 2014. [Google Scholar]
- Philbrick, B.; Adamson, C. DNX-2401: An investigational drug for the treatment of recurrent glioblastoma. Expert Opin. Investig. Drugs 2019, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shin, D.H.; Nguyen, T.T.; Fueyo, J.; Fan, X.; Henry, V.; Carrillo, C.C.; Yi, Y.; Alonso, M.M.; Collier, T.L.; et al. Localized Treatment with Oncolytic Adenovirus Delta-24-RGDOX Induces Systemic Immunity against Disseminated Subcutaneous and Intracranial Melanomas. Clin. Cancer Res. 2019, 25, 6801–6814. [Google Scholar] [CrossRef] [PubMed]
- Tufaro, F.; Fueyo-Margareto, J.; Gomez-Manzano, C.; Conrad, C.; Yung, A.W.; Jiang, H. Adenovirus Expressing Immune Cell Stimulatory Receptor Agonist(s). WIPO Patent No. WO 2015/077624 Al, 28 May 2015. [Google Scholar]
- Smitt, P.S.; Driesse, M.; Wolbers, J.; Kros, M.; Avezaat, C. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol. Ther. 2003, 7, 851–858. [Google Scholar] [CrossRef]
- Kasparov, S. Suitability of hCMV for viral gene expression in the brain. Nat. Methods 2007, 4, 379. [Google Scholar] [CrossRef]
- Germano, I.M.; Fable, J.; Gultekin, S.H.; Silvers, A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: Preliminary results of a phase I trial in patients with recurrent malignant gliomas. J. Neuro-Oncol. 2003, 65, 279–289. [Google Scholar] [CrossRef]
- Smith, R.L.; Traul, D.L.; Schaack, J.; Clayton, G.H.; Staley, K.J.; Wilcox, C.L. Characterization of Promoter Function and Cell-Type-Specific Expression from Viral Vectors in the Nervous System. J. Virol. 2000, 74, 11254–11261. [Google Scholar] [CrossRef]
- Lowenstein, P.; Castro, M.G. Evolutionary basis of a new gene- and immune-therapeutic approach for the treatment of malignant brain tumors: From mice to clinical trials for glioma patients. Clin. Immunol. 2018, 189, 43–51. [Google Scholar] [CrossRef]
- Vilaboa, N.; Boellmann, F.; Voellmy, R.W. Gene Switches for Deliberate Regulation of Transgene Expression: Recent Advances in System Development and Uses. J. Genet. Syndr. Gene Ther. 2011, 2. [Google Scholar] [CrossRef]
- Barrett, J.A.; Cai, H.; Miao, J.; Khare, P.D.; Gonzalez, P.; Dalsing-Hernandez, J.; Sharma, G.; Chan, T.; Cooper, L.J.; Lebel, F. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 2018, 25, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Johnston, S.C.; Tang, J.; Stahl, M.; Tobin, J.F.; Somers, W.S. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J. 2000, 19, 3530–3541. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Smith, K.M.; McKinney, B.; Palmer, C.; Rosenfeld, S.; Lillehei, K.; Hamilton, A.; DeMasters, B.K.; Judy, K.; Kirn, D. A Phase I Trial of Ad.hIFN-β Gene Therapy for Glioma. Mol. Ther. 2008, 16, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Yung, W.K.; Prados, M.; Levin, V.A.; Fetell, M.R.; Bennett, J.; Mahaley, M.S.; Salcman, M.; Etcubanas, E. Intravenous Recombinant Interferon beta in Patients With Recurrent Malignant Gliomas: A Phase I/II Study. J. Clin. Oncol. 1991, 9, 1945–1949. [Google Scholar] [CrossRef]
- Breitbart, E.; Leubitz, A.; Feige, E.; Penson, R. Treatment Methods Using Adenovirus. WIPO Patent No. WO 2014/060848 A2, 24 April 2014. [Google Scholar]
- Artusi, S.; Miyagawa, Y.; Goins, W.F.; Cohen, J.B.; Glorioso, J.C. Herpes Simplex Virus Vectors for Gene Transfer to the Central Nervous System. Diseases 2018, 6, 74. [Google Scholar] [CrossRef]
- Gale, M.; Katze, M.G. Molecular Mechanisms of Interferon Resistance Mediated by Viral-Directed Inhibition of PKR, the Interferon-Induced Protein Kinase. Pharmacol. Ther. 1998, 78, 29–46. [Google Scholar] [CrossRef]
- Cheng, G.; Gross, M.; Brett, M.-E.; He, B. AlaArg Motif in the Carboxyl Terminus of the γ134.5 Protein of Herpes Simplex Virus Type 1 Is Required for the Formation of a High-Molecular-Weight Complex That Dephosphorylates eIF-2α. J. Virol. 2001, 75, 3666–3674. [Google Scholar] [CrossRef][Green Version]
- Mostafa, H.H.; Thompson, T.W.; Konen, A.J.; Haenchen, S.D.; Hilliard, J.G.; Macdonald, S.J.; Morrison, L.A.; Davido, D.J. Herpes Simplex Virus 1 Mutant with Point Mutations inUL39Is Impaired for Acute Viral Replication in Mice, Establishment of Latency, and Explant-Induced Reactivation. J. Virol. 2018, 92, 1–12. [Google Scholar] [CrossRef]
