CA125 and Ovarian Cancer: A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Prognosis and Survival in Ovarian Cancer
3. Early Detection of Ovarian Cancer
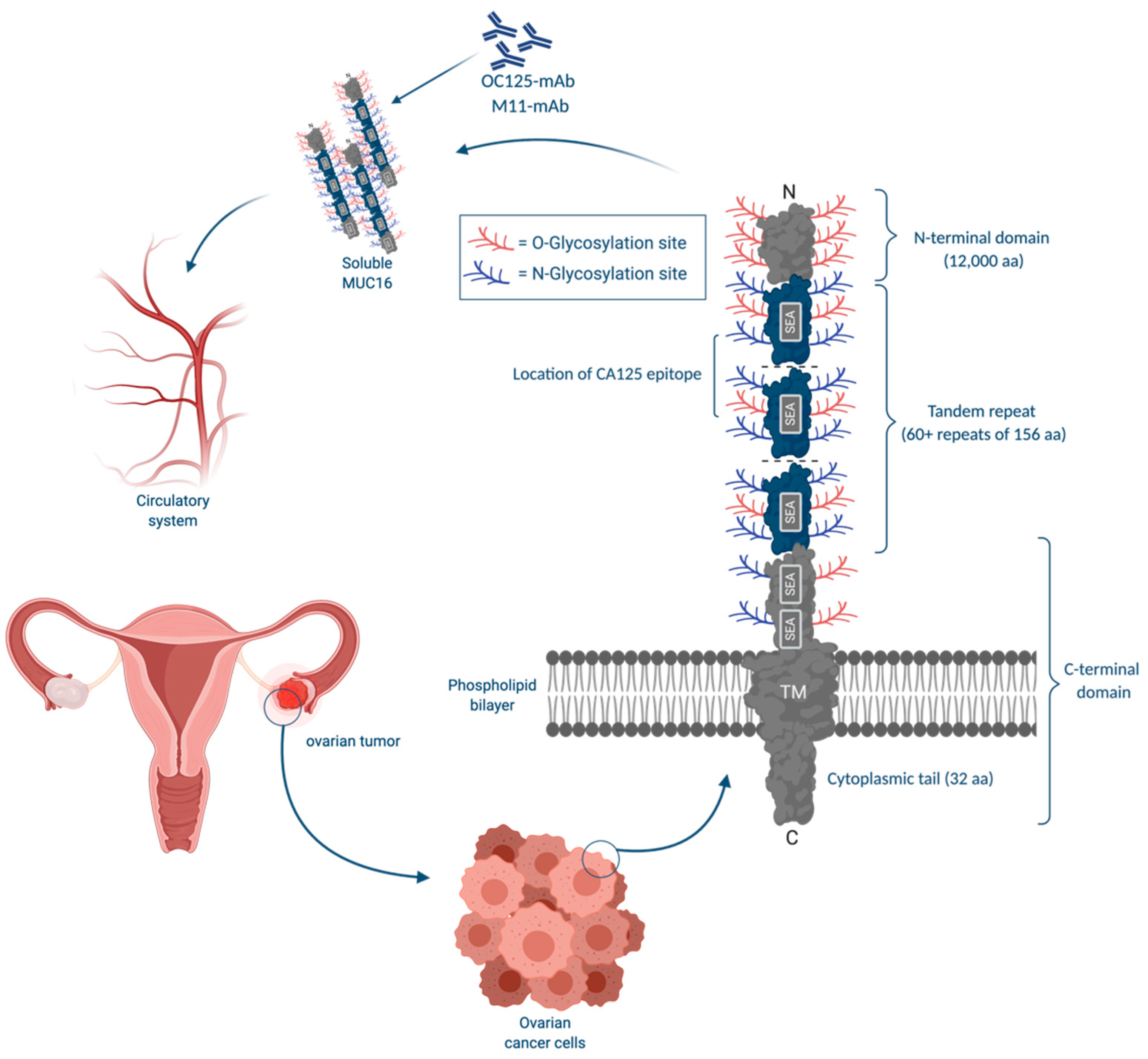
3.1. CA125
3.1.1. CA125: Physiological Functions
3.1.2. Factors Influencing Serum CA125 Concentrations
3.1.3. CA125 in Combination with Other Biomarkers
3.1.4. Biomarker Based Algorithms Involving CA125
3.1.5. Modifications of CA125 Cutoff Value
4. Effect of Ovarian Cancer Screening with CA125 on Mortality
5. Recurrent Ovarian Cancer and CA125
5.1. CA125: Early Detection of Relapse
5.2. CA125: Prognostic Value
6. Future Directions: Ovarian Cancer Screening
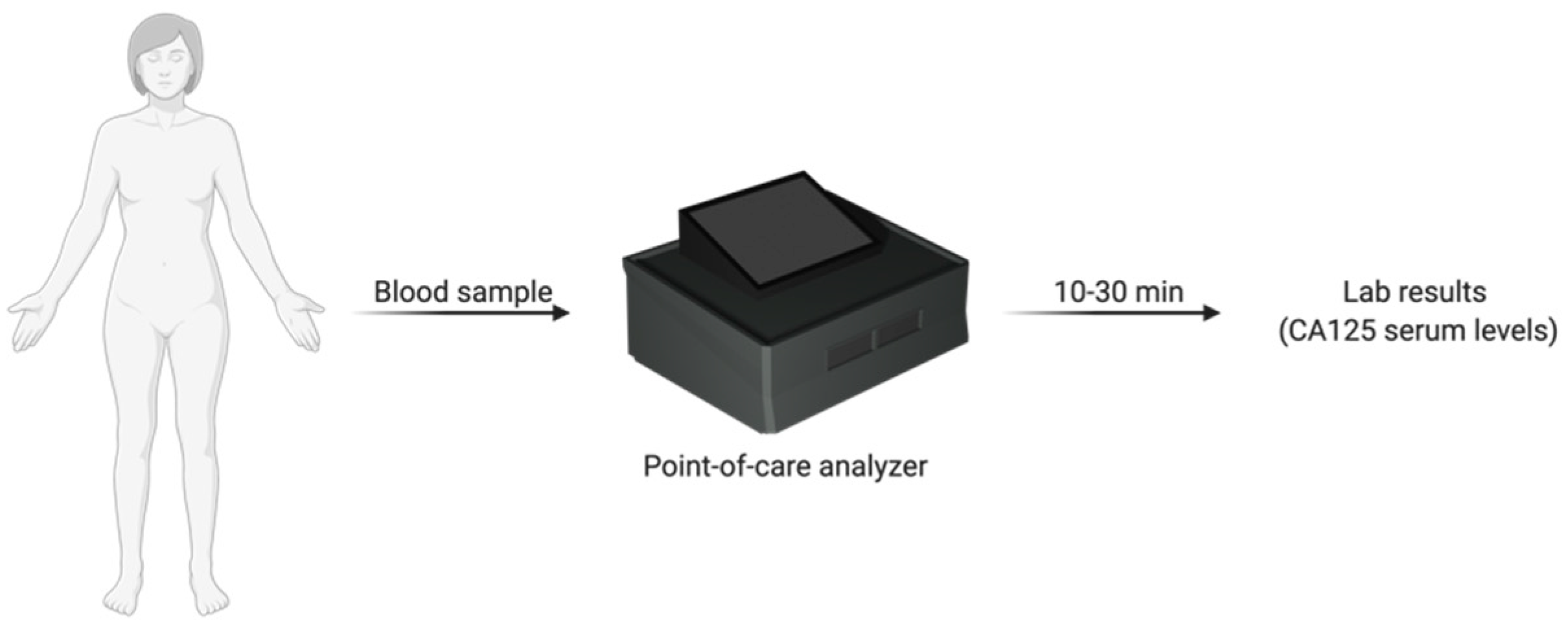
Chip-Based and Cartridge-Based Biosensors
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Zeppernick, F.; Meinhold-Heerlein, I.; Meinhold-Heerlein, Á.I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch. Gynecol Obs. 2014, 290, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.B.; Herzog, T.J.; Lewin, S.N.; Deutsch, I.; Sun, X.; Burke, W.M.; Wright, J.D. Natural history and outcome of mucinous carcinoma of the ovary. Am. J. Obstet. Gynecol. 2011, 205, 480.e1–480.e8. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020.
- Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar] [CrossRef]
- Alsop, K.; Fereday, S.; Meldrum, C.; DeFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian ovarian cancer study group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef]
- Moschetta, M.; George, A.; Kaye, S.B.; Banerjee, S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann. Oncol. 2016, 27, 1449–1455. [Google Scholar] [CrossRef]
- Enakpene, C.A.; Omigbodun, A.O.; Goecke, T.W.; Odukogbe, A.T.; Beckmann, M.W. Preoperative evaluation and triage of women with suspicious adnexal masses using risk of malignancy index. J. Obstet. Gynaecol. Res. 2009, 35, 131–138. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Hunn, J.; Rodriguez, G.C. Ovarian cancer: Etiology, risk factors, and epidemiology. Clin. Obs. Gynecol. 2012, 55, 3–23. [Google Scholar] [CrossRef]
- Mehra, K.; Mehrad, M.; Chen, E.; Ning, G.; Drapkin, R.; McKeon, F.D.; Xian, W.; Crum, C.P. STICS, SCOUTs and p53 signatures; A new language for pelvic serous carcinogenesis. Front. Biosci. 2011, 3, 625–634. [Google Scholar]
- Kim, J.; Coffey, D.M.; Ma, L.; Matzuk, M.M. The ovary is an alternative site of origin for high-grade serous ovarian cancer in mice. Endocrinology 2015, 156, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.J.; Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Behbakht, K.; Chen, L.M.; Copeland, L.; Crispens, M.A.; De Rosa, M.; Dorigo, O.; et al. Ovarian cancer, version 1.2016: Clinical practice guidelines in oncology. JNCCN J. Natl. Compr. Cancer Netw. 2016, 14, 1134–1163. [Google Scholar] [CrossRef] [PubMed]
- Prahm, K.P.; Karlsen, M.A.; Høgdall, E.; Scheller, N.M.; Lundvall, L.; Nedergaard, L.; Christensen, I.J.; Høgdall, C. The prognostic value of dividing epithelial ovarian cancer into type I and type II tumors based on pathologic characteristics. Gynecol. Oncol. 2015, 136, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Utrilla-Layna, J.; Mínguez, J.Á.; Jurado, M. Clinical and ultrasound features of type I and type II epithelial ovarian cancer. Int. J. Gynecol. Cancer 2013, 23, 680–684. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer. shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. The dualistic model of ovarian carcinogenesis revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef]
- Van Haaften-Day, C.; Shen, Y.; Xu, F.; Yu, Y.; Berchuck, A.; Havrilesky, L.J.; de Bruijn, H.W.A.; van der Zee, A.G.J.; Bast, R.C.; Hacker, N.F. OVXL, macrophage-colony stimulating factor, and CA-125-II as tumor markers for epithelial ovarian carcinoma a critical appraisal. Cancer 2001, 92, 2837–2844. [Google Scholar] [CrossRef]
- Nustad, K.; Bast, R.C.; O’brien, T.J.; Nilsson, O.; Seguin, P.; Suresh, M.R.; Saga, T.; Nozawa, S.; Børmer, O.P.; de Bruijn, H.W.A.; et al. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: First report from the ISOBM TD-1 workshop. Tumor. Biol. 1996, 17, 196–219. [Google Scholar] [CrossRef]
- Henderson, J.T.; Webber, E.M.; Sawaya, G.F. Screening for ovarian cancer updated evidence report and systematic review for the US preventive services task force. JAMA J. Am. Med. Assoc. 2018, 319, 595–606. [Google Scholar] [CrossRef]
- Cress, R.D.; Chen, Y.S.; Morris, C.R.; Petersen, M.; Leiserowitz, G.S. Characteristics of long-term survivors of epithelial ovarian cancer. Obs. Gynecol. 2015, 126, 491–497. [Google Scholar] [CrossRef]
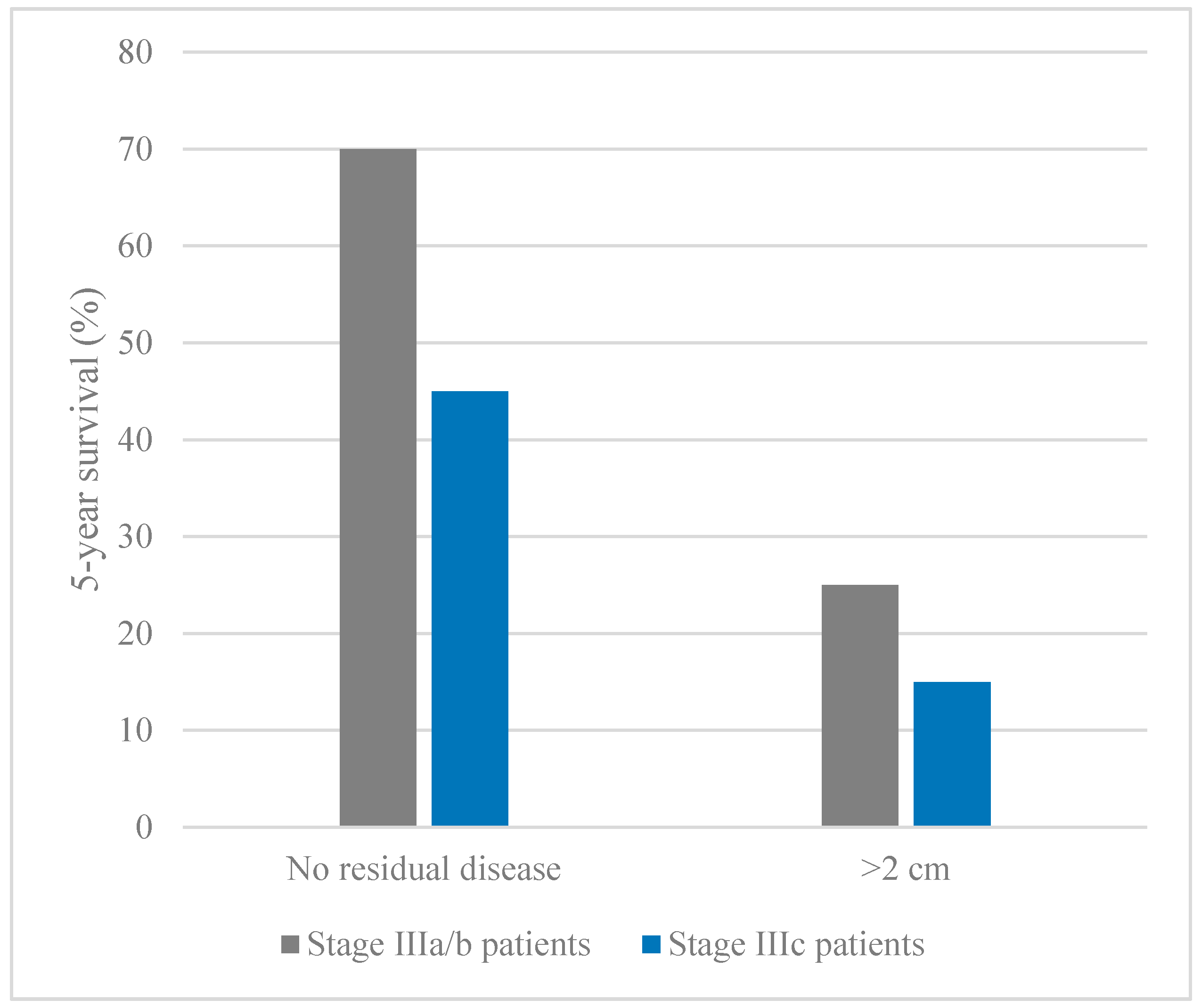
- Chi, D.S.; Musa, F.; Dao, F.; Zivanovic, O.; Sonoda, Y.; Leitao, M.M.; Levine, D.A.; Gardner, G.J.; Abu-Rustum, N.R.; Barakat, R.R. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol. Oncol. 2012, 124, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.; Laframboise, S.; Ferguson, S.; Dodge, J.; Bernardini, M.; Murphy, J.; Segev, Y.; Sun, P.; Narod, S.A. The impacts of neoadjuvant chemotherapy and of debulking surgery on survival from advanced ovarian cancer. Gynecol. Oncol. 2014, 134, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Eisenhauer, E.L.; Lang, J.; Huh, J.; Haddad, L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Hensley, M.; Barakat, R.R. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol. Oncol. 2006, 103, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Makar, A.P.; Baekelandt, M.; Tropé, C.O.; Kristensen, G.B. The prognostic significance of residual disease, figo substage, tumor histology, and grade in patients with figo stage III ovarian cancer. Gynecol. Oncol. 1995, 56, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef]
- Horowitz, N.S.; Miller, A.; Rungruang, B.; Richard, S.D.; Rodriguez, N.; Bookman, M.A.; Hamilton, C.A.; Krivak, T.C.; Maxwell, G.L. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: An analysis of GOG 182. J. Clin. Oncol. 2015, 33, 937–943. [Google Scholar] [CrossRef]
- Amate, P.; Huchon, C.; Dessapt, A.L.; Bensaid, C.; Medioni, J.; Le Frère Belda, M.A.; Bats, A.S.; Lécuru, F.R. Ovarian cancer: Sites of recurrence. Int. J. Gynecol. Cancer 2013, 23, 1590–1596. [Google Scholar] [CrossRef]
- Corrado, G.; Salutari, V.; Palluzzi, E.; Distefano, M.G.; Scambia, G.; Ferrandina, G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev. Anticancer Ther. 2017, 17, 1147–1158. [Google Scholar] [CrossRef]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Subtypes of ovarian cancer and ovarian cancer screening. Diagnostics 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Piek, J.M.J.; Van Diest, P.J.; Zweemer, R.P.; Jansen, J.W.; Poort-Keesom, R.J.J.; Menko, F.H.; Gille, J.J.P.; Jongsma, A.P.M.; Pals, G.; Kenemans, P.; et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J. Pathol. 2001, 195, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Sopik, V.; Rosen, B.; Giannakeas, V.; Narod, S.A. Why have ovarian cancer mortality rates declined? Part III. Prospects for the future. Gynecol. Oncol. 2015, 138, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef]
- Roy, H.K.; Khandekar, J.D. Biomarkers for the early detection of cancer: An inflammatory concept. Arch. Intern. Med. 2007, 167, 1822–1824. [Google Scholar] [CrossRef]
- Clarke-Pearson, D.L. Clinical practice. Screening for ovarian cancer. N. Engl. J. Med. 2009, 361, 170–177. [Google Scholar] [CrossRef]
- Nossov, V.; Amneus, M.; Su, F.; Lang, J.; Janco, J.M.T.; Reddy, S.T.; Farias-Eisner, R. The early detection of ovarian cancer: From traditional methods to proteomics. Can we really do better than serum CA-125? Am. J. Obs. Gynecol. 2008, 199, 215–223. [Google Scholar] [CrossRef]
