Current and New Biomarkers for Early Detection, Prognostic Stratification, and Management of Gallbladder Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
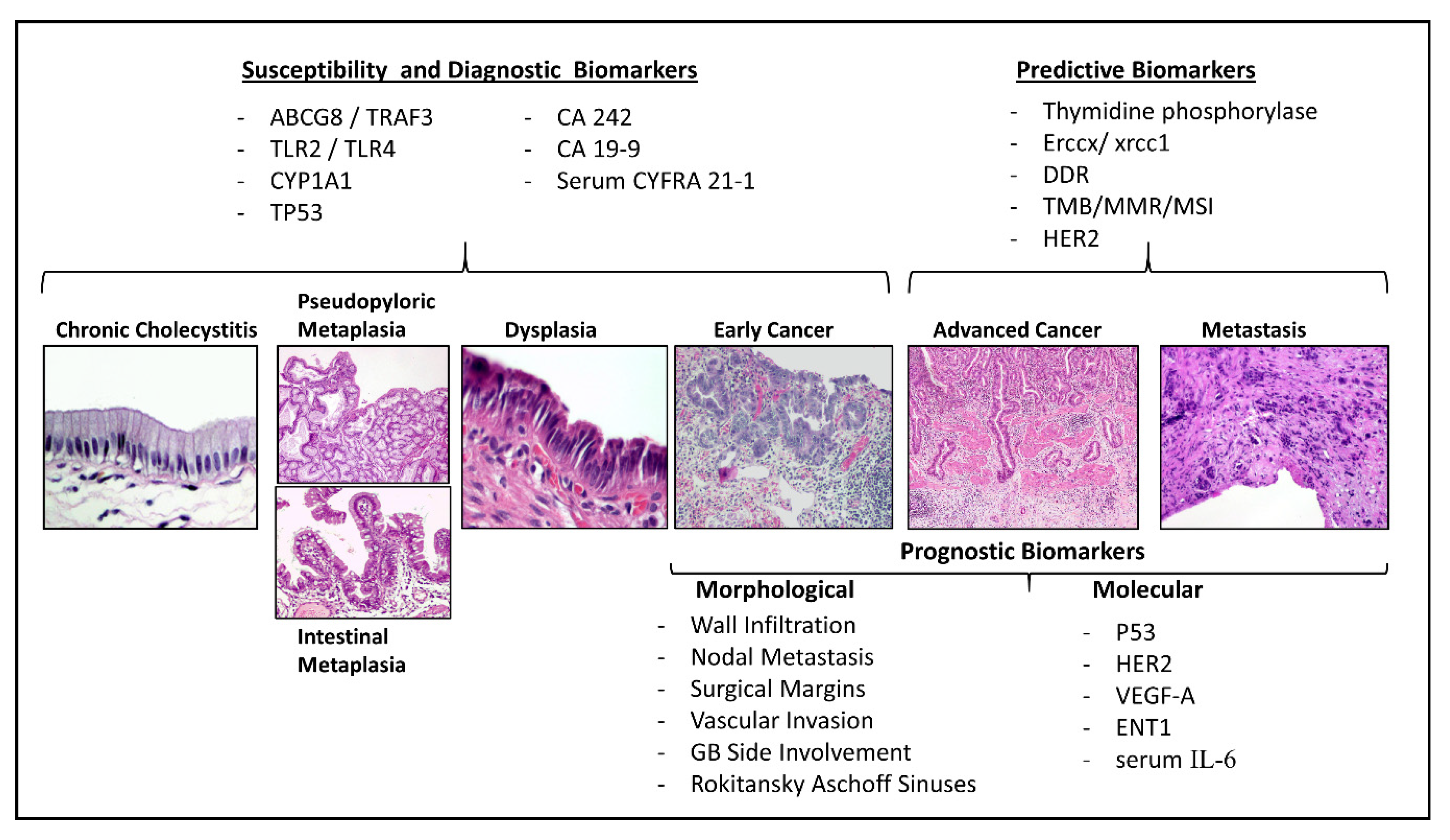
2. Genetic Susceptibility Biomarkers
3. Diagnosis Biomarkers
4. Prognostic Biomarkers in GBC
4.1. Pathological Prognostic Markers for Early and Advanced Gallbladder Cancer
4.2. Molecular Prognostic Biomarkers
5. Prognostic and Predictive Biomarkers for Treatment Selection
5.1. Predictors of Response to Adjuvant Treatment
5.2. Predictors of Response to Palliative Chemotherapy
5.3. Response to Targeted Therapies
5.4. Immunotherapy and Potential Predictive Factors
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, E.; Hundal, R. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Srivastava, A.; Mittal, B. Potential biomarkers in gallbladder cancer: Present status and future directions. Biomarkers 2012, 18, 1–9. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Montalvo-Jave, E.E.; Rahnemai-Azar, A.A.; Papaconstantinou, D.; Deloiza, M.E.; Tsilimigras, D.I.; Moris, D.; Mendoza-Barrera, G.E.; Weber, S.M.; Pawlik, T.M. Molecular pathways and potential biomarkers in gallbladder cancer: A comprehensive review. Surg. Oncol. 2019, 31, 83–89. [Google Scholar] [CrossRef]
- Andrén-Sandberg, Å. Diagnosis and management of gallbladder cancer. N. Am. J. Med. Sci. 2012, 4, 293–299. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Kim, R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: A review of the literature and future directions. Cancer Treat. Rev. 2009, 35, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, J.-K.; Ma, W.-J.; Yang, Q.; Hu, H.-J.; Li, F.-Y. Clinical value of preoperative CA19-9 levels in evaluating resectability of gallbladder carcinoma. ANZ J. Surg. 2018, 89, E76–E80. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39, 108–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, A.; Shah, S.A. Screening high risk populations for cancer: Hepatobiliary. J. Surg. Oncol. 2019, 120, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Kapacee, Z.; Breeze, M.; Bell, C.; Belcher, D.; Staiger, H.; Taylor, C.; McNamara, M.G.; Hubner, R.A.; Valle, J.W. Molecular Profiling in Daily Clinical Practice: Practicalities in Advanced Cholangiocarcinoma and Other Biliary Tract Cancers. J. Clin. Med. 2020, 9, 2854. [Google Scholar] [CrossRef]
- Lamarca, A.; Frizziero, M.; McNamara, M.G.; Valle, J.W. Clinical and Translational Research Challenges in Biliary Tract Cancers. Curr. Med. Chem. 2020, 27, 4756–4777. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020, 73, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Liebe, R.; Milkiewicz, P.; Krawczyk, M.; Bonfrate, L.; Portincasa, P.; Krawczyk, M. Modifiable Factors and Genetic Predisposition Associated with Gallbladder Cancer. A Concise Review. J. Gastrointest. Liver Dis. 2015, 24, 339–348. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Marcano-Bonilla, L.; Roberts, L.R. Gallbladder cancer: Epidemiology and genetic risk associations. Chin. Clin. Oncol. 2019, 8, 31. [Google Scholar] [CrossRef]
- Sakoda, L.C.; Gao, Y.-T.; Chen, B.E.; Chen, J.; Rosenberg, P.S.; Rashid, A.; Deng, J.; Shen, M.-C.; Wang, B.-S.; Han, T.-Q.; et al. Prostaglandin-endoperoxide synthase 2 (PTGS2) gene polymorphisms and risk of biliary tract cancer and gallstones: A population-based study in Shanghai, China. Carcinogenesis 2005, 27, 1251–1256. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, K.; Srivastava, A.; Pandey, S.N.; Kumar, A.; Mittal, B. Functional polymorphisms of the cyclooxygenase (PTGS2) gene and risk for gallbladder cancer in a North Indian population. J. Gastroenterol. 2009, 44, 774–780. [Google Scholar] [CrossRef]
- Srivastava, K.; Srivastava, A.; Kumar, A.; Mittal, B. Significant association between toll-like receptor gene polymorphisms and gallbladder cancer. Liver Int. 2010, 30, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Gao, Y.-T.; Corbel, A.; Kemp, T.J.; Shen, M.-C.; Hildesheim, A.; Hsing, A.W.; Rashid, A.; Wang, B.; Pfeiffer, R.M.; et al. Circulating inflammatory proteins and gallbladder cancer: Potential for risk stratification to improve prioritization for cholecystectomy in high-risk regions. Cancer Epidemiol. 2018, 54, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Tsuchiya, Y.; Lang, I.; Zoltan, S.; Nakadaira, H.; Ajioka, Y.; Kiyohara, C.; Oyama, M.; Nakamura, K. Effect of genetic predisposition on the risk of gallbladder cancer in Hungary. Asian Pac. J. Cancer Prev. 2008, 9, 391–396. [Google Scholar] [PubMed]
- Tsuchiya, Y.; Kiyohara, C.; Sato, T.; Nakamura, K.; Kimura, A.; Yamamoto, M. Polymorphisms of cytochrome P450 1A1, glutathione S-transferase class mu, and tumour protein p53 genes and the risk of developing gallbladder cancer in Japanese. Clin. Biochem. 2007, 40, 881–886. [Google Scholar] [CrossRef]
- Park, S.K.; Andreotti, G.; Sakoda, L.C.; Gao, Y.-T.; Rashid, A.; Chen, J.; Chen, B.E.; Rosenberg, P.S.; Shen, M.-C.; Wang, B.-S.; et al. Variants in hormone-related genes and the risk of biliary tract cancers and stones: A population-based study in China. Carcinogens 2009, 30, 606–614. [Google Scholar] [CrossRef]
- Sakai, K.; Loza, E.; Roig, G.V.-G.; Nozaki, R.; Asai, T.; Ikoma, T.; Tsuchiya, Y.; Kiyohara, C.; Yamamoto, M.; Nakamura, K. CYP1A1, GSTM1, GSTT1 and TP53 Polymorphisms and Risk of Gallbladder Cancer in Bolivians. Asian Pac. J. Cancer Prev. 2016, 17, 781–784. [Google Scholar] [CrossRef] [Green Version]
- Asai, T.; Tsuchiya, Y.; Mishra, K.; Behari, A.; Shukla, P.; Ikoma, T.; Kapoor, V.K.; Nakamura, K. Carcinogen Metabolism Pathway and Tumor Suppressor Gene Polymorphisms and Gallbladder Cancer Risk in North Indians: A Hospital-Based Case-Control Study. Asian Pac. J. Cancer Prev. 2019, 20, 3643–3647. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Baez, S.; Calvo, A.; Pruyas, M.; Nakamura, K.; Kiyohara, C.; Oyama, M.; Ikegami, K.; Yamamoto, M. Evidence that Genetic Variants of Metabolic Detoxication and Cell Cycle Control Are Not Related to Gallbladder Cancer Risk in Chilean Women. Int. J. Biol. Markers 2010, 25, 75–78. [Google Scholar] [CrossRef]