- Streby, K.A.; Geller, J.I.; Currier, M.A.; Warren, P.S.; Racadio, J.M.; Towbin, A.J.; Vaughan, M.R.; Triplet, M.; Ott-Napier, K.; Dishman, D.J.; et al. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin. Cancer Res. 2017, 23, 3566–3574. [Google Scholar] [CrossRef]
- Cassady, K.A.; Bauer, D.F.; Roth, J.; Chambers, M.R.; Shoeb, T.; Coleman, J.; Prichard, M.; Gillespie, G.Y.; Markert, J.M. Pre-clinical Assessment of C134, a Chimeric Oncolytic Herpes Simplex Virus, in Mice and Non-human Primates. Mol. Ther. Oncolytics 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Cinatl, J.; Michaelis, M.; Driever, P.H.; Činátl, J.; Hraběta, J.; Suhan, T.; Doerr, H.W.; Vogel, J.-U. Multimutated Herpes Simplex Virus G207 Is a Potent Inhibitor of Angiogenesis1. Neoplasia 2004, 6, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Nakashima, H.; Kasai, K.; Fernandez, S.A.; Oglesbee, M. Preclinical Toxicology of rQNestin34.5v.2: An Oncolytic Herpes Virus with Transcriptional Regulation of the ICP34.5 Neurovirulence Gene. Mol. Ther. Methods Clin. Dev. 2020, 17, 871–893. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Yoshimura, H.; Suzuki, T.; Ishiwata, T. Nestin: Neural Stem/Progenitor Cell Marker in Brain Tumors. In Evolution of the Molecular Biology of Brain Tumors and the Therapeutic Implications; IntechOpen: London, UK, 2013. [Google Scholar]
- Peters, C.; Rabkin, S.D. Designing herpes viruses as oncolytics. Mol. Ther. Oncolytics 2015, 2, 15010. [Google Scholar] [CrossRef]
- Strong, J.E.; Coffey, M.C.; Tang, D.; Sabinin, P.; Lee, P.W. The molecular basis of viral oncolysis: Usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998, 17, 3351–3362. [Google Scholar] [CrossRef]
- Werner, K. Use of a Virus Regimen for the Treatment of Diseases. WIPO Patent No. PCT/EP2009/003074, 12 November 2009. [Google Scholar]
- Biederer, C.; Ries, S.; Brandts, C.H.; McCormick, F. Replication-selective viruses for cancer therapy. J. Mol. Med. 2001, 80, 163–175. [Google Scholar] [CrossRef]
- Lazar, I.; Yaacov, B.; Shiloach, T.; Eliahoo, E.; Kadouri, L.; Lotem, M.; Perlman, R.; Zakay-Rones, Z.; Panet, A.; Ben-Yehuda, D. The Oncolytic Activity of Newcastle Disease Virus NDV-HUJ on Chemoresistant Primary Melanoma Cells Is Dependent on the Proapoptotic Activity of the Inhibitor of Apoptosis Protein Livin. J. Virol. 2009, 84, 639–646. [Google Scholar] [CrossRef]
- Marchini, A.; Bonifati, S.; Scott, E.M.; Angelova, A.L.; Rommelaere, J. Oncolytic parvoviruses: From basic virology to clinical applications. Virol. J. 2015, 12, 1–16. [Google Scholar] [CrossRef]
- Merrill, M.K.; Bernhardt, G.; Sampson, J.H.; Wikstrand, C.J.; Bigner, D.D.; Gromeier, M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro Oncol. 2004, 6, 208–217. [Google Scholar] [CrossRef]
- Perez, O.D.; Logg, C.R.; Hiraoka, K.; Diago, O.; Burnett, R.; Inagaki, A.; Jolson, D.; Amundson, K.; Buckley, T.; Lohse, D.; et al. Design and Selection of Toca 511 for Clinical Use: Modified Retroviral Replicating Vector With Improved Stability and Gene Expression. Mol. Ther. 2012, 20, 1689–1698. [Google Scholar] [CrossRef]
- Hogan, D.J.; Zhu, J.-J.; Diago, O.R.; Gammon, D.K.; Haghighi, A.; Lu, G.; Das, A.; Gruber, H.E.; Jolly, D.J.; Ostertag, D. Molecular Analyses Support the Safety and Activity of Retroviral Replicating Vector Toca 511 in Patients. Clin. Cancer Res. 2018, 24, 4680–4693. [Google Scholar] [CrossRef]
- Huang, T.T.; Hlavaty, J.; Ostertag, D.; Espinoza, F.L.; Martin, B.; Petznek, H.; Rodriguez-Aguirre, M.; E Ibanez, C.; Kasahara, N.; Gunzburg, W.; et al. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013, 20, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics 2019, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- E Hruby, D. Vaccinia virus vectors: New strategies for producing recombinant vaccines. Clin. Microbiol. Rev. 1990, 3, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Splinter, P.L.; Greiner, S.; Myers, R.; Peng, K.-W.; Federspiel, M.J.; Russell, S.J.; LaRusso, N.F. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology 2006, 44, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- A Database of Privately and Publicly Funded Clinical Studies Conducted Around the World (ClinicalTrials.com Database). Available online: https://clinicaltrials.gov/ (accessed on 5 November 2020).