- Bast, J.; Xu, F.J.; Yu, Y.H.; Barnhill, S.; Zhang, Z.; Mills, G.B. CA 125: The past and the future. Int. J. Biol. Markers 1998, 13, 179–187. [Google Scholar] [CrossRef]
- Jacobs, I.; Prys Davies, A.; Bridges, J.; Stabile, I.; Fay, T.; Lower, A.; Grudzinskas, J.G.; Oram, D. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. Br. Med. J. 1993, 306, 1030–1034. [Google Scholar] [CrossRef]
- Bast, R.C.; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef]
- Bast, R.C.; Klug, T.L.; John, E.S.; Jenison, E.; Niloff, J.M.; Lazarus, H.; Berkowitz, R.S.; Leavitt, T.; Griffiths, C.T.; Parker, L.; et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med. 1983, 309, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.W.T.; Lloyd, K.O. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. J. Biol. Chem. 2001, 276, 27371–27375. [Google Scholar] [CrossRef]
- Lloyd, K.O.; Yin, B.W.T.; Kudryashov, V. Isolation and characterization of ovarian cancer antigen CA 125 using a new monoclonal antibody (VK-8): Identification as a mucin-type molecule. Int. J. Cancer 1997, 71, 842–850. [Google Scholar] [CrossRef]
- Hardardottir, H.; Parmley, T.H.; Quirk, J.G.; Sanders, M.M.; Miller, F.C.; O’Brien, T.J. Distribution of CA 125 in embryonic tissues and adult derivatives of the fetal periderm. Am. J. Obstet. Gynecol. 1990, 163, 1925–1931. [Google Scholar] [CrossRef]
- Jacobs, I.J.; Menon, U. Progress and challenges in screening for early detection of ovarian cancer. Mol. Cell. Proteomics 2004, 3, 355–366. [Google Scholar] [CrossRef]
- Tamakoshi, K.; Kikkawa, F.; Hasegawa, N.; Ishikawa, H.; Mizuno, K.; Kawai, M.; Tomoda, Y. Clinical value of a new serum tumor marker, ca125ii, in gynecologic disease: Comparison with ca125. Gynecol. Obstet. Investig. 1995, 39, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008, 5, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Leandersson, P.; Kalapotharakos, G.; Henic, E.; Borgfeldt, H.; Petzold, M.; Høyer-Hansen, G.; Borgfeldt, C. A biomarker panel increases the diagnostic performance for epithelial ovarian cancer type I and II in young women. Anticancer Res. 2016, 36, 957–965. [Google Scholar]
- Kristjansdottir, B.; Levan, K.; Partheen, K.; Sundfeldt, K. Diagnostic performance of the biomarkers HE4 and CA125 in type i and type II epithelial ovarian cancer. Gynecol. Oncol. 2013, 131, 52–58. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Cheng, W.; Chang, D.Y.; Huang, J.; Wang, X.; Jia, L.; Rosen, D.G.; Zhang, W.; Yang, D.; et al. CA-125 level as a prognostic indicator in type i and type ii epithelial ovarian cancer. Int. J. Gynecol. Cancer 2013, 23, 815–822. [Google Scholar] [CrossRef]
- Gąsiorowska, E.; Ginekologicznej, K.O. Clinical application of HE4 and CA125 in ovarian cancer Type I and Type II detection and differential diagnosis. Ginekol. Pol. 2015, 86, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Yanaranop, M.; Jantarateptewan, N.; Tiyayon, J.; Nakrangsee, S. Significance of serum human epididymis protein 4 and cancer antigen 125 in distinguishing type I and type II epithelial ovarian cancers. Int. J. Gynecol. Cancer 2018, 28, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, L.; Indima, N.; Peng, K.; Li, Q.; Hua, T.; Tang, G. CT and MRI findings of type I and type II epithelial ovarian cancer. Eur. J. Radiol. 2017, 90, 225–233. [Google Scholar] [CrossRef]
- Fujiwara, H.; Suzuki, M.; Takeshima, N.; Takizawa, K.; Kimura, E.; Nakanishi, T.; Yamada, K.; Takano, H.; Sasaki, H.; Koyama, K.; et al. Evaluation of human epididymis protein 4 (HE4) and Risk of Ovarian Malignancy Algorithm (ROMA) as diagnostic tools of type I and type II epithelial ovarian cancer in Japanese women. Tumor. Biol. 2015, 36, 1045–1053. [Google Scholar] [CrossRef]
- Lu, D.; Kuhn, E.; Bristow, R.E.; Giuntoli, R.L.; Kjær, S.K.; Shih, I.-M.; Roden, R.B.S. Comparison of candidate serologic markers for type I and type II ovarian cancer. Gynecol. Oncol. 2011, 122, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Suidan, R.S.; Ramirez, P.T.; Sarasohn, D.M.; Teitcher, J.B.; Mironov, S.; Iyer, R.B.; Zhou, Q.; Iasonos, A.; Paul, H.; Hosaka, M.; et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol. Oncol. 2014, 134, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hattrup, C.L.; Gendler, S.J. Structure and Function of the Cell Surface (Tethered) Mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, H.W.A.; van Beeck Calkoen-Carpay, T.; Jager, S.; Duk, J.M.; Aalders, J.G.; Fleuren, G.J. The tumor marker CA 125 is a common constituent of normal cervical mucus. Am. J. Obs. Gynecol. 1986, 154, 1088–1091. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Hardin, J.W.; Bannon, G.A.; Norris, J.S.; Quirk, G. CA 125 antigen in human amniotic fluid and fetal membranes. Am. J. Obs. Gynecol. 1986, 155, 50–55. [Google Scholar] [CrossRef]
- Hanisch, F.G.; Uhlenbruck, G.; Dienst, C.; Stottrop, M.; Hippauf, E. Ca 125 and Ca 19-9: Two cancer-associated sialylsaccharide antigens on a mucus glycoprotein from human milk. Eur. J. Biochem. 1985, 149, 323–330. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Endo, K.; Kawamura, Y.; Yoshida, T.; Saga, T.; Watanabe, Y.; Koizumi, M.; Nakashima, T.; Konishi, J.; Yamaguchi, N.; et al. Normal bronchial mucus contains high levels of cancer-associated antigens, CA125, CA19-9, and carcinoembryonic antigen. Cancer 1990, 65, 506–510. [Google Scholar] [CrossRef]
- Kabawat, S.E.; Bast, R.C.; Bhan, A.K.; Welch, W.R.; Knapp, R.C.; Colvin, R.B. Tissue distribution of a coelomic- epithelium-related antigen recognized by the monoclonal antibody OC125. Int. J. Gynecol. Pathol. 1983, 2, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Wang, Y.; Gao, X.; Wang, W.; Liu, H.; He, H.; Liang, Y.; Pan, K.; Wu, H.; et al. Relationship of tumor marker CA125 and ovarian tumor stem cells: Preliminary identification. J. Ovarian Res. 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buamah, P. Benign conditions associated with raised serum, CA-125 concentration. J. Surg. Oncol. 2000, 75, 264–265. [Google Scholar] [CrossRef]
