Role of the Main Non HLA-Specific Activating NK Receptors in Pancreatic, Colorectal and Gastric Tumors Surveillance
Abstract
:Simple Summary
Abstract
1. Introduction
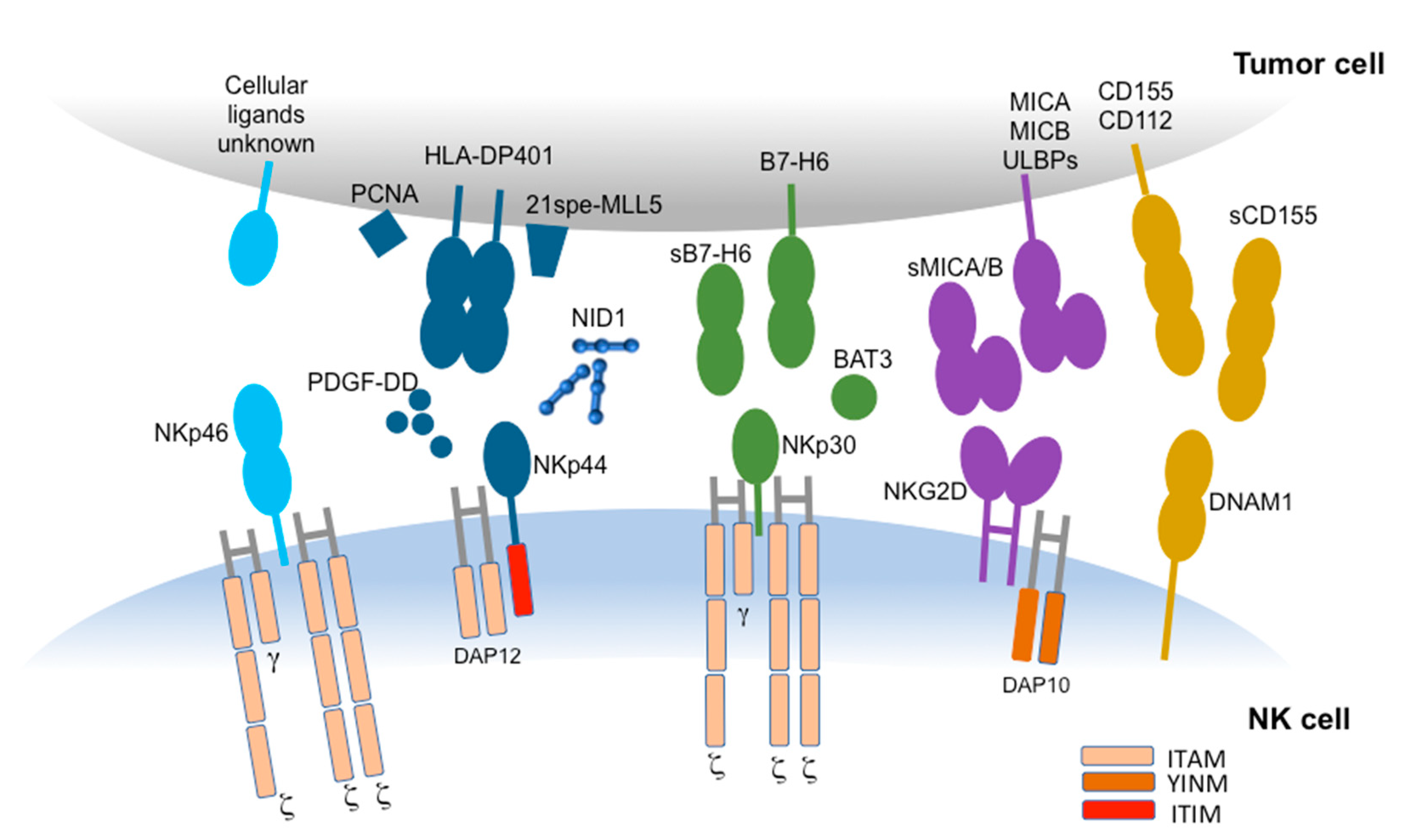
2. Non-HLA-Specific Activating NK Receptors
2.1. Natural Cytotoxicity Receptors (NCRs)
2.2. DNAM-1
2.3. NKG2D
3. Gastrointestinal Cancers
3.1. PDAC
3.2. CRC
3.3. GC
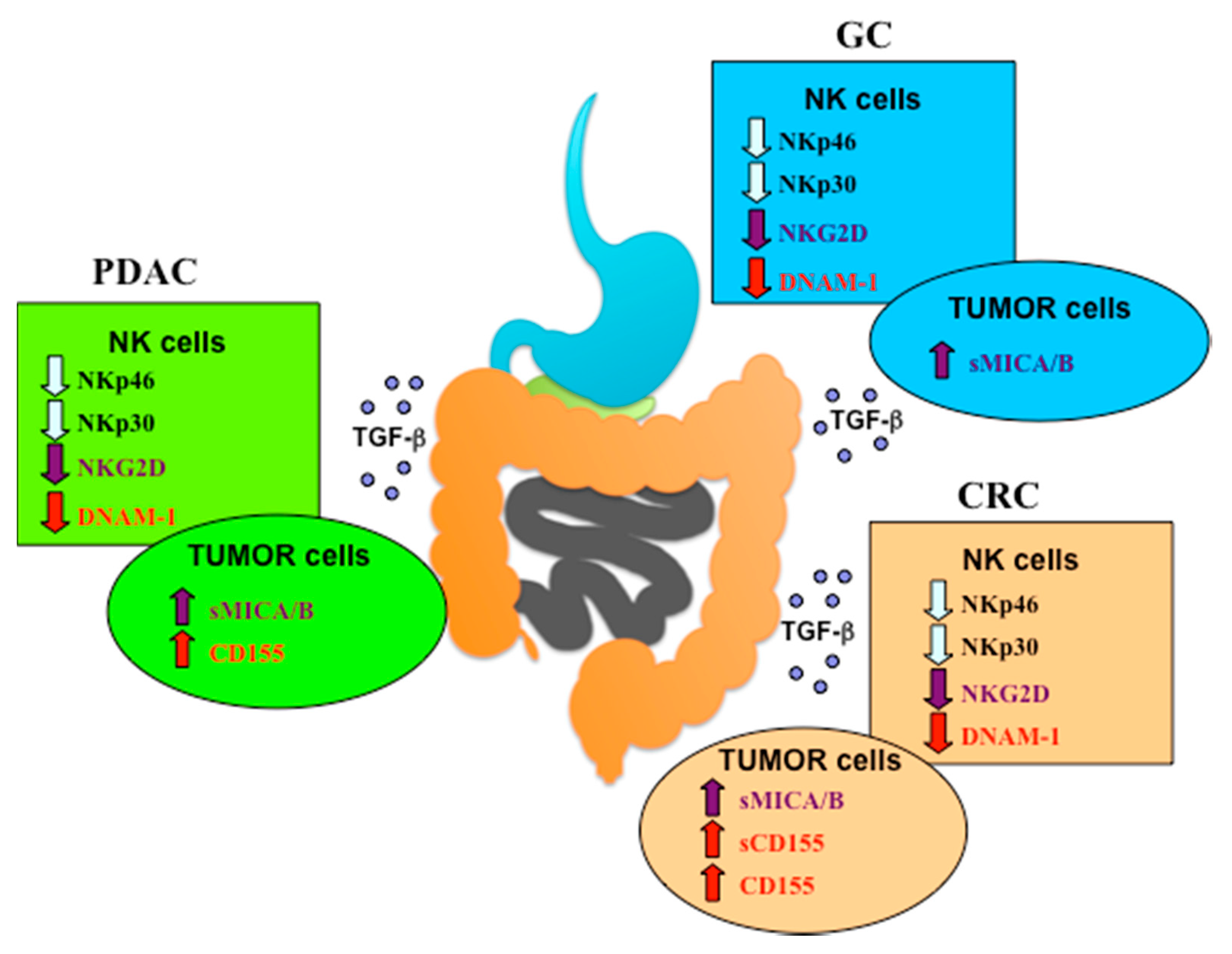
4. NCRs Role in PDAC, CRC and GC
4.1. PDAC
4.2. CRC
4.3. GC
5. NKG2D Role in PDAC, CRC and GC
5.1. PDAC
5.2. CRC
5.3. GC
6. DNAM-1 Role in PDAC, CRC and GC
6.1. PDAC
6.2. CRC
6.3. GC
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331. [Google Scholar] [CrossRef] [Green Version]
- Marcenaro, E.; Carlomagno, S.; Pesce, S.; Moretta, A.; Sivori, S. Bridging innate NK cell functions with adaptive immunity. Adv. Exp. Med. Biol. 2011, 780, 45–55. [Google Scholar] [CrossRef]
- Vitale, M.; Cantoni, C.; Della Chiesa, M.; Ferlazzo, G.; Carlomagno, S.; Pende, D.; Falco, M.; Pessino, A.; Muccio, L.; De Maria, A.; et al. An historical overview: The discovery of how NK cells can kill enemies, recruit defense troops, and more. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Elliott, J.M.; Yokoyama, W.M. Unifying concepts of MHC-dependent natural killer cell eduction. Trends Immunol. 2011, 32, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Shifrin, N.; Raulet, D.H.; Ardolino, M. NK cell self tolerance, responsiveness and missing self recognition. Semin Immunol. 2014, 26, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Ljunggren, H.G.; Karre, K. In search of the missing self—Mhc molecules and Nk cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 2018, 175, 1731. [Google Scholar] [CrossRef] [Green Version]
- Vey, N.; Goncalves, A.; Karlin, L.; Lebouvier-Sadot, S.; Broussais, F.; Marie, D.; Berton-Rigaud, D.; Andre, P.; Zerbib, R.A.; Buffet, R.; et al. A phase 1 dose-escalation study of IPH2102 (lirilumab, BMS-986015, LIRI), a fully human anti KIR monoclonal antibody (mAb) in patients (pts) with various hematologic (HEM) or solid malignancies (SOL). J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Sivori, S.; Vacca, P.; del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inoges, S.; Melero, I.; Berraondo, P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 2017, 95, 347–355. [Google Scholar] [CrossRef]
- Bowles, J.A.; Weiner, G.J. CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J. Immunol. Methods 2005, 304, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Burchard, P.R.; Malhotra, S.; Kaur, P.; Tsongalis, G.J. Detection of the FCGR3a polymorphism using a real-time polymerase chain reaction assay. Cancer Genet. 2013, 206, 130–134. [Google Scholar] [CrossRef]
- Mailliard, R.B.; Alber, S.M.; Shen, H.M.; Watkins, S.C.; Kirkwood, J.M.; Herberman, R.B.; Kalinski, P. IL-18-induced CD83(+)CCR7(+) NK helper cells. J. Exp. Med. 2005, 202, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Pesce, S.; Carlomagno, S.; Moretta, A.; Sivori, S.; Marcenaro, E. Uptake of CCR7 by KIR2DS4(+) NK Cells is induced upon recognition of certain HLA-C Alleles. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.H.; Cooley, S.; Verneris, M.; Walcheck, B.; et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013, 121, 3599–3608. [Google Scholar] [CrossRef]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.H.; Caligiuri, M.A. The broad spectrum of human natural killer cell diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [Green Version]
- Minetto, P.; Guolo, F.; Pesce, S.; Greppi, M.; Obino, V.; Ferretti, E.; Sivori, S.; Genova, C.; Lemoli, R.M.; Marcenaro, E. Harnessing NK cells for cancer treatment. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Cantoni, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001, 19, 197–223. [Google Scholar] [CrossRef]