- Pandey, S.N.; Jain, M.; Nigam, P.; Choudhuri, G.; Mittal, B. Genetic polymorphisms in GSTM1, GSTT1, GSTP1, GSTM3 and the susceptibility to gallbladder cancer in North India. Biomarkers 2006, 11, 250–261. [Google Scholar] [CrossRef]
- Srivastava, K.; Srivastava, A.; Mittal, B. Polymorphisms in ERCC2, MSH2, and OGG1 DNA repair genes and gallbladder cancer risk in a population of Northern India. Cancer 2010, 116, 3160–3169. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, W.-Y.; Andreotti, G.; Gao, Y.-T.; Rashid, A.; Chen, J.; Sakoda, L.C.; Shen, M.-C.; Wang, B.-S.; Chanock, S.; et al. Variants of DNA Repair Genes and the Risk of Biliary Tract Cancers and Stones: A Population-Based Study in China. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2123–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, R.; Sharma, K.L.; Sharma, S.; Misra, S.; Kumar, A.; Mittal, B. Death Receptor (DR4) Haplotypes Are Associated with Increased Susceptibility of Gallbladder Carcinoma in North Indian Population. PLoS ONE 2014, 9, e90264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, K.; Srivastava, A.; Mittal, B. Caspase-8 polymorphisms and risk of gallbladder cancer in a Northern Indian population. Mol. Carcinog. 2010, 49, 684–692. [Google Scholar] [CrossRef]
- Cha, P.-C.; Zembutsu, H.; Takahashi, A.; Kubo, M.; Kamatani, N.; Nakamura, Y. A genome-wide association study identifies SNP in DCC is associated with gallbladder cancer in the Japanese population. J. Hum. Genet. 2012, 57, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, S.; Wang, Z.; Nagrani, R.; Badwe, R.; Chiplunkar, S.; Mittal, B.; Yadav, S.; Zhang, H.; Chung, C.C.; Patil, P.; et al. Common genetic variation and risk of gallbladder cancer in India: A case-control genome-wide association study. Lancet Oncol. 2017, 18, 535–544. [Google Scholar] [CrossRef]
- Boekstegers, F.; Marcelain, K.; Ponce, C.B.; Benavides, P.F.B.; Müller, B.; De Toro, G.; Retamales, J.; Barajas, O.; Ahumada, M.; Morales, E.; et al. ABCB1/4 gallbladder cancer risk variants identified in India also show strong effects in Chileans. Cancer Epidemiol. 2020, 65, 101643. [Google Scholar] [CrossRef] [PubMed]
- Bustos, B.I.; Pérez-Palma, E.; Buch, S.; Azócar, L.; Riveras, E.; Ugarte, G.D.; Toliat, M.; Nürnberg, P.; Lieb, W.; Franke, A.; et al. Variants in ABCG8 and TRAF3 genes confer risk for gallstone disease in admixed Latinos with Mapuche Native American ancestry. Sci. Rep. 2019, 9, 772. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, K.L.; Gupta, A.; Yadav, A.; Kumar, A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J. Gastroenterol. 2017, 23, 3978–3998. [Google Scholar] [CrossRef]
- Soreide, K.; Guest, R.V.; Harrison, E.M.; Kendall, T.J.; Garden, O.J.; Wigmore, S.J. Systematic review of management of incidental gallbladder cancer after cholecystectomy. BJS 2018, 106, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Levy, A.D.; Murakata, L.A.; Rohrmann, C.A. Gallbladder Carcinoma: Radiologic-Pathologic Correlation. Radiographics 2001, 21, 295–314. [Google Scholar] [CrossRef] [Green Version]
- Ratanaprasatporn, L.; Uyeda, J.W.; Wortman, J.R.; Richardson, I.; Sodickson, A.D. Multimodality Imaging, including Dual-Energy CT, in the Evaluation of Gallbladder Disease. Radiography 2018, 38, 75–89. [Google Scholar] [CrossRef]
- Abhishek, V.; Vijayakumar, A.; Patil, V.; Mallikarjuna, M.N.; Shivaswamy, B.S. Early Diagnosis of Gallbladder Carcinoma: An Algorithm Approach. ISRN Radiol. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Kapoor, A.; Kapoor, A.; Mahajan, G. Differentiating Malignant From Benign Thickening of the Gallbladder Wall by the Use of Acoustic Radiation Force Impulse Elastography. J. Ultrasound Med. 2011, 30, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Tavolari, S.; Brandi, G. Circulating Tumor DNA in Biliary Tract Cancer: Current Evidence and Future Perspectives. Cancer Genom.-Proteom. 2020, 17, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Tewari, S.; Husain, N.; Agarwal, A.; Pandey, A.; Singhal, A.; Lohani, M. Quantification of Circulating Free DNA as a Diagnostic Marker in Gall Bladder Cancer. Pathol. Oncol. Res. 2016, 23, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Husain, N.; Agarwal, A.; Neyaz, A.; Gupta, S.; Chaturvedi, A.; Lohani, M.; Sonkar, A.A. Diagnostic Value of Circulating Free DNA Integrity and Global Methylation Status in Gall Bladder Carcinoma. Pathol. Oncol. Res. 2018, 25, 925–936. [Google Scholar] [CrossRef]
- Kinugasa, H.; Nouso, K.; Ako, S.; Dohi, C.; Matsushita, H.; Matsumoto, K.; Kato, H.; Okada, H. Liquid biopsy of bile for the molecular diagnosis of gallbladder cancer. Cancer Biol. Ther. 2018, 19, 934–938. [Google Scholar] [CrossRef]
- Shen, N.; Zhang, D.; Yin, L.; Qiu, Y.; Liu, J.; Yu, W.; Fu, X.; Zhu, B.; Xu, X.; Duan, A.; et al. Bile cell-free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol. Rep. 2019, 42, 549–560. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Chen, J. Diagnostic Value of Serum Biomarkers for Intrahepatic Cholangiocarcinoma. J. Coll. Physicians Surg. Pak. 2019, 29, 962–966. [Google Scholar] [CrossRef]
- Molina, V.; Visa, L.; Conill, C.; Navarro, S.; Escudero, J.M.; Auge, J.M.; Filella, X.; Lopez-Boado, M.A.; Ferrer, J.; Fernandez-Cruz, L.; et al. CA 19–9 in pancreatic cancer: Retrospective evaluation of patients with suspicion of pancreatic cancer. Tumor Biol. 2012, 33, 799–807. [Google Scholar] [CrossRef]