- Szubert, M.; Suzin, J.; Wierzbowski, T.; Kowalczyk-Amico, K. CA-125 concentration in serum and peritoneal fluid in patients with endometriosis—Preliminary results. Arch. Med. Sci. 2012, 8, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Seong, W.J. Amniotic fluid CA-125 as a marker of intra-amniotic inflammation associated with preterm delivery: A preliminary single center study. Arch. Gynecol. Obs. 2016, 293, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Taylor, P.T.; Gordon, A.; Cunningham, M.J.; Finkler, N.; Orr, J.; Rivkin, S.; Schultes, B.C.; Whiteside, T.L.; Nicodemus, C.F. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J. Clin. Oncol. 2004, 22, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, P.; Harter, P.; Scambia, G.; Sehouli, J.; Meier, W.; Wimberger, P.; Baumann, K.H.; Kurzeder, C.; Schmalfeldt, B.; Cibula, D.; et al. Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: A phase III trial of the AGO OVAR, COGI, GINECO, and GEICO-the MIMOSA study. J. Clin. Oncol. 2013, 31, 1554–1561. [Google Scholar] [CrossRef]
- Thériault, C.; Pinard, M.; Comamala, M.; Migneault, M.; Beaudin, J.; Matte, I.; Boivin, M.; Piché, A.; Rancourt, C. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol. Oncol. 2011, 121, 434–443. [Google Scholar] [CrossRef]
- Kim, N.; Hong, Y.; Kwon, D.; Yoon, S. Somatic mutaome profile in human cancer tissues. Genom. Inform. 2013, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Batra, S.K. Understanding the unique attributes of muc16 (ca125): Potential implications in targeted therapy. Cancer Res. 2015, 75, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC61 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Song, J.; Yang, W.; Wang, H.; Huo, Q.; Yang, J.; Yu, X.; Liu, Y.; Xu, C.; Bao, H. The effect of CA125 on metastasis of ovarian cancer: Old marker new function. Oncotarget 2017, 8, 50015–50022. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gubbels, J.A.A.; Felder, M.; Horibata, S.; Belisle, J.A.; Kapur, A.; Holden, H.; Petrie, S.; Migneault, M.; Rancourt, C.; Connor, J.P.; et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer 2010, 9. [Google Scholar] [CrossRef]
- Das, S.; Majhi, P.D.; Al-Mugotir, M.H.; Rachagani, S.; Sorgen, P.; Batra, S.K. Membrane proximal ectodomain cleavage of MUC16 occurs in the acidifying Golgi/post-Golgi compartments. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Dharma Rao, T.; Park, K.J.; Smith-Jones, P.; Iasonos, A.; Linkov, I.; Soslow, R.A.; Spriggs, D.R. Novel monoclonal antibodies against the proximal (carboxy-terminal) portions of MUC16. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 462–472. [Google Scholar] [CrossRef]
- Chekmasova, A.A.; Rao, T.D.; Nikhamin, Y.; Park, K.J.; Levine, D.A.; Spriggs, D.R.; Brentjens, R.J. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin. Cancer Res. 2010, 16, 3594–3606. [Google Scholar] [CrossRef]
- Skates, S.J.; Mai, P.; Horick, N.K.; Piedmonte, M.; Drescher, C.W.; Isaacs, C.; Armstrong, D.K.; Buys, S.S.; Rodriguez, G.C.; Horowitz, I.R.; et al. Large prospective study of ovarian cancer screening in high-risk women: CA125 cut-point defined by menopausal status. Cancer Prev. Res. 2011, 4, 1401–1408. [Google Scholar] [CrossRef]
- Zanaboni, F.; Vergadoro, F.; Presti, M.; Gallotti, P.; Lombardi, F.; Bolis, G. Tumor antigen CA 125 as a marker of ovarian epithelial carcinoma. Gynecol. Oncol. 1987, 28, 61–67. [Google Scholar] [CrossRef]
- Rosen, D.G.; Wang, L.; Atkinson, J.N.; Yu, Y.; Lu, K.H.; Diamandis, E.P.; Hellstrom, I.; Mok, S.C.; Liu, J.; Bast, R.C. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol. Oncol. 2005, 99, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Hollingworth, J.; Reynolds, T.M. The role of CA125 in clinical practice. J. Clin. Pathol. 2005, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Pauler, D.K.; Menon, U.; Mcintosh, M.; Symecko, H.L.; Skates, S.J.; Jacobs, I.J.; Bartholomew’s, S.; Royal, T. Factors influencing serum CA125II levels in healthy postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 489–493. [Google Scholar]
- Hirsch, M.; Duffy, J.; Davis, C.; Nieves Plana, M.; Khan, K. Diagnostic accuracy of cancer antigen 125 for endometriosis: A systematic review and meta-analysis. BJOG An. Int. J. Obs. Gynaecol. 2016, 123, 1761–1768. [Google Scholar] [CrossRef]
- Lehtovirta, P.; Apter, D.; Stenman, U.H. Serum CA 125 levels during the menstrual cycle. BJOG An Int. J. Obs. Gynaecol. 1990, 97, 930–933. [Google Scholar] [CrossRef]
- Szecsi, P.B.; Andersen, M.R.; Bjørngaard, B.; Hedengran, K.K.; Stender, S. Cancer antigen 125 after delivery in women with a normal pregnancy: A prospective cohort study. Acta Obs. Gynecol. Scand. 2014, 93, 1295–1301. [Google Scholar] [CrossRef]
- Erbağci, A.B.; Yilmaz, N.; Kutlar, I. Menstrual cycle dependent variability for serum tumor markers CEA, AFP, CA 19-9, CA 125 and CA 15-3 in healthy women. Dis. Markers 1999, 15, 259–267. [Google Scholar] [CrossRef]
- Morshed, G.; Fathy, S.M. Impact of post-laparoscopic sleeve gastrectomy weight loss on C-reactive protein, lipid profile and CA-125 in morbidly obese women. Wideochirurgia I Inne Tech. Maloinwazyjne 2015, 10, 521–526. [Google Scholar] [CrossRef]
- Urban, N.; Thorpe, J.; Karlan, B.Y.; McIntosh, M.W.; Palomares, M.R.; Daly, M.B.; Paley, P.; Drescher, C.W. Interpretation of single and serial measures of HE4 and CA125 in asymptomatic women at high risk for ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2087–2094. [Google Scholar] [CrossRef]
- Monteiro, S.; Franco, F.; Costa, S.; Monteiro, P.; Vieira, H.; Coelho, L.; Oliveira, L.; Providência, L.A. Prognostic value of CA125 in advanced heart failure patients. Int. J. Cardiol. 2010, 140, 115–118. [Google Scholar] [CrossRef]
- Akinwunmi, B.O.; Babic, A.; Vitonis, A.F.; Cramer, D.W.; Titus, L.; Tworoger, S.S.; Terry, K.L. Chronic medical conditions and CA125 levels among women without ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Cao, Y.; Liu, X.; Zeng, X.T.; Li, Y. Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget 2017, 8, 63963–63970. [Google Scholar] [CrossRef] [PubMed]
- Cedrés, S.; Nuñez, I.; Longo, M.; Martinez, P.; Checa, E.; Torrejón, D.; Felip, E. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin. Lung Cancer 2011, 12, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sjövall, K.; Nilsson, B.; Einhorn, N. The significance of serum CA 125 elevation in malignant and nonmalignant diseases. Gynecol. Oncol. 2002, 85, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, H.X.; Wang, W.Q.; Wu, C.T.; Xiang, J.F.; Liu, C.; Long, J.; Xu, J.; Fu, D.L.; Ni, Q.X.; et al. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden. Oncotarget 2016, 7, 5943–5956. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, M.; Xu, T.; Hu, F.; Li, X.; Xiong, B. Predictive value of serum CEA, CA19-9 and CA-125 in diagnosis of liver metastases from colorectal cancer in the Chinese population. Hepatogastroenterology 2013, 60, 1297–1301. [Google Scholar]