- Sivori, S.; Vitale, M.; Morelli, L.; Sanseverino, L.; Augugliaro, R.; Bottino, C.; Moretta, L.; Moretta, A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J. Exp. Med. 1997, 186, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Pessino, A.; Sivori, S.; Bottino, C.; Malaspina, A.; Morelli, L.; Moretta, L.; Biassoni, R.; Moretta, A. Molecular cloning of NKp46: A novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J. Exp. Med. 1998, 188, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Vitale, M.; Bottino, C.; Sivori, S.; Sanseverino, L.; Castriconi, R.; Marcenaro, E.; Augugliaro, R.; Moretta, L.; Moretta, A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J. Exp. Med. 1998, 187, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Parolini, S.; Pessino, A.; Sivori, S.; Augugliaro, R.; Morelli, L.; Marcenaro, E.; Accame, L.; Malaspina, A.; Biassoni, R.; et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 1999, 190, 1505–1516. [Google Scholar] [CrossRef]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The Natural cytotoxicity receptors in health and disease. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Della Chiesa, M.; De Maria, A.; Muccio, L.; Bozzano, F.; Sivori, S.; Moretta, L. Human NK cells and herpesviruses: Mechanisms of recognition, response and adaptation. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immun. 2017, 139, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.S.; Yusa, S.; Kikuchi-Maki, A.; Catina, T.L. NKP44 triggers NK cell activation through DAP12 association that is not influenced by a putative cytoplasmic inhibitory sequence. J. Immunol. 2004, 172, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivori, S.; Pende, D.; Bottino, C.; Marcenaro, E.; Pessino, A.; Biassoni, R.; Moretta, L.; Moretta, A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 1999, 29, 1656–1666. [Google Scholar] [CrossRef]
- Di Vito, C.; Mikulak, J.; Zaghi, E.; Pesce, S.; Marcenaro, E.; Mavilio, D. NK cells to cure cancer. Semin. Immunol. 2019, 41. [Google Scholar] [CrossRef]
- Rosental, B.; Brusilovsky, M.; Hadad, U.; Oz, D.; Appel, M.Y.; Afergan, F.; Yossef, R.; Rosenberg, L.A.; Aharoni, A.; Cerwenka, A.; et al. Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J. Immunol. 2011, 187, 5693–5702. [Google Scholar] [CrossRef]
- Reiners, K.S.; Topolar, D.; Henke, A.; Simhadri, V.R.; Kessler, J.; Sauer, M.; Bessler, M.; Hansen, H.P.; Tawadros, S.; Herling, M.; et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013, 121, 3658–3665. [Google Scholar] [CrossRef]
- Von Strandmann, E.P.; Simhadri, V.R.; von Tresckow, B.; Sasse, S.; Reiners, K.S.; Hansen, H.P.; Rothe, A.; Boll, B.; Simhadri, V.L.; Borchmann, P.; et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 2007, 27, 965–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baychelier, F.; Sennepin, A.; Ermonval, M.; Dorgham, K.; Debre, P.; Vieillard, V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood 2013, 122, 2935–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narni-Mancinelli, E.; Gauthier, L.; Baratin, M.; Guia, S.; Fenis, A.; Deghmane, A.E.; Rossi, B.; Fourquet, P.; Escaliere, B.; Kerdiles, Y.M.; et al. Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaggero, S.; Bruschi, M.; Petretto, A.; Parodi, M.; del Zotto, G.; Lavarello, C.; Prato, C.; Santucci, L.; Barbuto, A.; Bottino, C.; et al. Nidogen-1 is a novel extracellular ligand for the NKp44 activating receptor. Oncoimmunology 2018, 7. [Google Scholar] [CrossRef]
- Niehrs, A.; Garcia-Beltran, W.F.; Norman, P.J.; Watson, G.M.; Holzemer, A.; Chapel, A.; Richert, L.; Pommerening-Roser, A.; Korner, C.; Ozawa, M.; et al. A subset of HLA-DP molecules serve as ligands for the natural cytotoxicity receptor NKp44. Nat. Immunol. 2019, 20, 1129. [Google Scholar] [CrossRef]
- Parodi, M.; Pedrazzi, M.; Cantoni, C.; Averna, M.; Patrone, M.; Cavaletto, M.; Spertino, S.; Pende, D.; Balsamo, M.; Pietra, G.; et al. Natural Killer (NK)/melanoma cell interaction induces NK-mediated release of chemotactic High Mobility Group Box-1 (HMGB1) capable of amplifying NK cell recruitment. Oncoimmunology 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Della Chiesa, M.; Carlomagno, S.; Frumento, G.; Balsamo, M.; Cantoni, C.; Conte, R.; Moretta, L.; Moretta, A.; Vitale, M. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46-and NKG2D-activating receptors and regulates NK-cell function. Blood 2006, 108, 4118–4125. [Google Scholar] [CrossRef]