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Alonso, M.M.; García-Moure, M.; Gonzalez-Huarriz, M.; Marigil, M.; Hernandez-Alcoceba, R.; Buñales, M.; Hervás, S.; Gallego, J.; Gomez-Manzano, C.; Fueyo, J.; et al. Abstract CT027: Oncolytic virus DNX-2401 with a short course of temozolomide for glioblastoma at first recurrence: Clinical data and prognostic biomarkers. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar] [CrossRef]
- Regeneron. Phase 1b open-label randomized study of the oncolytic adenovirus DNX- 2401 administered with or without interferon gamma for recurrent glioblastoma. J. Clin. Oncol. 2017, 35, 3008. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Abbed, K.M.; Tatter, S.; Louis, D.N.; Hochberg, F.H.; Barker, F.; Kracher, J.; Grossman, S.A.; Fisher, J.D.; Carson, K.; et al. A Phase I Open-Label, Dose-Escalation, Multi-Institutional Trial of Injection with an E1B-Attenuated Adenovirus, ONYX-015, into the Peritumoral Region of Recurrent Malignant Gliomas, in the Adjuvant Setting. Mol. Ther. 2004, 10, 958–966. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Manzanera, A.G.; Bell, S.D.; Cavaliere, R.; McGregor, J.M.; Grecula, J.C.; Newton, H.B.; Lo, S.S.; Badie, B.; Portnow, J.; et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro-oncology 2016, 18, 1137–1145. [Google Scholar] [CrossRef]
- Ji, N.; Weng, D.; Liu, C.; Gu, Z.; Chen, S.; Guo, Y.; Fan, Z.; Wang, X.; Chen, J.; Zhao, Y.; et al. Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget 2015, 7, 4369–4378. [Google Scholar] [CrossRef]
- Lowenstein, P.R.; A Orringer, D.; Sagher, O.; Heth, J.; Hervey-Jumper, S.L.; Mammoser, A.G.; Junck, L.; Leung, D.; Umemura, Y.; Lawrence, T.S.; et al. First-in-human phase I trial of the combination of two adenoviral vectors expressing HSV1-TK and FLT3L for the treatment of newly diagnosed resectable malignant glioma: Initial results from the therapeutic reprogramming of the brain immune system. J. Clin. Oncol. 2019, 37, 2019. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Yu, J.S.; Lukas, R.V.; Solomon, I.H.; Ligon, K.L.; Nakashima, H.; Triggs, D.A.; Reardon, D.A.; Wen, P.; Stopa, B.M.; et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci. Transl. Med. 2019, 11, eaaw5680. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.J.; Cohen, Y.; Vredenburgh, J.J.; Peters, K.B.; Breitbart, E.; Bangio, L.; Sher, N.; Harats, D.; Wen, P.Y. Phase I/II dose-escalation study of VB-111, an antiangiogenic gene therapy, in patients with recurrent glioblastoma multiforme. J. Clin. Oncol. 2013. [Google Scholar] [CrossRef]
- Cloughesy, T.; Brenner, A.; De Groot, J.F.; A Butowski, N.; Zach, L.; Campian, J.L.; Ellingson, B.M.; Freedman, L.S.; Cohen, Y.C.; Lowenton-Spier, N.; et al. A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 2019, 22, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Rampling, R.; Cruickshank, G.; Papanastassiou, V.; Nicoll, J.A.R.; Hadley, D.M.; Brennan, D.C.; Petty, R.; MacLean, A.; Harland, J.; A McKie, E.; et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000, 7, 859–866. [Google Scholar] [CrossRef]
- Papanastassiou, V.; Rampling, R.; Fraser, M.; Petty, R.; Hadley, D.; Nicoll, J.; Harland, J.; Mabbs, R.; Brown, M. The potential for efficacy of the modified (ICP 34.5−) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: A proof of principle study. Gene Ther. 2002, 9, 398–406. [Google Scholar] [CrossRef]
- Harrow, S.; Papanastassiou, V.; Harland, J.; Mabbs, R.; Petty, R.D.; Fraser, M.J.; Hadley, D.M.; Patterson, J.; Brown, S.M.; Rampling, R. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: Safety data and long-term survival. Gene Ther. 2004, 11, 1648–1658. [Google Scholar] [CrossRef]