- Pinar Cilesiz Goksedef, B.; Gorgen, H.; Baran, S.Y.; Api, M.; Cetin, A. Preoperative serum CA 125 level as a predictor for metastasis and survival in endometrioid endometrial cancer. J. Obs. Gynaecol. Can. 2011, 33, 844–850. [Google Scholar] [CrossRef]
- Emoto, S.; Ishigami, H.; Yamashita, H.; Yamaguchi, H.; Kaisaki, S.; Kitayama, J. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer 2012, 15, 154–161. [Google Scholar] [CrossRef]
- Ferraro, S.; Braga, F.; Lanzoni, M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: A systematic review. J. Clin. Pathol. 2013, 66, 273–281. [Google Scholar] [CrossRef]
- Dikmen, Z.G.; Colak, A.; Dogan, P.; Tuncer, S.; Akbiyik, F. Diagnostic performances of CA 125, HE4, and ROMA index in ovarian cancer. Eur. J. Gynaecol. Oncol. 2015, 36, 457–462. [Google Scholar]
- Yu, S.; Yang, H.J.; Xie, S.Q.; Bao, Y.X. Diagnostic value of HE4 for ovarian cancer: A meta-analysis. Clin. Chem. Lab. Med. 2012, 50, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.R.; Goff, B.A.; Lowe, K.A.; Scholler, N.; Bergan, L.; Drescher, C.W.; Paley, P.; Urban, N. Use of a Symptom index, CA125, and HE4 to predict ovarian cancer. Gynecol. Oncol. 2010, 116, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Urban, N.; Thorpe, J.D.; Bergan, L.A.; Forrest, R.M.; Kampani, A.V.; Scholler, N.; O’Briant, K.C.; Anderson, G.L.; Cramer, D.W.; Berg, C.D.; et al. Potential role of HE4 in multimodal screening for epithelial ovarian cancer. J. Natl. Cancer Inst. 2011, 103, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, H.; Chen, R.; He, J.; Wang, Y.; Huang, L.; Sun, L.; Duan, C.; Luo, X.; Yan, H. Development of a multimarker assay for differential diagnosis of benign and malignant pelvic masses. Clin. Chim. Acta 2015, 440, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, T.; Cadron, I.; Despierre, E.; Daemen, A.; Leunen, K.; Amant, F.; Timmerman, D.; De Moor, B.; Vergote, I. HE4 and CA125 as a diagnostic test in ovarian cancer: Prospective validation of the Risk of Ovarian Malignancy Algorithm. Br. J. Cancer 2011, 104, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.L.; McIntosh, M.; Wu, L.; Barnett, M.; Goodman, G.; Thorpe, J.D.; Bergan, L.; Thornquist, M.D.; Scholler, N.; Kim, N.; et al. Assessing lead time of selected ovarian cancer biomarkers: A nested case–control study. JNCI J. Natl. Cancer Inst. 2010, 102, 26–38. [Google Scholar] [CrossRef]
- Yurkovetsky, Z.; Skates, S.; Lomakin, A.; Nolen, B.; Pulsipher, T.; Modugno, F.; Marks, J.; Godwin, A.; Gorelik, E.; Jacobs, I.; et al. Development of a multimarker assay for early detection of ovarian cancer. J. Clin. Oncol. 2010, 28, 2159–2166. [Google Scholar] [CrossRef]
- Russell, M.R.; Graham, C.; D’Amato, A.; Gentry-Maharaj, A.; Ryan, A.; Kalsi, J.K.; Whetton, A.D.; Menon, U.; Jacobs, I.; Graham, R.L.J. Diagnosis of epithelial ovarian cancer using a combined protein biomarker panel. Br. J. Cancer 2019, 121, 483–489. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Xu, F.; Berchuck, A.; van Haaften-Day, C.; Havrilesky, L.J.; de Bruijn, H.W.A.; van der Zee, A.G.J.; Woolas, R.P.; Jacobs, I.J.; et al. Combining multiple serum tumor markers improves detection of stage I epithelial ovarian cancer. Gynecol. Oncol. 2007, 107, 526–531. [Google Scholar] [CrossRef]
- Cramer, D.W.; Bast, R.C., Jr.; Berg, C.D.; Diamandis, E.P.; Godwin, A.K.; Hartge, P.; Lokshin, A.E.; Lu, K.H.; McIntosh, M.W.; Mor, G.; et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev. Res. 2011, 4, 365–374. [Google Scholar] [CrossRef]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. BJOG An Int. J. Obs. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef]
- Kumari, S. Serum biomarker based algorithms in diagnosis of ovarian cancer: A review. Indian J. Clin. Biochem. 2018, 33, 382–386. [Google Scholar] [CrossRef]
- Jacobs, I.J.; Skates, S.J.; Macdonald, N.; Menon, U.; Rosenthal, A.N.; Davies, A.P.; Woolas, R.; Jeyarajah, A.R.; Sibley, K.; Lowe, D.G.; et al. Screening for ovarian cancer: A pilot randomised controlled trial. Lancet 1999, 353, 1207–1210. [Google Scholar] [CrossRef]
- Skates, S.J.; Pauler, D.K.; Jacobs, I.J. Screening Based on the risk of cancer calculation from bayesian hierarchical changepoint and mixture models of longitudinal markers. J. Am. Stat. Assoc. 2001, 96, 429–439. [Google Scholar] [CrossRef]
- Skates, S.J.; Menon, U.; MacDonald, N.; Rosenthal, A.N.; Oram, D.H.; Knapp, R.C.; Jacobs, I.J. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J. Clin. Oncol. 2003, 21. [Google Scholar] [CrossRef]
- Menon, U.; Ryan, A.; Kalsi, J.; Gentry-Maharaj, A.; Dawnay, A.; Habib, M.; Apostolidou, S.; Singh, N.; Benjamin, E.; Burnell, M.; et al. Risk algorithm using serial biomarker measurements doubles the number of screen-detected cancers compared with a single-threshold rule in the United Kingdom collaborative trial of ovarian cancer screening. J. Clin. Oncol. 2015, 33, 2062–2071. [Google Scholar] [CrossRef]
- Drescher, C.W.; Shah, C.; Thorpe, J.; O’Briant, K.; Anderson, G.L.; Berg, C.D.; Urban, N.; McIntosh, M.W. Longitudinal screening algorithm that incorporates change over time in CA125 levels identifies ovarian cancer earlier than a single-threshold rule. J. Clin. Oncol. 2012, 31, 387–392. [Google Scholar] [CrossRef]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef]
- Al Musalhi, K.; Al Kindi, M.; Al Aisary, F.; Ramadhan, F.; Al Rawahi, T.; Al Hatali, K.; Mula-Abed, W.A. Evaluation of HE4, CA-125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) in the preoperative assessment of patients with adnexal mass. Oman Med. J. 2016, 31, 336–344. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Chen, C.-A.; Nam, J.-H.; Ochiai, K.; Wilailak, S.; Choon, A.-T.; Sabaratnam, S.; Hebbar, S.; Sickan, J.; Schodin, B.A.; et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecol. Oncol. 2013, 128, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Jabre-raughley, M.; Brown, A.K.; Robison, K.M.; Craig MILLER, M.; Jeffery ALLARD, W.; Kurman, R.J.; Bast, R.C.; Skates, S.J.; Schlitzer, D.; et al. Comparison of a novel multiple marker assay versus the risk of malignancy index for the prediction of epithelial ovarian cancer in patients with a pelvic mass NIH public access. Am. J. Obs. Gynecol 2010, 203, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.A.; Høgdall, E.V.S.; Christensen, I.J.; Borgfeldt, C.; Kalapotharakos, G.; Zdrazilova-Dubska, L.; Chovanec, J.; Lok, C.A.R.; Stiekema, A.; Mutz-Dehbalaie, I.; et al. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer-An international multicenter study in women with an ovarian mass. Gynecol. Oncol. 2015, 138, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Amonkar, S.D.; Bertenshaw, G.P.; Chen, T.H.; Bergstrom, K.J.; Zhao, J.; Seshaiah, P.; Yip, P.; Mansfield, B.C. Development and preliminary evaluation of a multivariate index assay for ovarian cancer. PLoS ONE 2009, 4, e4599. [Google Scholar] [CrossRef] [PubMed]