- Castriconi, R.; Cantoni, C.; Chiesa, M.D.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [Green Version]
- Spaggiari, G.M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M.C.; Moretta, L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008, 111, 1327–1333. [Google Scholar] [CrossRef]
- Pietra, G.; Manzini, C.; Rivara, S.; Vitale, M.; Cantoni, C.; Petretto, A.; Balsamo, M.; Conte, R.; Benelli, R.; Minghelli, S.; et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012, 72, 1407–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesce, S.; Tabellini, G.; Cantoni, C.; Patrizi, O.; Coltrini, D.; Rampinelli, F.; Matta, J.; Vivier, E.; Moretta, A.; Parolini, S.; et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. Oncoimmunology 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the Ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabile, H.; Fionda, C.; Gismondi, A.; Santoni, A. Role of Distinct natural Killer Cell Subsets in Anticancer Response. Front Immunol 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Pesce, S.; Belgrano, V.; Greppi, M.; Carlomagno, S.; Squillario, M.; Barla, A.; Chiesa, M.D.; di Domenico, S.; Mavilio, D.; Moretta, L.; et al. Different features of tumor-associated NK cells in patients with low-grade or high-grade peritoneal carcinomatosis. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, A.; Campbell, D.; Hannum, C.; Yssel, H.; FranzBacon, K.; McClanahan, T.; Kitamura, T.; Nicholl, J.; Sutherland, G.R.; Lanier, L.L.; et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996, 4, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Martinet, L.; Smyth, M.J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015, 15, 243–254. [Google Scholar] [CrossRef]
- De Andrade, L.F.; Smyth, M.J.; Martinet, L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol Cell Biol 2014, 92, 237–244. [Google Scholar] [CrossRef]
- Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C.; Grassi, J.; Marcenaro, S.; Reymond, N.; et al. Identification of PVR (CD155) and nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 2003, 198, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Nabekura, T.; Kanaya, M.; Shibuya, A.; Fu, G.; Gascoigne, N.R.J.; Lanier, L.L. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity 2014, 40, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Stannard, K.A.; Lemoine, S.; Watserhouse, N.J.; Vari, F.; Chatenoud, L.; Gandhi, M.K.; Martinet, L.; Smyth, M.J.; Guillerey, C. Human peripheral blood DNAM-1(neg) NK cells are a terminally differentiated subset with limited effector functions. Blood Adv. 2019, 3, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Norell, H.; Bryceson, Y.T.; Poschke, I.; Schedvins, K.; Liunggren, H.G.; Kiessling, R.; Malmberg, K.J. Primary Human Tumor Cells Expressing CD155 Impair Tumor Targeting by Down-Regulating DNAM-1 on NK Cells. J. Immunol. 2009, 183, 4921–4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iguchi-Manaka, A.; Okumura, G.; Kojima, H.; Cho, Y.; Hirochika, R.; Bando, H.; Sato, T.; Yoshikawa, H.; Hara, H.; Shibuya, A.; et al. Increased soluble CD155 in the serum of cancer patients. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, G.; Iguchi-Manaka, A.; Murata, R.; Yamashita-Kanemaru, Y.; Shibuya, A.; Shibuya, K. Tumor-derived soluble CD155 inhibits DNAM-1-mediated antitumor activity of natural killer cells. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Lanier, L.L. NKG2D receptor and its ligands in host defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.W.; Jung, H.Y. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.F.; Wang, S.J.; Xin, J.; Wang, J.; Yao, C.P.; Zhang, Z.X. Role of NKG2D and its ligands in cancer immunotherapy. Am. J. Cancer Res. 2019, 9, 2064–2078. [Google Scholar]
- Dhar, P.; Wu, J.D. NKG2D and its ligands in cancer. Curr. Opin. Immunol. 2018, 51, 55–61. [Google Scholar] [CrossRef]