- Markert, J.M.; Medlock, M.D.; Rabkin, S.D.; Gillespie, G.Y.; Todo, T.; Hunter, W.D.; A Palmer, C.; Feigenbaum, F.; Tornatore, C.; Tufaro, F.; et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000, 7, 867–874. [Google Scholar] [CrossRef]
- Markert, J.M.; Liechty, P.G.; Wang, W.; Gaston, S.; Braz, E.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Lakeman, A.D.; A Palmer, C.; et al. Phase Ib Trial of Mutant Herpes Simplex Virus G207 Inoculated Pre-and Post-tumor Resection for Recurrent GBM. Mol. Ther. 2009, 17, 199–207. [Google Scholar] [CrossRef]
- Markert, J.M.; Razdan, S.N.; Kuo, H.-C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A Phase 1 Trial of Oncolytic HSV-1, G207, Given in Combination With Radiation for Recurrent GBM Demonstrates Safety and Radiographic Responses. Mol. Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef]
- A Forsyth, P.; Roldán, G.; George, D.J.; Wallace, C.; Palmer, C.A.; Morris, D.; Cairncross, G.; Matthews, M.V.; Markert, J.M.; Gillespie, Y.; et al. A Phase I Trial of Intratumoral Administration of Reovirus in Patients with Histologically Confirmed Recurrent Malignant Gliomas. Mol. Ther. 2008, 16, 627–632. [Google Scholar] [CrossRef]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Geletneky, K.; Hüsing, J.; Rommelaere, J.; Schlehofer, J.; Leuchs, B.; Dahm, M.; Krebs, O.; Doeberitz, M.V.K.; Huber, B.; Hajda, J. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer 2012, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.I.; Zakay-Rones, Z.; Gomori, J.M.; Linetsky, E.; Rasooly, L.; Greenbaum, E.; Rozenman-Yair, S.; Panet, A.; Libson, E.; Irving, C.S.; et al. Phase I/II Trial of Intravenous NDV-HUJ Oncolytic Virus in Recurrent Glioblastoma Multiforme. Mol. Ther. 2006, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Ii, J.E.H.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Petrecca, K.; Walbert, T.; Butowski, N.; Salacz, M.; Perry, J.; Damek, D.; Bota, D.; Bettegowda, C.; Zhu, J.-J.; et al. Effect of Vocimagene Amiretrorepvec in Combination With Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients With Recurrent High-Grade Glioma: A Randomized Clinical Trial. JAMA Oncol. 2020, 33612. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Hogan, D.J.; Bloomfield, S.; Carter, B.; Chen, C.C.; Elder, J.B.; Kalkanis, S.N.; Kesari, S.; Lai, A.; et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016, 8, 341ra75. [Google Scholar] [CrossRef]
- Jolly, D.J.; Robbins, J.M.; Ostertag, D.; Ibañez, C.; Kasahara, N.; Gruber, H.; Kalkanis, S.N.; Vogelbaum, M.; Aghi, M.K.; Cloughesy, T.; et al. 61. Ascending Dose Trials of a Retroviral Replicating Vector (Toca 511) in Patients with Recurrent High-Grade Glioma: Clinical Update, Molecular Analyses, and Proposed Mechanism of Action. Mol. Ther. 2016, 24, S27. [Google Scholar] [CrossRef]
- Cloughesy, T.; Landolfi, J.; A Vogelbaum, M.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology 2018, 20, 1383–1392. [Google Scholar] [CrossRef]
- Kalkanis, S.N.; Aghi, M.K.; Cloughsy, T.F.; Kaptain, G.; Portnow, J.; Vogelbaum, M.A.; Kesari, S.; Mikkelsen, T.; Elder, J.B.; Chen, C.C.; et al. DDEL-06 Preliminary Safety of Toca 511, a Retroviral Replicating Vector, in Patients with Recurrent High Grade Glioma across Three Separate Phase 1 Studies. Neuro Oncol. 2015, 17, v74. [Google Scholar] [CrossRef]
- Lyle, C.; McCormick, F. Integrin αvβ5 is a primary receptor for adenovirus in CAR-negative cells. Virol. J. 2010, 7, 148. [Google Scholar] [CrossRef]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Lane, S.; Korsak, A.; Paton, J.F.R.; Gourine, A.V.; Kasparov, S.; Teschemacher, A.G. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 2014, 5, 3284. [Google Scholar] [CrossRef] [PubMed]