- Wang, Y.-F. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J. Gastroenterol. 2014, 20, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Dutta, U.; Kochhar, R.; Rana, S.V.; Gupta, R.; Pal, R.; Jain, K.; Srinivasan, R.; Nagi, B.; Nain, C.K.; et al. Evaluation of CA 242 as a Tumor Marker in Gallbladder Cancer. J. Gastrointest. Cancer 2011, 43, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, T.; Yamazaki, O.; Tanaka, H.; Takemura, S.; Yamamoto, T.; Tanaka, S.; Nishiguchi, S.; Kubo, S. Serum Cytokeratin 19 Fragment (CYFRA21-1) as a Prognostic Factor in Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2007, 15, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, W.; Liang, P.; Hu, W.; Zhang, K.; Shen, S.; Chen, J.; Zhang, Z.; Chen, B.; Han, Y.; et al. Serum CYFRA 21-1 in Biliary Tract Cancers: A Reliable Biomarker for Gallbladder Carcinoma and Intrahepatic Cholangiocarcinoma. Dig. Dis. Sci. 2014, 60, 1273–1283. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumari, N.; Krishnani, N. Molecular pathogenesis of gallbladder cancer: An update. Mutat. Res. Mol. Mech. Mutagen. 2019, 111674. [Google Scholar] [CrossRef]
- Sicklick, J.K.; Fanta, P.T.; Shimabukuro, K.; Kurzrock, R. Genomics of gallbladder cancer: The case for biomarker-driven clinical trial design. Cancer Metastasis Rev. 2016, 35, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Artico, M.; Bronzetti, E.; Alicino, V.; Ionta, B.; Bosco, S.; Grande, C.; Bruno, M.; Leali, F.M.T.; Ionta, G.; Fumagalli, L. Human gallbladder carcinoma: Role of neurotrophins, MIB-1, CD34 and CA15-3. Eur. J. Histochem. 2010, 54, e10. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Liu, Z.; Zhang, Y.; Wang, P.; Gao, H. Combination of CA19-9 and the Neutrophil-to-Lymphocyte Ratio for the Differential Diagnosis of Gallbladder Carcinoma. Cancer Manag. Res. 2020, 12, 4475–4482. [Google Scholar] [CrossRef]
- Amini, N.; Kim, Y.; Wilson, A.; Margonis, G.A.; Ethun, C.G.; Poultsides, G.; Tran, T.; Idrees, K.; Isom, C.A.; Fields, R.C.; et al. Prognostic Implications of Lymph Node Status for Patients with Gallbladder Cancer: A Multi-Institutional Study. Ann. Surg. Oncol. 2016, 23, 3016–3023. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, H.J.; Chen, Y. Establishment of a Gallbladder Cancer-Specific Survival Model to Predict Prognosis in Non-metastatic Gallbladder Cancer Patients After Surgical Resection. Dig. Dis. Sci. 2018, 63, 2251–2258. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ms, J.M.H.; Paredes, A.Z.; Moris, D.; Beal, E.W.; Merath, K.; Mehta, R.; Ejaz, A.; Cloyd, J.M.; Pawlik, T.M. The optimal number of lymph nodes to evaluate among patients undergoing surgery for gallbladder cancer: Correlating the number of nodes removed with survival in 6531 patients. J. Surg. Oncol. 2019, 119, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Kayahara, M.; Nagakawa, T.; Nakagawara, H.; Kitagawa, H.; Ohta, T. Prognostic Factors for Gallbladder Cancer in Japan. Ann. Surg. 2008, 248, 807–814. [Google Scholar] [CrossRef] [PubMed]
- De Aretxabala, X.; Roa, I.; Hepp, J.; Maluenda, F.; Mordojovich, G.; León, J.; Roa, J.C. Early gallbladder cancer: Is further treatment necessary? J. Surg. Oncol. 2009, 100, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E. Systematic review on the surgical treatment for T1 gallbladder cancer. World J. Gastroenterol. 2011, 17, 174–180. [Google Scholar] [CrossRef] [PubMed]
- FuksJean, D.; Regimbeau, J.-M.; Le Treut, Y.-P.; Bachellier, P.; Raventos, A.; Pruvot, F.-R.; Chiche, L.; Farges, O. Incidental Gallbladder Cancer by the AFC-GBC-2009 Study Group. World J. Surg. 2011, 35, 1887–1897. [Google Scholar] [CrossRef]
- Barreto, S.G.; Pawar, S.; Shah, S.; Talole, S.; Goel, M.; Shrikhande, S. Patterns of Failure and Determinants of Outcomes Following Radical Re-resection for Incidental Gallbladder Cancer. World J. Surg. 2013, 38, 484–489. [Google Scholar] [CrossRef]
- Chong, A.J.U.; Lee, W.J. Oncologic Outcomes of Extended Lymphadenectomy without Liver Resection for T1/T2 Gallbladder Cancer. Yonsei Med. J. 2019, 60, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kwon, W.; Han, Y.; Kim, J.R.; Kim, S.-W.; Jang, J.-Y. Optimal extent of surgery for early gallbladder cancer with regard to long-term survival: A meta-analysis. J. Hepato-Biliary-Pancreat. Sci. 2017, 25, 131–141. [Google Scholar] [CrossRef]
- Yuza, K.; Sakata, J.; Prasoon, P.; Hirose, Y.; Ohashi, T.; Toge, K.; Miura, K.; Nagahashi, M.; Kobayashi, T.; Wakai, T. Long-term outcomes of surgical resection for T1b gallbladder cancer: An institutional evaluation. BMC Cancer 2020, 20, 20–29. [Google Scholar] [CrossRef]
- Lee, S.E.; Korean Pancreas Surgery Club; Jang, J.-Y.; Kim, S.-W.; Han, H.-S.; Kim, H.-J.; Yun, S.-S.; Cho, B.-H.; Yu, H.C.; Lee, W.J.; et al. Surgical Strategy for T1 Gallbladder Cancer: A Nationwide Multicenter Survey in South Korea. Ann. Surg. Oncol. 2014, 21, 3654–3660. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Miyakawa, S.; Nagino, M.; Takada, T.; Miyazaki, M. Updated clinical practice guidelines for the management of biliary tract cancers: Revision concepts and major revised points. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Park, J.W.; Kim, H.; Han, Y.; Kwon, W.; Kim, S.-W.; Hwang, Y.J.; Kim, S.G.; Kwon, H.J.; Vinuela, E.; et al. Optimal surgical treatment in patients with T1b gallbladder cancer: An international multicenter study. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Adsay, V.; Saka, B.; Basturk, O.; Roa, J.C. Criteria for pathologic sampling of gallbladder specimens. Am. J. Clin. Pathol. 2013, 140, 278–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roa, J.C.; Tapia, O.; Manterola, C.; Villaseca, M.; Guzman, P.; Araya, J.C.; Bagci, P.; Saka, B.; Adsay, V. Early gallbladder carcinoma has a favorable outcome but Rokitansky–Aschoff sinus involvement is an adverse prognostic factor. Virchows Archiv 2013, 463, 651–661. [Google Scholar] [CrossRef]
- Roa, I.; Araya, J.C.; Villaseca, M.; Roa, J.; De Aretxabala, X.; Ibacache, G. Gallbladder cancer in a high risk area: Morphological features and spread patterns. Hepatogastroenterology 1999, 46, 1540–1546. [Google Scholar]