- Longoria, T.C.; Ueland, F.R.; Zhang, Z.; Chan, D.W.; Smith, A.; Fung, E.T.; Munroe, D.G.; Bristow, R.E. Clinical performance of a multivariate index assay for detecting early-stage ovarian cancer. Am. J. Obstet. Gynecol. 2014, 210, 78.e1–78.e9. [Google Scholar] [CrossRef] [PubMed]
- Havrilesky, L.J.; Dinan, M.; Sfakianos, G.P.; Curtis, L.H.; Barnett, J.C.; Gorp, V.; Myers, E.R. Costs, effectiveness, and workload impact of management strategies for women with an adnexal mass. J. Natl. Cancer Inst. 2015, 107, 322. [Google Scholar] [CrossRef]
- Diagnosis, D. Practice Bulletin No. 174 Summary: Evaluation and Management of Adnexal Masses. Obs. Gynecol. 2016, 128, 1193–1195. [Google Scholar]
- Bon, G.G.; Kenemans, P.; Verstraeten, R.; Van Kamp, G.J.; Hilgers, J. Serum tumor marker immunoassays in gynecologic oncology: Establishment of reference values. Am. J. Obs. Gynecol. 1996, 174, 107–114. [Google Scholar] [CrossRef]
- Bonfrer, J.M.; Korse, C.M.; Verstraeten, R.A.; van Kamp, G.J.; Hart, G.A.; Kenemans, P. Clinical evaluation of the Byk LIA-mat CA125 II assay: Discussion of a reference value. Clin. Chem. 1997, 43, 491–497. [Google Scholar] [CrossRef]
- Van Calster, B.; Valentin, L.; Van Holsbeke, C.; Zhang, J.; Jurkovic, D.; Lissoni, A.A.; Testa, A.C.; Czekierdowski, A.; Fischerová, D.; Domali, E.; et al. A novel approach to predict the likelihood of specific ovarian tumor pathology based on serum CA-125: A multicenter observational study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2420–2428. [Google Scholar] [CrossRef]
- Winarto, H.; Laihad, B.J.; Nuranna, L. Modification of cutoff values for HE4, CA125, the risk of malignancy index, and the risk of Malignancy Algorithm for ovarian cancer detection in Jakarta, Indonesia. Asian Pacific J. Cancer Prev. 2014, 15, 1949–1953. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhong, R.; He, J.; Ding, R.; Lin, H.; Deng, Y.; Zhou, L.; Li, X.; Jiang, J.; Bao, Y.; et al. Modification of cut-off values for HE4, CA125 and the ROMA algorithm for early-stage epithelial ovarian cancer detection: Results from 1021 cases in South China. Clin. Biochem. 2016, 49, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Ye, X.; Dong, L.; Cheng, H.; Cheng, Y.; Zhu, L.; Liao, Q.; Zhao, Y.; Tian, L.; Fu, T.; et al. Human epididymis protein 4 (HE4) as a serum tumor biomarker in patients with ovarian carcinoma. Int. J. Gynecol. Cancer 2011, 21, 852–858. [Google Scholar] [CrossRef]
- Jacobs, I.J.; Skates, S.; Davies, A.P.; Woolas, R.P.; Jeyerajah, A.; Weidemann, P.; Sibley, K.; Oram, D.H. Risk of diagnosis of ovarian cancer after raised serum CA 125 concentration: A prospective cohort study. Br. Med. J. 1996, 313, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. JAMA J. Am. Med. Assoc. 2011, 305, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F.; Yu, K.; Kramer, B.S.; Black, A.; Buys, S.S.; Partridge, E.; Gohagan, J.; Berg, C.D.; Prorok, P.C. Extended mortality results for ovarian cancer screening in the PLCO trial with median 15 years follow-up. Gynecol. Oncol. 2016, 143, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Temkin, S.M.; Miller, E.A.; Samimi, G.; Berg, C.D.; Pinsky, P.; Minasian, L. Outcomes from ovarian cancer screening in the PLCO trial: Histologic heterogeneity impacts detection, overdiagnosis and survival. Eur. J. Cancer 2017, 87, 182–188. [Google Scholar] [CrossRef]
- Xu, J.L.; Commins, J.; Partridge, E.; Riley, T.L.; Prorok, P.C.; Johnson, C.C.; Buys, S.S. Longitudinal evaluation of CA-125 velocity and prediction of ovarian cancer. Gynecol. Oncol. 2012, 125, 70–74. [Google Scholar] [CrossRef][Green Version]
- Pinsky, P.F.; Zhu, C.; Skates, S.J.; Black, A.; Partridge, E.; Buys, S.S.; Berg, C.D. Potential effect of the risk of ovarian cancer algorithm (ROCA) on the mortality outcome of the prostate, lung, colorectal and ovarian (PLCO) trial. Int. J. Cancer 2013, 132, 2127–2133. [Google Scholar] [CrossRef]
- Van Nagell, J.R.; Miller, R.W.; Desimone, C.P.; Ueland, F.R.; Podzielinski, I.; Goodrich, S.T.; Elder, J.W.; Huang, B.; Kryscio, R.J.; Pavlik, E.J. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obs. Gynecol. 2011, 118, 1212–1221. [Google Scholar] [CrossRef]
- Kumar Gupta, K.; Kumar Gupta, V.; Wendel Naumann, R.; Birmingham Hospitals, W. Ovarian cancer: Screening and future directions. Int. J. Gynecol. Cancer 2019, 29, 195–200. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Miller, A.; Kramer, B.S.; Church, T.; Reding, D.; Prorok, P.; Gelmann, E.; Schoen, R.E.; Buys, S.; Hayes, R.B.; et al. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am. J. Epidemiol. 2007, 165, 874–881. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. Genetic/familial high-risk assessment: Breast and ovarian, version 2.2017: Featured updates to the NCCN guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2017, 15, 9–20. [Google Scholar] [CrossRef]
- Van der Velde, N.M.; Mourits, M.J.; Arts, H.J.; de Vries, J.; Leegte, B.K.; Dijkhuis, G.; Oosterwijk, J.C.; de Bock, G.H. Time to stop ovarian cancer screening in BRCA1/2 mutation carriers? Int. J. Cancer 2009, 124, 919–923. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Sanders, G.D.; Kulasingam, S.; Chino, J.P.; Berchuck, A.; Marks, J.R.; Myers, E.R. Development of an ovarian cancer screening decision model that incorporates disease heterogeneity. Cancer 2011, 117, 545–553. [Google Scholar] [CrossRef]
- How to Check for Ovarian Cancer. Ovarian Cancer Screening. The American Cancer Society, 2020. Available online: https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/detection.html (accessed on 20 July 2020).