- Duale, H.; Kasparov, S.; Paton, J.F.R.; Teschemacher, A.G. Differences in transductional tropism of adenoviral and lentiviral vectors in the rat brainstem. Exp. Physiol. 2005, 90, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Paton, J.F.R.; Kasparov, S. Viral vectors based on bidirectional cell-specific mammalian promoters and transcriptional amplification strategy for use in vitro and in vivo. BMC Biotechnol. 2008, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Li, H.-P.; Hung, Y.-H.; Leu, Y.-W.; Wu, W.-H.; Wang, F.-S.; Lee, K.-D.; Chang, P.-J.; Wu, C.-S.; Lu, Y.-J.; et al. Targeted methylation of CMV and E1A viral promoters. Biochem. Biophys. Res. Commun. 2010, 402, 228–234. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Tian, Z.; Zhang, X.; Xu, D.; Li, Q.; Zhang, J.; Wang, T. The EF-1α promoter maintains high-level transgene expression from episomal vectors in transfected CHO-K1 cells. J. Cell. Mol. Med. 2017, 21, 3044–3054. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D. Glioblastoma Heterogeneity and Cancer Cell Plasticity. Crit. Rev. Oncog. 2014, 19, 327–336. [Google Scholar] [CrossRef]
- Sharma, P.; Khuc, K. Summary Basis for Regulatory Action. 2018. Available online: https://www.fda.gov/media/125157/download (accessed on 10 October 2020).
- Al-Zaidy, S.A.; Mendell, J.R. From Clinical Trials to Clinical Practice: Practical Considerations for Gene Replacement Therapy in SMA Type 1. Pediatr. Neurol. 2019, 100, 3–11. [Google Scholar] [CrossRef]
- Chan, K.Y.; Jang, M.J.; Yoo, B.B.; Greenbaum, A.; Ravi, N.; Wu, W.-L.; Sánchez-Guardado, L.; Lois, C.; Mazmanian, S.K.; E Deverman, B.; et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017, 20, 1172–1179. [Google Scholar] [CrossRef]

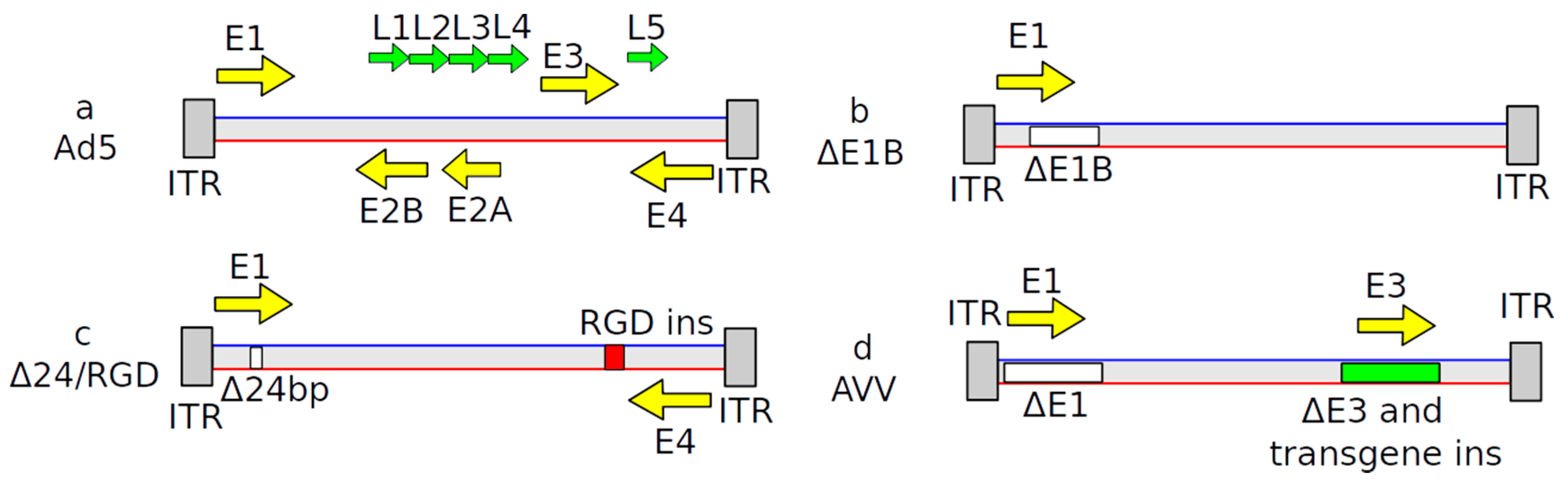
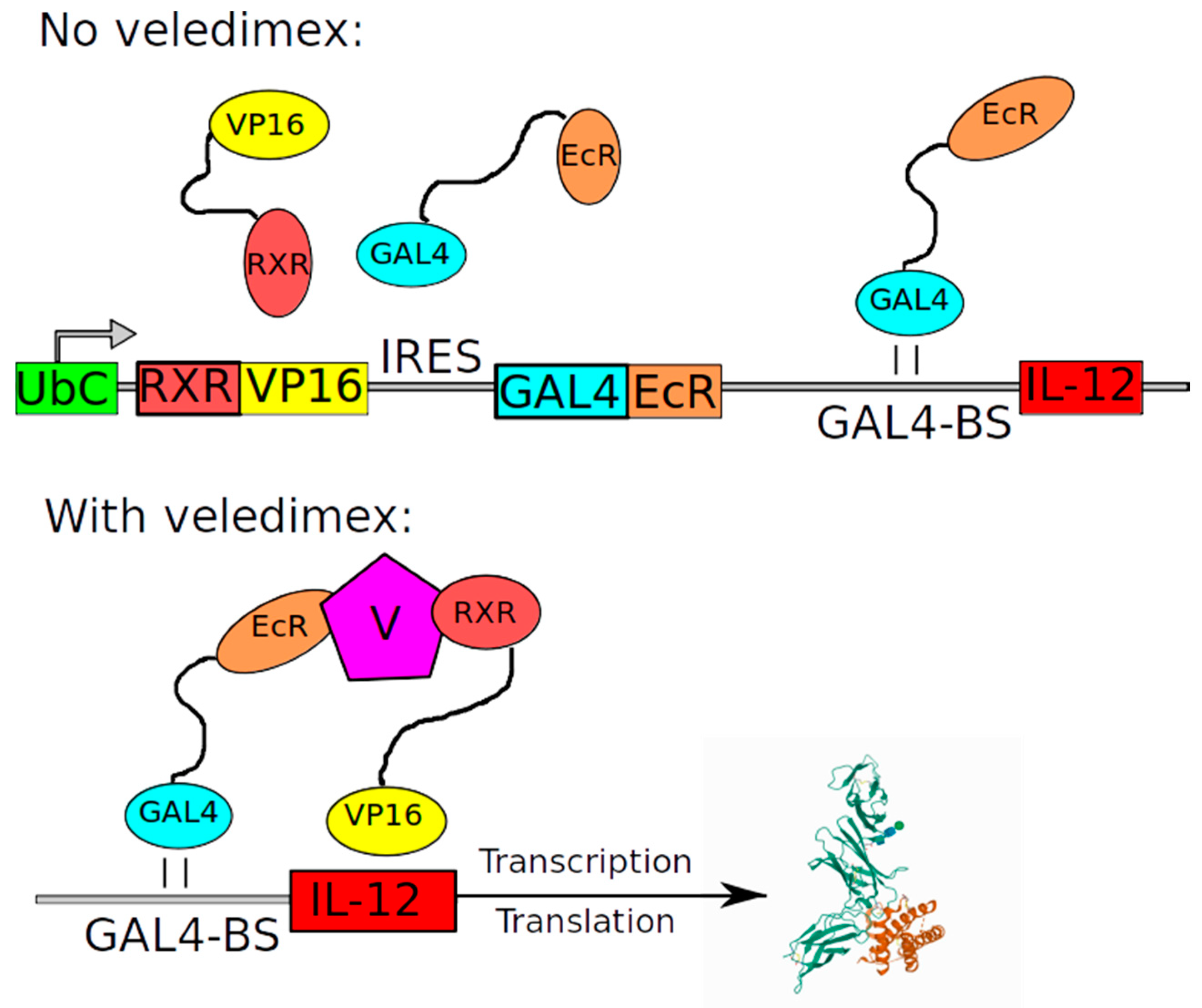
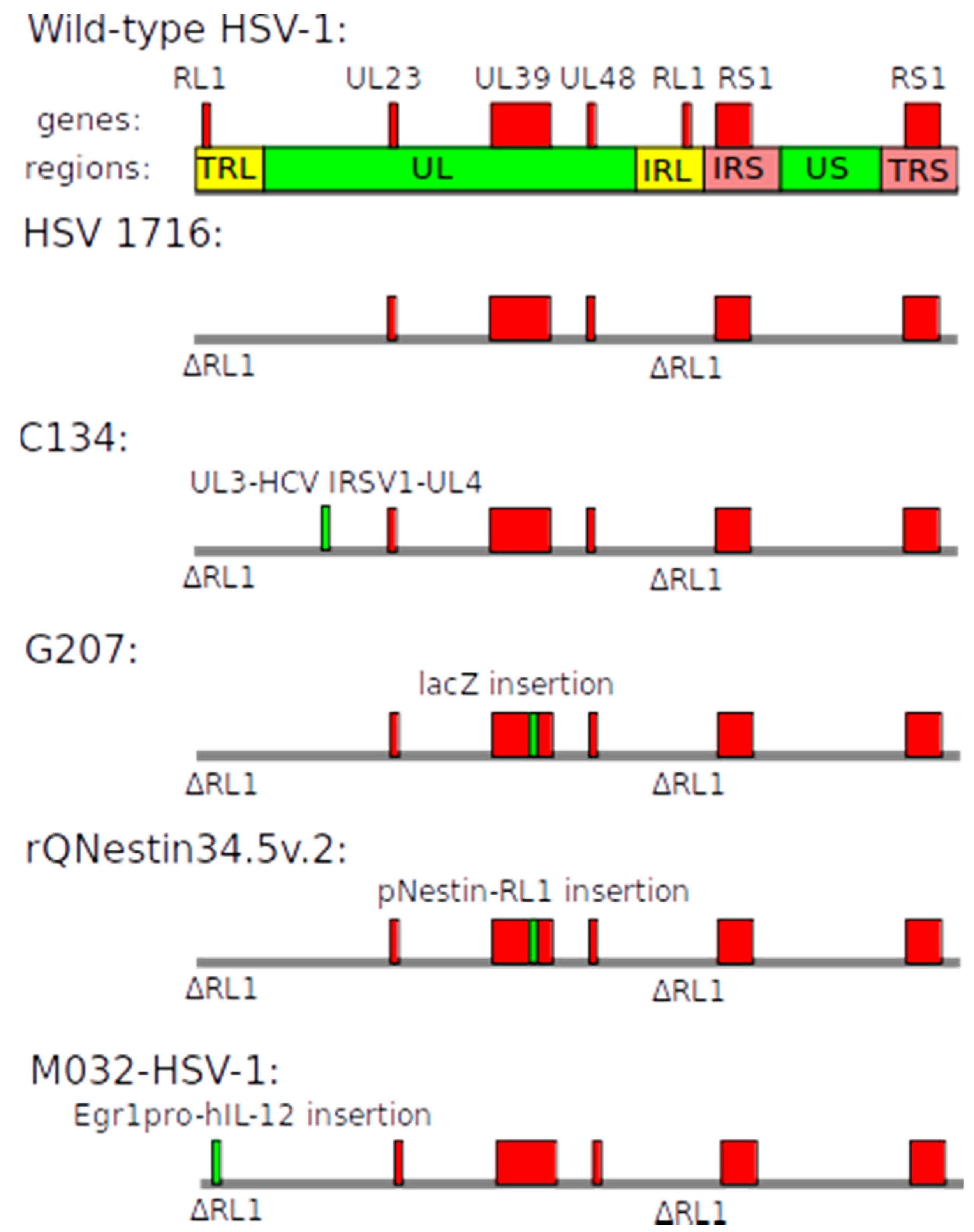
| Name | Structure of Vector | Mechanism of Action | Specificity | Replication Competent |
|---|---|---|---|---|
| DNX2401 | Ad5 | Lytic viral cycle in targeted cells | Replicate in cells defective in the Rb/p16 tumor suppressor pathway and expressing integrins αvβ3 and αvβ5 | ± |
| DNX2440 | Ad5 | Lytic viral cycle in targeted cells and immunomodulatory effect | Replicate in cells defective in the Rb/p16 tumor suppressor pathway and expressing integrins αvβ3 and αvβ5 | ± |
| ONYX-015 | chimeric Ad2 and Ad5 | Lytic viral cycle in targeted cells | Replicate in tumor cells with altered p53 pathway | ± |
| Ad-hCMV-TK | Ad5 | Converts harmless ganciclovir to toxic product in transduced cells | Transduce CAR-expressing cells. CMV-dependent expression mechanism | − |
| ADV/HSV-tk | Ad5 | Converts harmless ganciclovir to toxic product in transduced cells | Transduce CAR-expressing cells. RSV-dependent expression mechanism | − |
| Ad-hCMV-Flt3L | Ad5 | Immunomodulatory effect by stimulating both the proliferation of dendritic cells (DCs) and their migration to the tumor site | Transduce CAR expressing cells | − |
| Ad-RTS-hIL12 | Ad5 | Immunomodulatory effect by activation of immune system via IL-12 release | Transduce CAR-expressing cells | − |
| Ad.hIFN-β | Ad5 | Immunomodulatory effect by activation of immune system via human Interferon-β release | Transduce CAR-expressing cells | − |
| VB-111 | Ad5 | Decrease excessive angiogenesis via inhibition of endothelial cells | Transduce CAR-expressing cells, promotor initializes transcription only in endothelial cells undergoing angiogenesis | − |