- Albores-Saavedra, J.; Shukla, D.; Carrick, K.; Henson, D.E. In Situ and Invasive Adenocarcinomas of the Gallbladder Extending Into or Arising From Rokitansky-Aschoff Sinuses. Am. J. Surg. Pathol. 2004, 28, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Gleisner, A.L.; Vigano, L.; Kooby, D.A.; Bauer, T.W.; Frilling, A.; Adams, R.B.; Staley, C.A.; Trindade, E.N.; Schulick, R.D.; et al. Incidence of Finding Residual Disease for Incidental Gallbladder Carcinoma: Implications for Re-resection. J. Gastrointest. Surg. 2007, 11, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Butte, J.M.; Kingham, T.P.; Gönen, M.; D’Angelica, M.I.; Allen, P.J.; Fong, Y.; DeMatteo, R.P.; Jarnagin, W.R. Residual Disease Predicts Outcomes after Definitive Resection for Incidental Gallbladder Cancer. J. Am. Coll. Surg. 2014, 219, 416–429. [Google Scholar] [CrossRef] [Green Version]
- Creasy, J.M.; Goldman, D.A.; Gonen, M.; Dudeja, V.; Askan, G.; Basturk, O.; Balachandran, V.P.; Allen, P.J.; DeMatteo, R.P.; D’Angelica, M.I.; et al. Predicting Residual Disease in Incidental Gallbladder Cancer: Risk Stratification for Modified Treatment Strategies. J. Gastrointest. Surg. 2017, 21, 1254–1261. [Google Scholar] [CrossRef]
- Ramos, E.; Lluis, N.; Llado, L.; Torras, J.; Busquets, J.; Rafecas, A.; Serrano, T.; Mils, K.; Leiva, D.; Fabregat, J. Prognostic value and risk stratification of residual disease in patients with incidental gallbladder cancer. World J. Surg. Oncol. 2020, 18, 18. [Google Scholar] [CrossRef]
- Gil, L.; De Aretxabala, X.; Lendoire, J.; Duek, F.; Hepp, J.; Imventarza, O. Incidental Gallbladder Cancer: How Residual Disease Affects Outcome in Two Referral HPB Centers from South America. World J. Surg. 2018, 43, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Krell, R.W.; Wei, A.C. Gallbladder cancer: Surgical management. Chin. Clin. Oncol. 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lim, T.-W.; Park, P.-J.; Choi, S.-B.; Kim, W.-B. Clinicopathological Differences in T2 Gallbladder Cancer According to Tumor Location. Cancer Control. 2020, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindoh, J.; De Aretxabala, X.; Aloia, T.A.; Roa, J.C.; Roa, I.; Zimmitti, G.; Javle, M.; Conrad, C.; Maru, D.M.; Aoki, T.; et al. Tumor Location Is a Strong Predictor of Tumor Progression and Survival in T2 Gallbladder Cancer. Ann. Surg. 2015, 261, 733–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Choi, D.W.; Park, J.-Y.; Youn, S.; Kwon, W.; Heo, J.S.; Choi, S.-H.; Jang, K.-T. Surgical Strategy for T2 Gallbladder Cancer According to Tumor Location. Ann. Surg. Oncol. 2014, 22, 2779–2786. [Google Scholar] [CrossRef]
- Ethun, C.G.; Postlewait, L.M.; Le Bs, N.; Pawlik, T.M.; Buettner, S.; Poultsides, G.; Tran, T.; Idrees, K.; Isom, C.A.; Fields, R.C.; et al. A Novel Pathology-Based Preoperative Risk Score to Predict Locoregional Residual and Distant Disease and Survival for Incidental Gallbladder Cancer: A 10-Institution Study from the U.S. Extrahepatic Biliary Malignancy Consortium. Ann. Surg. Oncol. 2017, 24, 1343–1350. [Google Scholar] [CrossRef]
- Mochizuki, T.; Abe, T.; Amano, H.; Hanada, K.; Hattori, M.; Kobayashi, T.; Nakahara, M.; Ohdan, H.; Noriyuki, T. Efficacy of the Gallbladder Cancer Predictive Risk Score Based on Pathological Findings: A Propensity Score-Matched Analysis. Ann. Surg. Oncol. 2018, 25, 1699–1708. [Google Scholar] [CrossRef]
- Maruyama, S.; Kawaida, H.; Hosomura, N.; Amemiya, H.; Saito, R.; Shimizu, H.; Furuya, S.; Akaike, H.; Kawaguchi, Y.; Sudo, M.; et al. Indications for extrahepatic bile duct resection due to perineural invasion in patients with gallbladder cancer. World J. Surg. Oncol. 2019, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.J.; Yoo, B.C.; Kim, S.W.; Lee, B.L.; Kim, W.H. Significance of PML and p53 protein as molecular prognostic markers of gallbladder carcinomas. Pathol. Oncol. Res. 2007, 13, 326–335. [Google Scholar] [CrossRef]
- Kim, K.; Kim, N.-H.; Chae, S.W.; Shin, J.-H.; Kim, H.J.; Do, S.; Lee, H.J.; Koo, J.H.; Pyo, J.-S.; Sohn, J.H. Expression of Cell Cycle-Related Proteins, p16, p53 and p63 as Important Prognostic Markers in Gallbladder Adenocarcinoma. Pathol. Oncol. Res. 2013, 20, 409–415. [Google Scholar] [CrossRef]
- Singh, A.; Mishra, P.K.; Saluja, S.S.; Talikoti, M.A.; Kirtani, P.; Najmi, A.K. Prognostic Significance of HER-2 and p53 Expression in Gallbladder Carcinoma in North Indian Patients. Oncology 2016, 91, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Rashid, A.; Churi, C.; Kar, S.; Zuo, M.; Eterovic, A.K.; Nogueras-Gonzalez, G.M.; Janku, F.; Shroff, R.T.; Aloia, T.A.; et al. Molecular characterization of gallbladder cancer using somatic mutation profiling. Hum. Pathol. 2014, 45, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivaldi, C.; Fornaro, L.; Ugolini, C.; Niccoli, C.; Musettini, G.; Pecora, I.; Insilla, A.C.; Salani, F.; Pasquini, G.; Catanese, S.; et al. HER2 Overexpression as a Poor Prognostic Determinant in Resected Biliary Tract Cancer. Oncology 2020, 25, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, G.; Lerut, E.; Ectors, N.; Hendrickx, T.; Aerts, R.; Topal, B. The prognostic relevance of tumor hypoxia markers in resected carcinoma of the gallbladder. Eur. J. Surg. Oncol. 2011, 37, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Pais-Costa, S.R.; Farah, J.F.D.M.; Artigiani-Neto, R.; Martins, S.J.; Goldenberg, A. Evaluation of P53, E-cadherin, Cox-2, and EGFR protein imunnoexpression on prognostic of resected gallbladder carcinoma. ABCD. Arq. Bras. Cir. Dig. 2014, 27, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Letelier, P.; García, P.; Leal, P.; Ili, C.; Buchegger, K.; Riquelme, I.; Sandoval, A.; Tapia, O.; Roa, J.C. Immunohistochemical Expression of Vascular Endothelial Growth Factor A in Advanced Gallbladder Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 530–536. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.-L.; Wang, C.; Miao, X.; Liu, C.; Li, D.; Zou, Q.; Li, J.; Liang, L.; Zeng, G.; et al. The Expression of Notch 1 and Notch 3 in Gallbladder Cancer and Their Clinicopathological Significance. Pathol. Oncol. Res. 2015, 22, 483–492. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Liao, M.; Deng, Z.; Gong, L.; Jiang, J.; Lynn, L.; Wu, K.; Miao, X. Clinicopathologic and Prognostic Significance of CD24 in Gallbladder Carcinoma. Pathol. Oncol. Res. 2010, 17, 45–50. [Google Scholar] [CrossRef]