- Soriani, A.; Fionda, C.; Ricci, B.; Iannitto, M.L.; Cippitelli, M.; Santoni, A. Chemotherapy-elicited upregulation of NKG2D and DNAM-1 ligands as a therapeutic target in multiple myeloma. Oncoimmunology 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Blanco, A.; de la Cruz-Hernandez, E.; Dominguez, G.I.; Rodriguez-Cortez, O.; Alatorre, B.; Perez-Cardenas, E.; Chacon-Salinas, R.; Trejo-Becerril, C.; Taja-Chayeb, L.; Trujillo, J.E.; et al. Upregulation of NKG2D ligands and enhanced natural killer cell cytotoxicity by hydralazine and valproate. Int. J. Oncol. 2011, 39, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer (vol 18, pg 671, 2018). Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Peng, Y.P.; Zhang, J.J.; Liang, W.B.; Tu, M.; Lu, Z.P.; Wei, J.S.; Jiang, K.R.; Gao, W.T.; Wu, J.L.; Xu, Z.K.; et al. Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates natural killer cell dysfunction. BMC Cancer 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, V.; Reni, M.; Ychou, M.; Richel, D.J.; Macarulla, T.; Ducreux, M. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: Rationale and current evidence for new therapeutic strategies. Cancer Treat. Rev. 2014, 40, 118–128. [Google Scholar] [CrossRef]
- Lim, S.A.; Kim, J.; Jeon, S.; Shin, M.H.; Kwon, J.; Kim, T.J.; Im, K.; Han, Y.; Kwon, W.; Kim, S.W.; et al. Defective localization with impaired tumor cytotoxicity contributes to the immune escape of NK cells in pancreatic cancer patients. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Watt, J.; Kocher, H.M. The desmoplastic stroma of pancreatic cancer is a barrier to immune cell infiltration. Oncoimmunology 2013, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.S.; Pirola, R.C.; Apte, M.V. Stars and stripes in pancreatic cancer: Role of stellate cells and stroma in cancer progression. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.P.; Xi, C.H.; Zhu, Y.; Yin, L.D.; Wei, J.S.; Zhang, J.J.; Liu, X.C.; Guo, S.; Fu, Y.; Miao, Y. Altered expression of CD226 and CD96 on natural killer cells in patients with pancreatic cancer. Oncotarget 2016, 7, 66586–66594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.P.; Zhu, Y.; Zhang, J.J.; Xu, Z.K.; Qian, Z.Y.; Dai, C.C.; Jiang, K.R.; Wu, J.L.; Gao, W.T.; Li, Q.; et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J. Transl. Med. 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Maisonneuve, C.; Irrazabal, T.; Martin, A.; Girardin, S.E.; Philpott, D.J. The impact of the gut microbiome on colorectal cancer. Annu. Rev. Canc. Biol. 2018, 2, 229–249. [Google Scholar] [CrossRef]
- Harada, S.; Morlote, D. Molecular pathology of colorectal cancer. Adv. Anat. Pathol. 2020, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Turano, M.; Delrio, P.; Rega, D.; Cammarota, F.; Polverino, A.; Duraturo, F.; Izzo, P.; de Rosa, M. Promising colorectal cancer biomarkers for precision prevention and therapy. Cancers 2019, 11, 1932. [Google Scholar] [CrossRef] [Green Version]
- Augestad, K.M.; Merok, M.A.; Ignatovic, D. Tailored treatment of colorectal cancer: Surgical, molecular, and genetic considerations. Clin. Med. Insights On 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Mlecnik, B.; Marliot, F.; Ou, F.S.; Bifulco, C.B.; Lugli, A.; Zlobec, I.; Rau, T.T.; Hartmann, A.; Masucci, G.V.; et al. Validation of the Immunoscore (IM) as a prognostic marker in stage I/II/III colon cancer: Results of a worldwide consortium-based analysis of 1,336 patients. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Pages, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Tumor immunology and tumor evolution: Intertwined histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Sconocchia, G.; Eppenberger, S.; Spagnoli, G.C.; Tornillo, L.; Droeser, R.; Caratelli, S.; Ferrelli, F.; Coppola, A.; Arriga, R.; Lauro, D.; et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Coppola, A.; Arriga, R.; Lauro, D.; del Principe, M.I.; Buccisano, F.; Maurillo, L.; Palomba, P.; Venditti, A.; Sconocchia, G. NK cell inflammation in the clinical outcome of colorectal carcinoma. Front. Med. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Kulkarni, N.; Shilpi; Lal, G. Intratumoral natural killer cells show reduced effector and cytolytic properties and control the differentiation of effector Th1 cells. Oncoimmunology 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Rocca, Y.S.; Roberti, M.P.; Julia, E.P.; Pampena, M.B.; Bruno, L.; Rivero, S.; Huertas, E.; Loria, F.S.; Pairola, A.; Caignard, A.; et al. Phenotypic and functional dysregulated blood NK cells in colorectal cancer Patients can Be activated by cetuximab Plus IL-2 or IL-15. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharagozloo, M.; Kalantari, H.; Rezaei, A.; Maracy, M.R.; Salehi, M.; Bahador, A.; Hassannejad, N.; Narimani, M.; Sanei, M.H.; Bayat, B.; et al. The decrease in NKG2D+Natural Killer cells in peripheral blood of patients with metastatic colorectal cancer. Bratisl. Med. J. 2015, 116, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Lee, J.; Sano, T.; Janjigian, Y.Y.; Fan, D.M.; Song, S.M. Gastric adenocarcinoma. Nat. Rev. Dis. Primers 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Li, B.L.; Jiang, Y.M.; Li, G.X.; Fisher, G.A.; Li, R.J. Natural killer cell and stroma abundance are independently prognostic and predict gastric cancer chemotherapy benefit. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [Green Version]
- Li, T.J.; Zhang, Q.; Jiang, Y.M.; Yu, J.; Hu, Y.F.; Mou, T.Y.; Chen, G.H.; Li, G.X. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.S.; Zhang, J.Y.; Teng, Y.S.; Zhao, Y.L.; Wang, T.T.; Mao, F.Y.; Lv, Y.P.; Cheng, P.; Li, W.H.; Chen, N.; et al. Tumor-Associated monocytes/macrophages impair nk-cell function via TGF beta 1 in human gastric cancer. Cancer Immunol. Res. 2017, 5, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Gulubova, M.; Manolova, I.; Kyurkchiev, D.; Julianov, A.; Altunkova, I. Decrease in intrahepatic CD56+lymphocytes in gastric and colorectal cancer patients with liver metastases. Apmis 2009, 117, 870–879. [Google Scholar] [CrossRef]
- Wang, Z.G.; Si, X.L.; Xu, A.; Meng, X.N.; Gao, S.L.; Qi, Y.J.; Zhu, L.; Li, T.J.; Li, W.P.; Dong, L.Y. Activation of STAT3 in human gastric cancer cells via Interleukin (IL)-6-Type cytokine signaling correlates with clinical implications. PloS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Tomasello, E.; Yessaad, N.; Gregoire, E.; Hudspeth, K.; Luci, C.; Mavilio, D.; Hardwigsen, J.; Vivier, E. Mapping of NKp46(+) cells in healthy human lymphoid and non-lymphoid tissues. Front. Immunol. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggi, A.; Benelli, R.; Vene, R.; Costa, D.; Ferrari, N.; Tosetti, F.; Zocchi, M.R. Human gut-associated natural killer cells in health and disease. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussan, H.; Clinton, S.K.; Roberts, K.; Bailey, M.T. Fusobacterium’s link to colorectal neoplasia sequenced: A systematic review and future insights. World J. Gastroentero 2017, 23, 8626–8650. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastro. Hepat. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Fu, X.S. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl. Oncol. 2019, 12, 846–851. [Google Scholar] [CrossRef]
- Chaushu, S.; Wilensky, A.; Gur, C.; Shapira, L.; Elboim, M.; Halftek, G.; Polak, D.; Achdout, H.; Bachrach, G.; Mandelboim, O. Direct recognition of fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Bi, J.C.; Zheng, X.D.; Chen, Y.Y.; Wang, H.; Wu, W.Y.; Wang, Z.G.; Wu, Q.; Peng, H.; Wei, H.M.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723. [Google Scholar] [CrossRef]
- Halama, N.; Braun, M.; Kahlert, C.; Spille, A.; Quack, C.; Rahbari, N.; Koch, M.; Weitz, J.; Kloor, M.; Zoernig, I.; et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin. Cancer Res. 2011, 17, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Rocca, Y.S.; Roberti, M.P.; Arriaga, J.M.; Amat, M.; Bruno, L.; Pampena, M.B.; Huertas, E.; Loria, F.S.; Pairola, A.; Bianchini, M.; et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. London 2013, 19, 76–85. [Google Scholar] [CrossRef]