| HSV 1716 | HSV-1 | Lytic viral cycle in targeted cells and indirect T cell-mediated cell death | Replication in PKR-deficient cells | ± |
| G207 | HSV-1 | Lytic viral cycle in targeted cells and indirect T cell-mediated cell death | Replication in PKR-deficient and fast dividing cells | ± |
| C134 | HSV-1 | Lytic viral cycle in targeted cells and indirect T cell-mediated cell death | Replication in PKR-deficient and fast dividing cells | ± |
| rQNestin34.5v.2 | HSV-1 | Lytic viral cycle in targeted cells and indirect T-cell mediated cell death | Replication in PKR-deficient, Nestin-positive and fast dividing cells | ± |
| M032-HSV-1 | HSV-1 | Lytic viral cycle in targeted cells, indirect T-cell mediated cell death and immune system stimulation via IL12 release | Replication in PKR-defective and fast dividing cells | ± |
| Pelareorep (Reolysin) | Wild-type reovirus | Lytic viral cycle in targeted cells | Replication in ras-positive cells | + |
| ParvOryx | Wild-type parvovirus | Lytic viral cycle in targeted cells | Replication in fast dividing cells | + |
| NDV-HUJ | Wild-type HUJ strain of Newcastle disease virus | Livin-mediated apoptosis | Replication in fast dividing cells, apoptosis of livin-positive cells | + |
| PVSRIPO | Recombinant poliovirus type 1 | Lytic viral cycle in targeted cells | Replication restricted to CD155-expressing non-neuronal cells | + |
| Toca 511 | Recombinant Gammaretrovirus | CD-mediated prodrug conversion to cytotoxic drug in transduced cells | Replication in fast dividing cells | + |
| TG6002 | Recombinant vaccinia virus | Lytic viral cycle in targeted cells, CD-mediated prodrug conversion | Replication in cells expressing ribonucleotide reductase | + |
| MV-CEA | Recombinant measles virus | Lytic viral cycle in targeted cells | Transduce CD46-expressing cells | + |
| Vector | A Unique Identification Code Given to Clinical Study Registered on ClinicalTrials.gov | Study Date | Study Type (Safety/Trials in Recurrent GBM/Trials in Newly Diagnosed GBM) | Results/Comments |
|---|---|---|---|---|
| DNX2401 | NCT00805376 | 2008 | Dose-escalation study in recurrent GBM | Reported in 2018: DNX-2401 is safe, improves clinical outcome. Post-treatment histology examination of biopsy revealed sites of necrosis in GBM [64]. |
| - | NCT01582516 | 2012 | Dose-escalation study in recurrent GBM | No posted results. |
| - | NCT01956734 | 2013 | Safety and efficacy study in recurrent GBM DNX2401 + TMZ vs. TMZ alone | Reported in 2017: The safety objective of the trial was achieved with no severe toxicities related to DNX-2401 [65]. |
| - | NCT02197169 | 2014 | Safety and efficacy study in recurrent GBM, DNX2401 + IFN vs. DMX2401 alone | Reported in 2017: DNX-2401 was well tolerated as monotherapy. The addition of interferon did not improve survival [66]. |
| - | NCT02798406 | 2016 | Safety and efficacy study in recurrent GBM, DNX2401 + pembrolizumab | No posted results. |
| DNX2440 | NCT03714334 | 2018 | Safety and efficacy study in recurrent GBM, DNX2440 alone | No posted results. |
| ONYX-015 | Was not registered at ClinicalTrials.gov | - | Dose-escalation study | Reported in 2004: None of the 24 patients experienced serious adverse events related to ONYX-015 [67]. |
| ADV/HSV-tk | NCT00589875 | 2008 | Study of AdV-tk + valacyclovir Gene therapy in combination with standard radiation therapy for malignant glioma | Reported in 2016: Addition of ADV/HSV-tk to SoC improves outcome [68]. |
| - | NCT00870181 | 2009 | Safety and efficacy of intravenous-administered ADV/HSV-tk in recurrent GBM vs. surgery or systemic chemotherapy or palliative care | Reported in 2016: ADV/HSV-tk is safe and can provide benefits [69]. |