- García, P.; Rosa, L.; Vargas, S.; Weber, H.; Espinoza, J.A.; Suárez, F.; Romero-Calvo, I.; Elgueta, N.; Rivera, V.; Nervi, B.; et al. Hippo-YAP1 Is a Prognosis Marker and Potentially Targetable Pathway in Advanced Gallbladder Cancer. Cancers 2020, 12, 778. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.-C.; Yang, Z.-L.; Jiang, S. Identification of musashi-1 and ALDH1 as carcinogenesis, progression, and poor-prognosis related biomarkers for gallbladder adenocarcinoma. Cancer Biomark. 2011, 8, 113–121. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Z.; Yang, L.; Liu, J.; Miao, X. Expressive levels of MUC1 and MUC5AC and their clinicopathologic significances in the benign and malignant lesions of gallbladder. J. Surg. Oncol. 2011, 105, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.A.; García, P.; Bizama, C.; Leal, J.L.; Riquelme, I.; Weber, H.; Macanas, P.; Aguayo, G.; Viñuela, E.; Roa, J.C.; et al. Low expression of equilibrative nucleoside transporter 1 is associated with poor prognosis in chemotherapy-naïve pT2 gallbladder adenocarcinoma patients. Histopathology 2015, 68, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.; García, P.; Sandoval, A.; Letelier, P.; Brebi, P.; Ili, C.; Álvarez, H.; Tapia, O.; Roa, J.C. Immunohistochemical Expression of Phospho-mTOR Is Associated with Poor Prognosis in Patients with Gallbladder Adenocarcinoma. Arch. Pathol. Lab. Med. 2013, 137, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.-Z.; Kong, X.; Weng, M.-Z.; Zhang, M.-D.; Qin, Y.-Y.; Gong, W.; Zhang, W.-J.; Quan, Z. Long non-coding RNA-LET is a positive prognostic factor and exhibits tumor-suppressive activity in gallbladder cancer. Mol. Carcinog. 2015, 54, 1397–1406. [Google Scholar] [CrossRef]
- Wu, X.; Wang, F.; Li, H.; Hu, Y.; Jiang, L.; Zhang, F.; Li, M.; Wang, X.; Jin, Y.; Zhang, Y.; et al. Lnc RNA - PAGBC acts as a micro RNA sponge and promotes gallbladder tumorigenesis. EMBO Rep. 2017, 18, 1837–1853. [Google Scholar] [CrossRef]
- Jin, L.; Cai, Q.; Wang, S.; Wang, S.; Mondal, T.; Wang, J.; Quan, Z. Long noncoding RNA MEG3 regulates LATS2 by promoting the ubiquitination of EZH2 and inhibits proliferation and invasion in gallbladder cancer. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yan, Y.; Zhang, G.; Chen, C.; Shen, W.; Xing, P. Knockdown of LINC01694 inhibits growth of gallbladder cancer cells via miR-340-5p/Sox4. Biosci. Rep. 2020, 40, 40. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yu, Y.; Li, H.; Hu, Q.; Chen, X.; He, Y.; Xue, C.; Ren, F.; Ren, Z.; Li, J.; et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol. Cancer 2019, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.-C.; Jiang, L.; Hong, H.-J.; Meng, Z.-W.; Du, Q.; Zhou, L.-Y.; She, F.; Chen, Y. Serum vascular endothelial growth factors C and D as forecast tools for patients with gallbladder carcinoma. Tumor Biol. 2015, 36, 6305–6312. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Liu, M.; Cai, X.; Xie, L.; She, F.; Chen, Y. Serum vascular endothelial growth factor-C levels predict lymph node metastasis and prognosis of patients with gallbladder cancer. Oncol. Lett. 2018, 16, 6065–6070. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, J.; Chang, Q.; Yang, B.; Li, S.; Gu, C. The association between preoperative serum interleukin-6 levels and postoperative prognosis in patients with T2 gallbladder cancer. J. Surg. Oncol. 2018, 117, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Li, S.X.; Cui, X.; Huang, Y.; Zhu, S.; Wang, Y.; Tan, H.; Ma, X. The prognostic value of preoperative inflammatory indexes in gallbladder carcinoma with hepatic involvement. Cancer Biomark. 2018, 22, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Fassan, M.; Ruzzenente, A.; Mafficini, A.; Wood, L.D.; Corbo, V.; Melisi, D.; Malleo, G.; Vicentini, C.; Malpeli, G.; et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 2014, 5, 2839–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javle, M.; Bekaii-Saab, T.S.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, R.; Yamaguchi, K.; Ikenoue, T.; Terakado, Y.; Ohta, Y.; Yamashita, N.; Kainuma, O.; Yokoi, S.; Maru, Y.; Nagase, H.; et al. Genetic alterations in Japanese extrahepatic biliary tract cancer. Oncol. Lett. 2017, 14, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Neyaz, A.; Husain, N.; Gupta, S.; Kumari, S.; Arora, A.; Awasthi, N.P.; Malhotra, K.P.; Misra, S. Investigation of targetable predictive and prognostic markers in gallbladder carcinoma. J. Gastrointest. Oncol. 2018, 9, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.; Kim, D.; Yoo, C.; Kim, K.-P.; Jeong, J.H.; Chang, H.-M.; Lee, S.S.; Park, D.H.; Song, T.J.; Hwang, S.; et al. Therapeutic relevance of targeted sequencing in management of patients with advanced biliary tract cancer: DNA damage repair gene mutations as a predictive biomarker. Eur. J. Cancer 2019, 120, 31–39. [Google Scholar] [CrossRef]
- Yadav, S.; De Sarkar, N.; Kumari, N.; Krishnani, N.; Kumar, A.; Mittal, B. Targeted Gene Sequencing of Gallbladder Carcinoma Identifies High-impact Somatic and Rare Germline Mutations. Cancer Genom.-Proteom. 2017, 14, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Wardell, C.P.; Fujita, M.; Yamada, T.; Simbolo, M.; Fassan, M.; Karlic, R.; Polak, P.; Kim, J.; Hatanaka, Y.; Maejima, K.; et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J. Hepatol. 2018, 68, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Bagante, F.; Ruzzenente, A.; Conci, S.; Rusev, B.C.; Simbolo, M.; Campagnaro, T.; Pawlik, T.M.; Luchini, C.; Iacono, C.; Scarpa, A.; et al. Patterns of gene mutations in bile duct cancers: Is it time to overcome the anatomical classification? HPB 2019, 21, 1648–1655. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarca, A.; Palmer, D.; Wasan, H.; Ryder, W.; Davies, L.; Flight, H.; Rogan, J.; Hubner, R.; Bridgewater, J.; Valle, J. Abc-06: A Randomised Phase Iii, Multi-Centre, Open-Label Study of Active Symptom Control (Asc) Alone or Asc with Oxaliplatin/5-Fu Chemotherapy for Patients with Locally Advanced/Metastatic Biliary Tract Cancers (Abc) Previously Treated with Cisplatin/Gemcitabine Chemotherapy. Ann. Oncol. 