- Cella, M.; Fuchs, A.; Vermi, W.; Facchetti, F.; Otero, K.; Lennerz, J.K.M.; Doherty, J.M.; Mills, J.C.; Colonna, M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009, 457, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Cupedo, T.; Crellin, N.K.; Papazian, N.; Rombouts, E.J.; Weijer, K.; Grogan, J.L.; Fibbe, W.E.; Cornelissen, J.J.; Spits, H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127(+) natural killer-like cells. Nat. Immunol. 2009, 10, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Glatzer, T.; Killig, M.; Meisig, J.; Ommert, I.; Luetke-Eversloh, M.; Babic, M.; Paclik, D.; Bluthgen, N.; Seidl, R.; Seifarth, C.; et al. ROR gamma t(+) Innate Lymphoid Cells Acquire a Proinflammatory Program upon Engagement of the Activating Receptor NKp44. Immunity 2013, 38, 1223–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luci, C.; Reynders, A.; Ivanov, I.I.; Cognet, C.; Chiche, L.; Chasson, L.; Hardwigsen, J.; Anguiano, E.; Banchereau, J.; Chaussabel, D.; et al. Influence of the transcription factor ROR gamma t on the development of NKp46(+) cell populations in gut and skin. Nat. Immunol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Vosshenrich, C.A.J.; Lesjean-Pottier, S.; Sawa, S.; Lochner, M.; Rattis, F.; Mention, J.J.; Thiam, K.; Cerf-Bensussan, N.; Mandelboim, O.; et al. Microbial flora drives interleukin 22 production in intestinal NKp46(+) cells that provide innate mucosal immune defense. Immunity 2008, 29, 958–970. [Google Scholar] [CrossRef]
- Atreya, I.; Kindermann, M.; Wirtz, S. Innate lymphoid cells in intestinal cancer development. Semin. Immunol. 2019, 41. [Google Scholar] [CrossRef]
- Penny, H.A.; Hodge, S.H.; Hepworth, M.R. Orchestration of intestinal homeostasis and tolerance by group 3 innate lymphoid cells. Semin. Immunopathol 2018, 40, 357–370. [Google Scholar] [CrossRef] [Green Version]
- De Vries, N.L.; van Unen, V.; Ijsselsteijn, M.E.; Abdelaal, T.; van der Breggen, R.; Sarasqueta, A.F.; Mahfouz, A.; Peeters, K.C.M.J.; Hollt, T.; Lelieveldt, B.P.F.; et al. High-dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. Gut 2020, 69, 691–703. [Google Scholar] [CrossRef] [Green Version]
- Tallerico, R.; Todaro, M.; Di Franco, S.; Maccalli, C.; Garofalo, C.; Sottile, R.; Palmieri, C.; Tirinato, L.; Pangigadde, P.N.; La Rocca, R.; et al. Human NK cells selective targeting of colon cancer-initiating cells: A role for natural cytotoxicity receptors and MHC class I molecules. J. Immunol. 2013, 190, 2381–2390. [Google Scholar] [CrossRef] [Green Version]
- Krijgsman, D.; de Vries, N.L.; Skovbo, A.; Andersen, M.N.; Swets, M.; Bastiaannet, E.; Vahrmeijer, A.L.; van de Velde, C.J.H.; Heemskerk, M.H.M.; Hokland, M.; et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: The peripheral blood immune cell profile. Cancer Immunol. Immun. 2019, 68, 1011–1024. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.P.; Xie, M.Z.; Li, K.Z.; Li, J.L.; Cai, Z.M.; Hu, B.L. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol. 2020, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, F.; Qu, D.; Sun, R.Y.; Tao, H.; Si, J.R.; Xu, Y.Q. The role of circulating CD16+CD56+Natural killer cells in the screening, diagnosis, and staging of colorectal cancer before initial treatment. Dis. Markers 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Qu, D.; Sun, R.Y.; Nan, K.J. Circulating CD16+CD56+nature killer cells indicate the prognosis of colorectal cancer after initial chemotherapy. Med. Oncol. 2018, 36. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Mao, F.Y.; Zhao, Y.L.; Lv, Y.P.; Teng, Y.S.; Duan, M.B.; Chen, W.S.; Cheng, P.; Wang, T.T.; Liang, Z.Y.; et al. Altered NKp30, NKp46, NKG2D, and DNAM-1 expression on circulating NK cells is associated with tumor progression in human gastric cancer. J. Immunol. Res. 2018, 2018. [Google Scholar] [CrossRef]
- Chen, X.J.; Shen, J.; Zhang, G.B.; Chen, W.C. B7-H6 protein expression has no prognostic significance in human gastric carcinoma. Pathol. Oncol. Res. 2014, 20, 203–207. [Google Scholar] [CrossRef]
- Kono, K.; Takahashi, A.; Ichihara, F.; Sugai, H.; Fujii, H.; Matsumoto, Y. Impaired antibody-dependent cellular cytotoxicity mediated by Herceptin in patients with gastric cancer. Cancer Res. 2002, 62, 5813–5817. [Google Scholar]
- Duan, S.X.; Guo, W.H.; Xu, Z.X.; He, Y.B.; Liang, C.T.; Mo, Y.Z.; Wang, Y.; Xiong, F.; Guo, C.; Li, Y.; et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol. Cancer 2019, 18. [Google Scholar] [CrossRef]