| - | NCT03603405 | 2018 | Safety and efficacy study of standard treatment + ADV/HSV-tk in newly diagnosed GBM | No results posted. |
| - | NCT03596086 | 2018 | Safety and efficacy of ADV/HSV-tk in recurrent GBM | No results posted. |
| Ad-hCMV-Flt3L + 4. Ad-hCMV-TK (combination) | NCT01811992 | 2013 | Dose-escalation study in newly diagnosed GBM + standard treatment | Reported in 2019: Examination of tumor samples reveals increase in the infiltration of inflammatory cells. Preliminary data suggest that virotherapy can improve outcomes [70]. |
| Ad-RTS-hIL12 | NCT02026271 | 2014 | Safety and tolerability of a single tumor injection of Ad-RTS-hIL-12 given with oral veledimex (the activator of RTS promoter) in patients with recurrent or progressive GBM | Reported in 2019: The clinical trial demonstrated tolerability of veledimex-induced hIL-12 expression [71]. |
| - | NCT04006119 | 2019 | Safety and efficacy of intratumoral Ad-RTS-hIL-12 and oral veledimex in combination with cemiplimab-rwlc in patients with recurrent or progressive GBM | No results posted. |
| Ad.hIFN-β | Was not registered | - | Dose-escalation study | Reported in 2008: The most common adverse events were considered by the investigator as being unrelated to treatment [38]. |
| VB-111 | NCT01260506 | 2010 | Dose-escalation study of VB-111 in combination with bevacizumab in recurrent GBM. | Reported in 2013: VB-111 was safe and well tolerated in patients with recurrent GBM with repeat doses of up to 1 × 1013 VPs. Tumor responses were seen [72]. |
| - | NCT02511405 | 2015 | Comparison of VB-111 plus bevacizumab to bevacizumab in patients with recurrent GBM | Reported in 2020: Upfront concomitant administration of VB-111 and bevacizumab failed to improve outcomes [73]. |
| HSV 1716 | Was not registered | - | Safety and feasibility of intratumoral administration of HSV1716 | Reported in 2000: HSV1716 is safe when injected into sites around the post-resection tumor cavity [74]. |
| - | Was not registered | - | Efficacy of HSV1716 | Reported in 2002: HSV1716 replicates in HGG without causing toxicity [75]. |
| - | Was not registered | - | Efficacy of HSV1716 | Reported in 2004:Study demonstrates that HSV1716 injections can provide benefits [76]. |
| G207 | Was not registered | - | Dose-escalation study | Reported in 2000: No viral-related toxicity; evidence of antitumor activity. While adverse events were noted in some patients, no toxicity or serious adverse events could unequivocally be ascribed to G207 [77]. |
| - | NCT00028158 | 2001 | Dose-escalation study. Doses 1E9, 3E9 and 1E10 pfu were tested | Reported in 2009:No encephalitis; evidence of antitumor activity and viral replication [78]. |
| - | NCT00157703 | 2005 | De-escalation study. First patients received the highest dose (1E10 pfu). and if excessive toxicity had occurred, the dose would be reduced for the following patients | As reported in 2014: Treatment was well tolerated with signs of improving outcomes [79]. |
| C134 | NCT03657576 | 2018 | Dose-escalation study in recurrent/progressive GBM, anaplastic astrocytoma, or gliosarcoma | No results posted. |
| rQNestin34.5v.2 | NCT03152318 | 2017 | Dose-escalation study of in patients with recurrent GBM | No results posted. |
| M032-HSV-1 | NCT02062827 | 2014 | Dose escalation in recurrent/progressive GBM, anaplastic astrocytoma or gliosarcoma | No results posted. |
| Pelareorep (Reolysin) | NCT02444546 | 2015 | Dose-escalation study of Pelareorep in combination with sargramostim in recurrent/progressive GBM | No results posted. |