2014, 25, iv252. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, M.G.; Lopes, A.; Wasan, H.; Malka, D.; Goldstein, D.; Shannon, J.; Okusaka, T.; Knox, J.J.; Wagner, A.D.; André, T.; et al. Landmark survival analysis and impact of anatomic origin in prospective clinical trials of biliary tract cancer. J. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Lamarca, A.; McNamara, M.; Valle, J. PD-1 Systematic review and meta-analysis of the efficacy of chemotherapeutic regimens in advanced gallbladder cancer: Assessing current practice and treatment benefit. Ann. Oncol. 2020, 31, S212. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, B.H.; McLeod, H.L. Thymidine Phosphorylase and Capecitabine: A Predictive Marker for Therapy Selection? J. Clin. Oncol. 2006, 24, 4051–4053. [Google Scholar] [CrossRef]
- Won, H.S.; Lee, M.A.; Jung, E.S.; Kim, D.-G.; You, Y.K.; Hong, T.H.; Lee, I.S. Comparison of thymidine phosphorylase expression and prognostic factors in gallbladder and bile duct cancer. BMC Cancer 2010, 10, 564–568. [Google Scholar] [CrossRef] [Green Version]
- Mian, M.; McNamara, M.G.; Doherty, M.; Hedley, D.W.; Knox, J.J.; Serra, S. Predictive and prognostic values of ERCC1 and XRCC1 in biliary tract cancers. J. Clin. Pathol. 2016, 69, 695–701. [Google Scholar] [CrossRef]
- Tsavaris, N.; Kosmas, C.; Gouveris, P.; Gennatas, K.; Polyzos, A.; Mouratidou, D.; Tsipras, H.; Margaris, H.; Papastratis, G.; Tzima, E.; et al. Weekly Gemcitabine for the Treatment of Biliary Tract and Gallbladder Cancer. Investig. New Drugs 2004, 22, 193–198. [Google Scholar] [CrossRef]
- Eckel, F.; Schmid, R.M. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br. J. Cancer 2007, 96, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Kanai, M.; Kobayashi, S.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; Ishioka, C.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). Ann. Oncol. 2018, 29, viii205. [Google Scholar] [CrossRef]
- Belkouz, A.; De Vos-Geelen, J.; Eskens, F.; Mathot, R.A.; Van Gulik, T.; Van Oijen, M.G.; Punt, C.J.A.; Wilmink, J.; Kluempen, H.-J. Efficacy and safety of FOLFIRINOX in advanced biliary tract cancer after failure of gemcitabine plus cisplatin: A phase II trial. J. Clin. Oncol. 2019, 37, 4086. [Google Scholar] [CrossRef]
- Grunnet, M.; Christensen, I.J.; Lassen, U.; Jensen, L.H.; Lydolph, M.; Knox, J.J.; McNamara, M.G.; Jitlal, M.; Wasan, H.; Bridgewater, J.; et al. Decline in CA19-9 during chemotherapy predicts survival in four independent cohorts of patients with inoperable bile duct cancer. Eur. J. Cancer 2015, 51, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.; Lopes, A.; Wasan, H.; Malka, D.; Jensen, L.; Okusaka, T.; Knox, J.; Wagner, D.; Cunningham, D.; Shannon, J.; et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann. Oncol. 2016, 27, 134–140. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kan, M.; Kimura, G.; Umemoto, K.; Watanabe, K.; Sasaki, M.; Takahashi, H.; Hashimoto, Y.; Imaoka, H.; Ohno, I.; et al. Predictive factors of the treatment outcome in patients with advanced biliary tract cancer receiving gemcitabine plus cisplatin as first-line chemotherapy. J. Gastroenterol. 2019, 54, 281–290. [Google Scholar] [CrossRef] [Green Version]
- You, M.S.; Ryu, J.K.; Choi, Y.H.; Choi, J.H.; Huh, G.; Paik, W.H.; Lee, S.H.; Kim, Y.T. Therapeutic outcomes and prognostic factors in unresectable gallbladder cancer treated with gemcitabine plus cisplatin. BMC Cancer 2019, 19, 10. [Google Scholar] [CrossRef]
- Yoon, K.-A.; Lee, W.J.; Hong, E.K.; Jung, M.K.; Park, W.S.; Bae, K.; Han, S.-S.; Kim, T.H.; Koh, Y.H.; Park, S.-J.; et al. Cytidine Deaminase as a Molecular Predictor of Gemcitabine Response in Patients with Biliary Tract Cancer. Oncology 2015, 89, 345–350. [Google Scholar] [CrossRef]
- Backen, A.C.; Lopes, A.; Wasan, H.; Palmer, D.H.; Duggan, M.; Cunningham, D.; Anthoney, A.; Corrie, P.G.; Madhusudan, S.S.; Maraveyas, A.; et al. Circulating biomarkers during treatment in patients with advanced biliary tract cancer receiving cediranib in the UK ABC-03 trial. Br. J. Cancer 2018, 119, 27–35. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Biliary Tract Cancer: State of the Art and potential role of DNA Damage Repair. Cancer Treat. Rev. 2018, 70, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.; Subimerb, C.; Pairojkul, C.; Wongkham, S.; Cutcutache, I.; Yu, W.; McPherson, J.R.; Allen, G.E.; Ng, C.C.Y.; Wong, B.H.; et al. Exome sequencing of liver fluke–associated cholangiocarcinoma. Nat. Genet. 2012, 44, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Pawlik, T.M.; Anders, R.A.; Selaru, F.M.; Streppel, M.M.; Lucas, D.J.; Niknafs, N.; Guthrie, V.B.; Maitra, A.; Argani, P.; et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat. Genet. 2013, 45, 1470–1473. [Google Scholar] [CrossRef]
- Chan-On, W.; Nairismägi, M.-L.; Ong, C.K.; Lim, W.K.; Dima, S.; Pairojkul, C.; Lim, K.H.; McPherson, J.R.; Cutcutache, I.; Heng, H.L.; et al. Exome sequencing identifies distinct mutational patterns in liver fluke–related and non-infection-related bile duct cancers. Nat. Genet. 2013, 45, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Mafficini, A.; Amato, E.; Cataldo, I.; Rusev, B.C.; Bertoncello, L.; Corbo, V.; Simbolo, M.; Luchini, C.; Fassan, M.; Cantù, C.; et al. Ampulla of Vater Carcinoma. Ann. Surg. 2018, 267, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, R.; Ali, S.M.; Borad, M.J.; Shroff, R.T.; Kwong, L.; Vauthey, J.-N.; Koay, E.J.; Zuo, M.; Rashid, A.; Schrock, A.B.; et al. Variations in DNA repair genomic alterations and tumor mutation burden in biliary tract cancer (BTC) subtypes. J. Clin. Oncol. 2018, 36, 263. [Google Scholar] [CrossRef]