- Duan, X.H.; Deng, L.M.; Chen, X.; Lu, Y.B.; Zhang, Q.; Zhang, K.J.; Hu, Y.J.; Zeng, J.; Sun, W.J. Clinical significance of the immunostimulatory MHC class I chain-related molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med. Oncol. 2011, 28, 466–474. [Google Scholar] [CrossRef]
- Chung, H.W.; Jang, S.; Lim, J.B. Clinical implications and diagnostic usefulness of correlation between soluble major histocompatibility complex class I chain-related molecule a and protumorigenic cytokines in pancreatic ductal adenocarcinoma. Cancer Am. Cancer Soc. 2013, 119, 233–244. [Google Scholar] [CrossRef]
- Van Audenaerde, J.R.M.; De Waele, J.; Marcq, E.; Van Loenhout, J.; Lion, E.; van den Bergh, J.M.J.; Jesenofsky, R.; Masamune, A.; Roeyen, G.; Pauwels, P.; et al. Interleukin-15 stimulates natural killer cell-mediated killing of both human pancreatic cancer and stellate cells. Oncotarget 2017, 8, 56968–56979. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.B.; Hu, J.J.; Sun, W.J.; Duan, X.H.; Chen, X. Hypoxia-mediated immune evasion of pancreatic carcinoma cells. Mol. Med. Rep. 2015, 11, 3666–3672. [Google Scholar] [CrossRef] [PubMed]
- Chitadze, G.; Lettau, M.; Bhat, J.; Wesch, D.; Steinle, A.; Furst, D.; Mytilineos, J.; Kalthoff, H.; Janssen, O.; Oberg, H.H.; et al. Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: Heterogeneous involvement of the "a disintegrin and metalloproteases" 10 and 17. Int. J. Cancer 2013, 133, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.S.; Huang, M.; Xie, F.; Zhou, H.C.; Yang, J.; Huang, Q. Gemcitabine promotes immune escape of pancreatic cancer by down regulating the soluble ULBP2 protein. Oncotarget 2016, 7, 70092–70099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, T.; Miki, K.; Kamigaki, T.; Makino, I.; Nakagawara, H.; Tajima, H.; Takamura, H.; Kitagawa, H.; Fushida, S.; Ahmed, A.K.; et al. Low-dose gemcitabine induces major histocompatibility complex class I-related chain A/B expression and enhances an antitumor innate immune response in pancreatic cancer. Clin. Exp. Med. 2017, 17, 19–31. [Google Scholar] [CrossRef]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Chen, M.Y.; Smith, R.C.; Hagino, T.; Perez-Cunningham, J.; Sckisel, G.D.; Urayama, S.; et al. NK cells preferentially target tumor cells with a cancer stem cell phenotype. J. Immunol. 2015, 195, 4010–4019. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.J.; Lu, C.; Tian, W.J.; Wang, L.C.; Cui, B.; Jiao, Y.L.; Ma, C.Y.; Ju, Y.; Zhu, L.; Shao, C.H.; et al. Possible association of decreased NKG2D expression levels and suppression of the activity of natural killer cells in patients with colorectal cancer. Int. J. Oncol. 2012, 40, 1285–1290. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Stieber, P.; Peterfi, A.; Nagel, D.; Steinle, A.; Salih, H.R. Soluble MICA in malignant diseases. Int. J. Cancer 2006, 118, 684–687. [Google Scholar] [CrossRef]
- Watson, N.F.S.; Spendlovel, I.; Madjd, Z.; McGilvray, R.; Green, A.R.; Ellis, I.O.; Scholefield, J.H.; Durrant, L.G. Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. Int. J. Cancer 2006, 118, 1445–1452. [Google Scholar] [CrossRef]
- Chen, D.; Gyllensten, U. MICA polymorphism: Biology and importance in cancer. Carcinogenesis 2014, 35, 2633–2642. [Google Scholar] [CrossRef]
- Tang, S.W.; Fu, H.Y.; Xu, Q.H.; Zhou, Y. miR-20a regulates sensitivity of colorectal cancer cells to NK cells by targeting MICA. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.F.; Ma, Y.Y.; Zhu, W.F.; Pu, W.L.; Zhang, J.F.; Qian, F.; Zhou, Y.L.; Deng, Y.; Guo, S.C.; Wang, J.C.; et al. MICA*012:01allele facilitates the metastasis of KRAS-mutant colorectal cancer. Front. Genet. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wei, Y.C. Therapeutic potential of natural killer cells in gastric cancer. Front. Immunol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, R.; Yoshimura, K.; Yoshino, S.; Inoue, M.; Asao, T.; Fuse, M.; Wada, S.; Kuramasu, A.; Furuya-Kondo, T.; Oga, A.; et al. Expression levels of UL16 binding protein 1 and natural killer group 2 member D affect overall survival in patients with gastric cancer following gastrectomy. Oncol. Lett. 2018, 15, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Mimura, K.; Kamiya, T.; Shiraishi, K.; Kua, L.F.; Shabbir, A.; So, J.; Yong, W.P.; Suzuki, Y.; Yoshimoto, Y.; Nakano, T.; et al. Therapeutic potential of highly cytotoxic natural killer cells for gastric cancer. Int. J. Cancer 2014, 135, 1390–1398. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Xu, X.Y. DKK3 attenuates the cytotoxic effect of natural killer cells on CD133(+) gastric cancer cells. Mol. Carcinog. 2017, 56, 1712–1721. [Google Scholar] [CrossRef]