- Kurman, R.J.; Visvanathan, K.; Roden, R.; Wu, T.C.; Shih, I.-M. Early detection and treatment of ovarian cancer: Shifting from Early stage to minimal volume of disease based on a new model of carcinogenesis. Am. J. Obs. Gynecol 2008, 198, 351–356. [Google Scholar] [CrossRef]
- Salani, R.; Backes, F.J.; Fung Kee Fung, M.; Holschneider, C.H.; Parker, L.P.; Bristow, R.E.; Goff, B.A. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of gynecologic oncologists recommendations. Am. J. Obstet. Gynecol. 2011, 204, 466–478. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Sopik, V.; Iqbal, J.; Rosen, B.; Narod, S.A. Why have ovarian cancer mortality rates declined? Part II. Case-fatality. Gynecol. Oncol. 2015, 138, 750–756. [Google Scholar] [CrossRef]
- Colombo, N.; Lorusso, D.; Scollo, P. Impact of recurrence of ovarian cancer on quality of life and outlook for the future. Int. J. Gynecol. Cancer 2017, 27, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.S.; Larry Maxwell, G.; Darcy, K.M.; Hamilton, C.A.; Mcguire, W.P. Current Approaches and challenges in managing and monitoring treatment response in ovarian cancer. J. Cancer 2014, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Van Der Burg, M.E.L.; Lammes, F.B.; Verweij, J. The role of CA 125 in the early diagnosis of progressive disease in ovarian cancer. Ann. Oncol. 1990, 1, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Salani, R.; Santillan, A.; Zahurak, M.L.; Giuntoli, R.L.; Gardner, G.J.; Armstrong, D.K.; Bristow, R.E. Secondary cytoreductive surgery for localized, recurrent epithelial ovarian cancer. Cancer 2007, 109, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.S.; Nelstrop, A.E.; Tuxen, M.K.; Lambert, H.E. Defining progression of ovarian carcinoma during follow-up according to CA 125: A north thames ovary group study. Ann. Oncol. 1996, 7, 361–364. [Google Scholar] [CrossRef]
- Santillan, A.; Garg, R.; Zahurak, M.L.; Gardner, G.J.; Giuntoli, R.L.; Armstrong, D.K.; Bristow, R.E. Risk of epithelial ovarian cancer recurrence in patients with rising serum CA-125 levels within the normal range. J. Clin. Oncol. 2005, 23, 9338–9343. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.S.; Marples, M.; Nelstrop, A.E.; Mahmoudi, M.; Meyer, T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J. Clin. Oncol. 2001, 19, 4054–4057. [Google Scholar] [CrossRef]
- Wilder, J.L.; Pavlik, E.; Straughn, J.M.; Kirby, T.; Higgins, R.V.; DePriest, P.D.; Ueland, F.R.; Kryscio, R.J.; Whitley, R.J.; Van Nagell, J. Clinical implications of a rising serum CA-125 within the normal range in patients with epithelial ovarian cancer: A preliminary investigation. Gynecol. Oncol. 2003, 89, 233–235. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S. Surveillance of patients after initial treatment of ovarian cancer. Crit. Rev. Oncol. Hematol. 2009, 71, 43–52. [Google Scholar] [CrossRef]
- Wang, F.; Ye, Y.; Xu, X.; Zhou, X.; Wang, J.; Chen, X. CA-125-indicated asymptomatic relapse confers survival benefit to ovarian cancer patients who underwent secondary cytoreduction surgery. J. Ovarian Res. 2013, 6, 14. [Google Scholar] [CrossRef]
- Rustin, G.J.S.; Van Der Burg, M.E.L.; Griffin, C.L.; Guthrie, D.; Lamont, A.; Jayson, G.C.; Kristensen, G.; Mediola, C.; Coens, C.; Qian, W.; et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): A randomised trial. Lancet 2010, 376, 1155–1163. [Google Scholar] [CrossRef]
- Ron, I.-G.; Inbar, M.; Gelernter, I.; Lewysohn, O.; Ayalon, D.; Dale, J.; Chaitchik, S. Use of CA-125 response to predict survival parameters of patients with advanced ovarian carcinoma. Acta Obs. Gynecol. Scand. 1994, 73, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C. CA 125 and the detection of recurrent ovarian cancer: A reasonably accurate biomarker for a difficult disease. Cancer 2010, 116, 2850–2853. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, L. Cancer antigen 125 in ovarian cancer surveillance: A decision analysis model. Curr. Oncol. 2007, 14, 167–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanner, E.J.; Chi, D.S.; Eisenhauer, E.L.; Diaz-Montes, T.P.; Santillan, A.; Bristow, R.E. Surveillance for the detection of recurrent ovarian cancer: Survival impact or lead-time bias? Gynecol. Oncol. 2010, 117, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Federico, M.; Liu, P.Y.; Hannigan, E.; Alberts, D. Significance of early changes in the serum CA-125 antigen level on overall survival in advanced ovarian cancer. Gynecol. Oncol. 2006, 103, 195–198. [Google Scholar] [CrossRef]
- Riedinger, J.M.; Wafflart, J.; Ricolleau, G.; Eche, N.; Larbre, H.; Basuyau, J.P.; Dalifard, I.; Hacene, K.; Pichon, M.F. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: Results of a French multicentric study. Ann. Oncol. 2006, 17, 1234–1238. [Google Scholar] [CrossRef]
- Gadducci, A.; Zola, P.; Landoni, F.; Maggino, T.; Sartori, E.; Bergamino, T.; Cristofani, R. Serum half-life of CA 125 during early chemotherapy as an independent prognostic variable for patients with advanced epithelial ovarian cancer: Results of a multicentric italian study. Gynecol. Oncol. 1995, 58, 42–47. [Google Scholar] [CrossRef]
- Fleming, N.D.; Cass, I.; Walsh, C.S.; Karlan, B.Y.; Li, A.J. CA125 surveillance increases optimal resectability at secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Gynecol. Oncol. 2011, 121, 249–252. [Google Scholar] [CrossRef]
- Gronlund, B.; Dehny, H.; Høgdallyz, C.K.; Engelholm, S.A.; Jørgensen, M.; Nørgaard-Pedersenz, B.; Høgdallz, E.V.S. Cancer-associated serum antigen level: A novel prognostic indicator for survival in patients with recurrent ovarian carcinoma. Int. J. Gynecol. Cancer 2005, 15, 836–843. [Google Scholar] [CrossRef]