- Javle, M.; Catenacci, D.; Jain, A.; Young, L.; Wang, K.; Chung, J.; Hezel, A.F.; Schrock, A.B.; Goyal, L.; Gay, L.M.; et al. Precision medicine for gallbladder cancer using somatic copy number amplifications (SCNA) and DNA repair pathway gene alterations. J. Clin. Oncol. 2017, 35, 4076. [Google Scholar] [CrossRef]
- Kohya, N.; Miyazaki, K.; Matsukura, S.; Yakushiji, H.; Kitajima, Y.; Kitahara, K.; Fukuhara, M.; Nakabeppu, Y.; Sekiguchi, M. Deficient expression of O(6)-methylguanine-DNA methyltransferase combined with mismatch-repair proteins hMLH1 and hMSH2 is related to poor prognosis in human biliary tract carcinoma. Ann. Surg. Oncol. 2002, 9, 371–379. [Google Scholar] [CrossRef]
- Hwang, I.G.; Jang, J.-S.; Do, J.H.; Kang, J.H.; Lee, G.W.; Oh, S.Y.; Kwon, H.C.; Jun, H.J.; Lim, H.Y.; Lee, S.J.; et al. Different relation between ERCC1 overexpression and treatment outcomes of two platinum agents in advanced biliary tract adenocarcinoma patients. Cancer Chemother. Pharmacol. 2011, 68, 935–944. [Google Scholar] [CrossRef]
- Ahn, D.H.; Javle, M.; Ahn, C.W.; Jain, A.; Mikhail, S.; Noonan, A.M.; Wu, C.; Shroff, R.T.; Chen, J.L.; Bekaii-Saab, T. Next-generation sequencing survey of biliary tract cancer reveals the association between tumor somatic variants and chemotherapy resistance. Cancer 2016, 122, 3657–3666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borger, D.R.; Tanabe, K.K.; Fan, K.C.; Lopez, H.U.; Fantin, V.R.; Straley, K.S.; Schenkein, D.P.; Hezel, A.F.; Ancukiewicz, M.; Liebman, H.M.; et al. Frequent Mutation of Isocitrate Dehydrogenase (IDH)1 and IDH2 in Cholangiocarcinoma Identified Through Broad-Based Tumor Genotyping. Oncology 2011, 17, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holcombe, R.F.; Xiu, J.; Pishvaian, M.J.; Millis, S.Z.; Gatalica, Z.; Reddy, S.K.; Sicklick, J.K.; Fanta, P.T.; Kesari, S.; Morse, M. Tumor profiling of biliary tract carcinomas to reveal distinct molecular alterations and potential therapeutic targets. J. Clin. Oncol. 2015, 33, 285. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Javle, M.M.; Catenacci, D.V.T.; Shroff, R.T.; Ali, S.M.; Elvin, J.A.; Chmielecki, J.; Yelensky, R.; Lipson, D.; et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and frequency of clinically relevant genomic alterations. J. Clin. Oncol. 2015, 33, 4009. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, Y.; Wang, X.; Guo, W.; Gao, S.; Wei, L.; Shi, J.; Shi, G.; Wang, Z.; Zhang, Y.; et al. Activating Mutations in PTPN3 Promote Cholangiocarcinoma Cell Proliferation and Migration and Are Associated with Tumor Recurrence in Patients. Gastroenterology 2014, 146, 1397–1407. [Google Scholar] [CrossRef]
- Borad, M.J.; Champion, M.D.; Egan, J.B.; Liang, W.S.; Fonseca, R.; Bryce, A.H.; McCullough, A.E.; Barrett, M.T.; Hunt, K.; Patel, M.D.; et al. Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma. PLoS Genet. 2014, 10, e1004135. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Gay, L.; Al-Rohil, R.; Rand, J.V.; Jones, D.M.; Lee, H.J.; Sheehan, C.E.; Otto, G.A.; Palmer, G.; et al. New Routes to Targeted Therapy of Intrahepatic Cholangiocarcinomas Revealed by Next-Generation Sequencing. Oncology 2014, 19, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; ElZawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Tannapfel, A.; Sommerer, F.; Benicke, M.; Katalinic, A.; Uhlmann, D.; Witzigmann, H.; Hauss, J.; Wittekind, C. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut 2003, 52, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-K.; Van Es, J.H.; Born, M.V.D.; Clevers, H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc. Natl. Acad. Sci. USA 2015, 112, 7548–7550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drilon, A.; Siena, S.; Ou, S.-H.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhang, Z.; Li, X.; Ye, J.; Wu, X.; Tan, Z.; Liu, C.; Shen, B.; Wang, X.-A.; Wu, W.; et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat. Genet. 2014, 46, 872–876. [Google Scholar] [CrossRef]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef]
- Jaiswal, B.S.; Kljavin, N.M.; Stawiski, E.W.; Chan, E.; Parikh, C.; Durinck, S.; Chaudhuri, S.; Pujara, K.; Guillory, J.; Edgar, K.A.; et al. Oncogenic ERBB3 Mutations in Human Cancers. Cancer Cell 2013, 23, 603–617. [Google Scholar] [CrossRef] [Green Version]
- Kol, A.; Van Scheltinga, A.G.T.; Timmer-Bosscha, H.; Lamberts, L.E.; Bensch, F.; De Vries, E.G.; Schröder, C.P. HER3, serious partner in crime. Pharmacol. Ther. 2014, 143, 1–11. [Google Scholar] [CrossRef]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nat. Cell Biol. 2018, 554, 189–194. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Belani, C.P.; Singh, D.A.; Tanaka, M.; Lenz, H.-J.; Yen, Y.; Kindler, H.L.; Iqbal, S.; Longmate, J.; Mack, P.C.; et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother. Pharmacol. 2009, 64, 777–783. [Google Scholar] [CrossRef]
- Peck, J.; Wei, L.; Zalupski, M.; O’Neil, B.; Calero, M.V.; Bekaii-Saab, T. HER2/neu May Not Be an Interesting Target in Biliary Cancers: Results of an Early Phase II Study with Lapatinib. Oncology 2012, 82, 175–179. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Rankin, C.; Siegel, A.B.; Iqbal, S.; Gong, I.-Y.; Micetich, K.; Kayaleh, O.R.; Lenz, H.-J.; Blanke, C.D. S0941: A phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br. J. Cancer 2014, 110, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Aloysius, M.M.; Lobo, D.N.; Rowlands, B.J.; Madhusudan, S.S.; Ilyas, M.; Zaitoun, A.M. HER-2/Neu overexpression is a rare event in peri-ampullary cancer: Assessment using the HercepTestTM. Histopathology 2009, 55, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, W.; Wang, C.; Wang, L.; Yang, M.; Qi, M.; Su, H.; Sun, X.; Liu, Z.; Zhang, J.; et al. Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncol. Rep. 2014, 32, 700–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamoto, T.; Ishige, K.; Thomas, M.; Yamashita-Kashima, Y.; Shu, S.; Ishikura, N.; Ariizumi, S.; Yamamoto, M.; Kurosaki, K.; Shoda, J. Overexpression and gene amplification of EGFR, HER2, and HER3 in biliary tract carcinomas, and the possibility for therapy with the HER2-targeting antibody pertuzumab. J. Gastroenterol. 2014, 50, 467–479. [Google Scholar] [CrossRef]