- Saito, H.; Osaki, T.; Ikeguchi, M. Decreased NKG2D expression on NK cells correlates with impaired NK cell function in patients with gastric cancer. Gastric. Cancer 2012, 15, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.L.; Wang, H.J.; Nie, Y.Z.; Mi, Q.Y.; Chen, X.G.; Hou, Y.Y. Midkine upregulates MICA/B expression in human gastric cancer cells and decreases natural killer cell cytotoxicity. Cancer Immunol. Immun. 2012, 61, 1745–1753. [Google Scholar] [CrossRef]
- Shiraishi, K.; Mimura, K.; Kua, L.F.; Koh, V.; Siang, L.; Nakajima, S.; Fujii, H.; Shabbir, A.; Yong, W.P.; So, J.; et al. Inhibition of MMP activity can restore NKG2D ligand expression in gastric cancer, leading to improved NK cell susceptibility. J. Gastroenterol. 2016, 51, 1101–1111. [Google Scholar] [CrossRef]
- Hu, B.G.; Tian, X.K.; Li, Y.B.; Liu, Y.C.; Yang, T.; Han, Z.D.; An, J.J.; Kong, L.Q.; Li, Y.M. Epithelial-mesenchymal transition may be involved in the immune evasion of circulating gastric tumor cells via downregulation of ULBP1. Cancer Med. 2020, 9, 2686–2697. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Su, T.; He, L.; Wang, H.T.; Ji, G.; Liu, X.N.; Zhang, Y.; Dong, G.L. Identification and functional analysis of ligands for natural killer cell activating receptors in colon carcinoma. Tohoku J. Exp. Med. 2012, 226, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Karabulut, M.; Gunaldi, M.; Alis, H.; Afsar, C.U.; Karabulut, S.; Serilmez, M.; Akarsu, C.; Seyit, H.; Aykan, N.F. Serum nectin-2 levels are diagnostic and prognostic in patients with colorectal carcinoma. Clin. Transl. Oncol. 2016, 18, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Schnalzger, T.E.; Groot, M.H.D.; Zhang, C.C.; Mosa, M.H.; Michels, B.E.; Roder, J.; Darvishi, T.; Wels, W.S.; Farin, H.F. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. Embo. J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, H.X.; Ding, J.G.; Liu, H.Y.; Li, H.Z.; Li, H.L.; Lu, M.M.; Miao, Y.N.; Li, L.T.; Zheng, J.N. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J. Immunol. Res. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Felices, M.; Lenvik, T.R.; Davis, Z.B.; Miller, J.S.; Vallera, D.A. Generation of BiKEs and TriKEs to improve NK cell-mediated targeting of tumor cells. Methods Mol. Biol. 2016, 1441, 333–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferretti, E.; Carlomagno, S.; Pesce, S.; Muccio, L.; Obino, V.; Greppi, M.; Solari, A.; Setti, C.; Marcenaro, E.; Della Chiesa, M.; et al. Role of the Main Non HLA-Specific Activating NK Receptors in Pancreatic, Colorectal and Gastric Tumors Surveillance. Cancers 2020, 12, 3705. https://doi.org/10.3390/cancers12123705
Ferretti E, Carlomagno S, Pesce S, Muccio L, Obino V, Greppi M, Solari A, Setti C, Marcenaro E, Della Chiesa M, et al. Role of the Main Non HLA-Specific Activating NK Receptors in Pancreatic, Colorectal and Gastric Tumors Surveillance. Cancers. 2020; 12(12):3705. https://doi.org/10.3390/cancers12123705
Chicago/Turabian StyleFerretti, Elisa, Simona Carlomagno, Silvia Pesce, Letizia Muccio, Valentina Obino, Marco Greppi, Agnese Solari, Chiara Setti, Emanuela Marcenaro, Mariella Della Chiesa, and et al. 2020. "Role of the Main Non HLA-Specific Activating NK Receptors in Pancreatic, Colorectal and Gastric Tumors Surveillance" Cancers 12, no. 12: 3705. https://doi.org/10.3390/cancers12123705
APA StyleFerretti, E., Carlomagno, S., Pesce, S., Muccio, L., Obino, V., Greppi, M., Solari, A., Setti, C., Marcenaro, E., Della Chiesa, M., & Sivori, S. (2020). Role of the Main Non HLA-Specific Activating NK Receptors in Pancreatic, Colorectal and Gastric Tumors Surveillance. Cancers, 12(12), 3705. https://doi.org/10.3390/cancers12123705