- Gu, P.; Pan, L.-L.; Wu, S.-Q.; Sun, L.; Huang, G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma A systematic review and meta-analysis. Eur. J. Radiol. 2009, 71, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Heintz, A.; Odicino, F.; Maisonneuve, P.; Quinn, M.; Benedet, J.; Creasman, W.; Ngan, H.; Pecorelli, S.; Beller, U. Carcinoma of the ovary. FIGO 26th Annual report on the results of treatment in gynecological cancer. Int. J. Gynecol. Obs. 2006, 95. [Google Scholar] [CrossRef]
- Javadi, S.; Ganeshan, D.M.; Qayyum, A.; Iyer, R.B.; Bhosale, P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. Am. J. Roentgenol. 2016, 206, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Blyuss, O.; Burnell, M.; Ryan, A.; Gentry-Maharaj, A.; Mariño, I.P.; Kalsi, J.; Manchanda, R.; Timms, J.F.; Parmar, M.; Skates, S.J.; et al. Comparison of longitudinal CA125 algorithms as a first-line screen for ovarian cancer in the general population. Clin. Cancer Res. 2018, 24, 4726–4733. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zeng, Y.; Zeng, Y. Integrated microfluidic lectin barcode platform for high-performance focused glycomic profiling. Sci. Rep. 2016, 6, 20297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef]
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Singh, H.; Zhuang, S.; Misra, D.; Bhuyian, M.N.U.; Lee, E.S. Detection of cancer antigens (CA-125) using gold nano particles on interdigitated electrode-based microfluidic biosensor. Nano Converg. 2019, 6, 3. [Google Scholar] [CrossRef]
- Gedi, V.; Song, C.K.; Kim, G.B.; Lee, J.O.; Oh, E.; Shin, B.S.; Jung, M.; Shim, J.; Lee, H.; Kim, Y.-P. Sensitive on-chip detection of cancer antigen 125 using a DNA aptamer/carbon nanotube network platform. Sens. Actuators B 2018, 256, 89–97. [Google Scholar] [CrossRef]
- Whited, A.M. Investigation of Impedance Spectroscopy for Detection of Ovarian Cancer. Ph.D. Thesis, Portland State University, Portland, OR, USA, 1 December 2020. [Google Scholar] [CrossRef]
- Wang, S.; Ge, L.; Yan, M.; Yu, J.; Song, X.; Ge, S.; Huang, J. 3D microfluidic origami electrochemiluminescence immunodevice for sensitive point-of-care testing of carcinoma antigen 125. Sens. Actuators B 2013, 176, 1–8. [Google Scholar] [CrossRef]
- Shadfan, B.H.; Simmons, A.R.; Simmons, G.W.; Ho, A.; Wong, J.; Lu, K.H.; Bast, R.C.; McDevitt, J.T. A multiplexable, microfluidic platform for the rapid quantitation of a biomarker panel for early ovarian cancer detection at the point-of-care. Cancer Prev. Res. 2015, 8, 37–48. [Google Scholar] [CrossRef]
- Williams, R.M.; Lee, C.; Galassi, T.V.; Harvey, J.D.; Leicher, R.; Sirenko, M.; Dorso, M.A.; Shah, J.; Olvera, N.; Dao, F.; et al. Noninvasive ovarian cancer biomarker detection via an optical nanosensor implant. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Hosu, O.; Ravalli, A.; Lo Piccolo, G.M.; Cristea, C.; Sandulescu, R.; Marrazza, G. Smartphone-based immunosensor for CA125 detection. Talanta 2017, 166, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kricka, L.J. Prospects for the commercialization of chemiluminescence-based point-of-care and on-site testing devices. Anal. Bioanal. Chem. 2014, 406, 5631–5637. [Google Scholar] [CrossRef] [PubMed]
- CardioGenics—QL Care Analyzer. Available online: http://www.cardiogenics.com/ql_care_analyzer.html (accessed on 18 June 2020).
- La Franier, B.D.; Thompson, M. Early stage detection and screening of ovarian cancer: A research opportunity and significant challenge for biosensor technology. Biosens. Bioelectron. 2019, 135, 71–81. [Google Scholar] [CrossRef] [PubMed]
| Type I | Type II | |
|---|---|---|
| Subtypes | Endometrioid, clear cell, low-grade serous carcinomas (LGSC), mucinous carcinomas, seromucous carcinomas, malignant Brenner tumours | High-grade serous carcinoma (HGSC), carcinosarcoma, undifferentiated carcinoma |
| Genetic stability | Genetically stable | Genetically unstable |
| Diagnosis | Early-stage | Advanced-stage |
| Early detection | Frequent | Infrequent |
| Progression | Slow | Rapid |
| TP53 mutations | Infrequent | Frequent |
| Germline BRCA mutations | Infrequent | Frequent |
| Ki 67 proliferative index | 10–15% | 50–75% |
| Median CA125 levels | 53–413 U/mL | 395–1340 U/mL |
| Study | n | Benign | Type I | Type II |
|---|---|---|---|---|
| Alcázer et al. [15] (2013) | 244 | NA * | 78.9 | 490 |
| Leandersson et al. [50] (2016) † | 350 | 54 | 413 | 1340 |
| Kristjansdottir et al. [51] (2013) | 373 | 16 | 53 | 395 |
| Gąsiorowska et al. [53] (2015) | 206 | 25 | 45 | 936 |
| Yanaranop et al. [54] (2018) | 499 | 35.8 | 155.8 | 690.7 |
| Liu et al. [55] (2017) † | 65 | NA * | 141.1 | 299.9 |
| Fujiwara et al. [56] (2015) | 225 | 21.9 | 61.2 | 567.2 |
| Advantages | Disadvantages |
|---|---|
| Easily accessible at the physician’s office and patient’s bedside | Analysis of a few samples |
| Portable | Antibodies, aptamers, or lectins need to be in correct orientations |
| Low cost (equipment and personnel) | Quality control and calibration |
| Rapid results | Error management and interassay variations |
| Multiplex testing for several biomarkers | Analysis time of up to 3 h for some microfluidic devices |
| Minimal sample requirement | Distribution to primary care facilities |
| Minimal sample processing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. https://doi.org/10.3390/cancers12123730
Charkhchi P, Cybulski C, Gronwald J, Wong FO, Narod SA, Akbari MR. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers. 2020; 12(12):3730. https://doi.org/10.3390/cancers12123730
Chicago/Turabian StyleCharkhchi, Parsa, Cezary Cybulski, Jacek Gronwald, Fabian Oliver Wong, Steven A. Narod, and Mohammad R. Akbari. 2020. "CA125 and Ovarian Cancer: A Comprehensive Review" Cancers 12, no. 12: 3730. https://doi.org/10.3390/cancers12123730
APA StyleCharkhchi, P., Cybulski, C., Gronwald, J., Wong, F. O., Narod, S. A., & Akbari, M. R. (2020). CA125 and Ovarian Cancer: A Comprehensive Review. Cancers, 12(12), 3730. https://doi.org/10.3390/cancers12123730