| - | NCT00528684 | 2007 | Dose-escalation study of Pelareorep in recurrent GBM | Reported in 2008: The intratumoral administration of the genetically unmodified reovirus was well tolerated using these doses and schedule in patients with recurrent GBM [80]. |
| ParvOryx | NCT01301430 | 2011 | Dose-escalation study of ParvOryx in patients with progressive or recurrent GBM | Reported in 2012 and 2017: No dose-limiting toxicity was reported but clinical response did not depend on the dose or mode of ParvOryx administration. No statistical confirmation of efficacy [81,82]. |
| NDV-HUJ | Was not registered | - | Dose-escalation study of NDV-HUJ | Reported in 2006: Toxicity was minimal with Grade I/II constitutional fever being seen in five patients. Maximum tolerated dose was not achieved [83]. |
| - | NCT01174537 | 2010 | Safety and efficacy of single dose intravenously administered | No results posted. |
| PVSRIPO | NCT02986178 | 2016 | Safety and efficacy of single dose PVSRIPO administered intratumorally in patients with recurrent GBM | No results posted. |
| - | NCT03973879 | 2019 | Safety and efficacy of single dose PVSRIPO administered intratumorally with atezolizumab treatment in patients with recurrent GBM | Withdrawn. |
| - | NCT01491893 | 2011 | Dose-escalation study of PVSRIPO administered intratumorally in patients with recurrent GBM | Reported in 2018: Intratumoral infusion of PVSRIPO in patients with recurrent WHO grade IV malignant glioma confirmed the absence of neurovirulent potential [84]. |
| Toca 511 | NCT04105374 | 2019 | Toca 511, Toca FC and standard of care vs. standard of care in newly diagnosed GBM | Withdrawn. |
| - | NCT02414165 | 2015 | Toca 511/Toca FC vs. Lomustine, Temozolomide, or Bevacizumab in recurrent GBM | Reported in 2020: administration of Toca 511 and Toca FC, compared with SoC, did not improve overall survival (11.10 months vs. 12.22 months, respectively) or other end points [85]. |
| - | NCT01470794 | 2011 | Dose-escalation study of Toca 511/Toca FC administered by injections into resection cavity wall in patients with recurrent GBM | Reported in 2016, 2016, 2018: Toca 511/Toca FC is safe and can provide durable complete response in some patients [86,87,88]. |
| - | NCT01156584 | 2010 | Dose-escalation study of Toca 511/Toca FC administered by intratumoral injections in patients with recurrent GBM | Reported in 2015, 2016:Safe and well tolerated [87,88,89]. |
| - | NCT01985256 | 2013 | Dose-escalation study of Toca 511/Toca FC administered by intravenously in patients with recurrent GBM | Reported in 2016: Injections were well tolerated [87]. |
| TG6002 | NCT03294486 | 2017 | Dose-escalation study of TG6002 in patients with recurrent GBM | No results posted. |
| MV-CEA | NCT00390299 | 2006 | Dose-escalation study of MV-CEA in patients with recurrent GBM | No results posted. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozhei, O.; G. Teschemacher, A.; Kasparov, S. Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma. Cancers 2020, 12, 3724. https://doi.org/10.3390/cancers12123724
Mozhei O, G. Teschemacher A, Kasparov S. Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma. Cancers. 2020; 12(12):3724. https://doi.org/10.3390/cancers12123724
Chicago/Turabian StyleMozhei, Oleg, Anja G. Teschemacher, and Sergey Kasparov. 2020. "Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma" Cancers 12, no. 12: 3724. https://doi.org/10.3390/cancers12123724
APA StyleMozhei, O., G. Teschemacher, A., & Kasparov, S. (2020). Viral Vectors as Gene Therapy Agents for Treatment of Glioblastoma. Cancers, 12(12), 3724. https://doi.org/10.3390/cancers12123724