- Lamarca, A.; Galdy, S.; Barriuso, J.; Moghadam, S.; Beckett, E.; Rogan, J.; Backen, A.; Billington, C.; McNamara, M.G.; Hubner, R.A.; et al. The HER3 pathway as a potential target for inhibition in patients with biliary tract cancers. PLoS ONE 2018, 13, e0206007. [Google Scholar] [CrossRef]
- Javle, M.; Hainsworth, J.D.; Swanton, C.; Burris, H.A.; Kurzrock, R.; Sweeney, C.; Meric-Bernstam, F.; Spigel, D.R.; Bose, R.; Guo, S.; et al. Pertuzumab + trastuzumab for HER2-positive metastatic biliary cancer: Preliminary data from MyPathway. J. Clin. Oncol. 2017, 35, 402. [Google Scholar] [CrossRef]
- Harding, J.; Cleary, J.; Shapiro, G.; Braña, I.; Moreno, V.; Quinn, D.; Borad, M.; Loi, S.; Spanggaard, I.; Stemmer, S.; et al. Treating HER2-mutant advanced biliary tract cancer with neratinib: Benefits of HER2-directed targeted therapy in the phase 2 SUMMIT ‘basket’ trial. Ann. Oncol. 2019, 30, iv127. [Google Scholar] [CrossRef]
- Zhang, N.; Chang, Y.; Rios, A.; An, Z. HER3/ErbB3, an emerging cancer therapeutic target. Acta Biochim. Biophys. Sin. 2015, 48, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Shimada, K.; Kosuge, T.; Hiraoka, N. A significant subgroup of resectable gallbladder cancer patients has an HER2 positive status. Virchows Archiv 2016, 468, 431–439. [Google Scholar] [CrossRef]
- Elebro, J.; Heby, M.; Warfvinge, C.F.; Nodin, B.; Eberhard, J.; Jirström, K. Expression and Prognostic Significance of Human Epidermal Growth Factor Receptors 1, 2 and 3 in Periampullary Adenocarcinoma. PLoS ONE 2016, 11, e0153533. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Piha-Paul, S.A.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: Results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J. Clin. Oncol. 2019, 37, 4079. [Google Scholar] [CrossRef]
- Ueno, M.; Chung, H.; Nagrial, A.; Marabelle, A.; Kelley, R.; Xu, L.; Mahoney, J.; Pruitt, S.; Oh, D.-Y. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase II KEYNOTE-158 study. Ann. Oncol. 2018, 29, viii210. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suto, T.; Habano, W.; Sugai, T.; Uesugi, N.; Kanno, S.; Saito, K.; Nakamura, S. Infrequent microsatellite instability in biliary tract cancer. J. Surg. Oncol. 2001, 76, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.W.K.; Askan, G.; Daniel, T.D.; Lowery, M.; Klimstra, D.S.; Abou-Alfa, G.K.; Shia, J. Biliary carcinomas: Pathology and the role of DNA mismatch repair deficiency. Chin. Clin. Oncol. 2016, 5, 62. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Kim, R.D.; Kim, D.W.; Alese, O.B.; Li, D.; Shah, N.; Schell, M.J.; Zhou, J.M.; Chung, V. A phase II study of nivolumab in patients with advanced refractory biliary tract cancers (BTC). J. Clin. Oncol. 2019, 37, 4097. [Google Scholar] [CrossRef]
- Rashid, A.; Ueki, T.; Gao, Y.-T.; Houlihan, P.S.; Wallace, C.; Wang, B.-S.; Shen, M.-C.; Deng, J.; Hsing, A.W. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: A population-based study in China. Clin. Cancer Res. 2002, 8, 3156–3163. [Google Scholar]
- McNamara, M.; Jacobs, T.; Frizziero, M.; Pihlak, R.; Lamarca, A.; Hubner, R.; Valle, J.; Amir, E. Prognostic and predictive impact of high tumor mutation burden (TMB) in solid tumors: A systematic review and meta-analysis. Ann. Oncol. 2019, 30, v25. [Google Scholar] [CrossRef]
| Genetic Variant | Measurement Technique | Utility | Reference |
|---|---|---|---|
| TLR2 (Delta22) and TLR4 (rs4986791) | PCR-RFLP | Higher susceptibility in North Indian population (233 GBC and 257 controls OR = 1.54/1.96, 95% CI: 1.02–2.24/1.11–2.26). | [21] |
| CYP1A1 (rs1048943) | PCR-RFLP | Increased risk of GBC among Hungarian and Japanese women (37 GBC and 48 controls; OR = 8.9, 95% CI: 2.9–27.4; and 33 GBC and 91 controls; OR = 2.70, 95% CI: 1.14–6.40, respectively). | [23,24] |
| CYP1A1 (rs2606345) | TaqMan assay | Higher risk and susceptibility in Chinese population (237 GBC and 737 controls; OR = 2.0, 95% CI: 1.3–3.0) | [25] |
| GSTM1 null genotype | PCR-RFLP | Increased risk in Bolivian population (32 GBC and 86 controls; OR = 2.35, 95% CI: 1.03–5.37) | [26] |
| TP53 Arg72Pro | PCR-RFLP | Increased risk of GBC among Japanese men (21 GBC and 87 controls; OR = 4.32, 95% CI: 1.08–17.2) | [24] |
| MSH2 IVS1 + 9G>C, ERCC2 Asp312Asn, and OGG1 Ser326Cys | PCR-RFLP | Increased risk in Indian population (230 GBC and 230 controls; OR = 1.8, 95% CI: 1.1–3.1; OR = 2.1, 95% CI: 1.1–4.0; and OR = 2.5, 95% CI: 1.1 –5.4, respectively). | [30] |
| ABCG8 (rs11887534) and TRAF3 (rs12882491) | GWAS | Higher risk in Chilean Latinos with Mapuche Native American ancestry (397 GBC and 667 controls; OR = 1.77, 95% CI: 1.27–2.45; and OR = 1.24, 95% CI: 1.004–1.53, respectively). | [37] |
| Biomarker | Measurement Technique | Utility | Reference |
|---|---|---|---|
| CA 242 | ELISA | Diagnosis (sensitivity 64%, specificity 83%; positive predictive value 88%, negative predictive values 53%) | [52] |
| CA 19-9 | ECLIA | Diagnosis (sensitivity 71.7%, specificity 96.1%) | [51] |
| CYFRA 21-1 | ECLIA | Diagnosis (cut-off values 3.27 ng/mL; sensitivity 93.7%, specificity 96.2%) | [54] |
| Biomarker | Measurement Technique | Prognostic Significance | Reference |
|---|---|---|---|
| p53 | IHC | Overexpression associated with reduced survival rates (n = 138, 96, and 60 GBC in each study). | [89,90,91] |
| HER2 | IHC | Overexpression associated with poor survival (n = 60) | [91] |
| EGFR | IHC | High expression was related to worse prognosis (n = 34 and 39 in each study) | [94,95] |
| VEGF-A | IHC | High expression associated with a poor prognosis in advanced GBC (n = 224) | [96] |
| NOTCH | IHC | Notch 1 and Notch 3 expression correlated with poor prognosis (n = 126) | [97] |
| CD24 | IHC | Positive expression was related to decreased OS (n = 207) | [98] |
| YAP1 | IHC | High nuclear expression associated with poor survival in pT2 tumors (n = 132) | [99] |
| Msi-1 and ALDH1 | IHC | Positive expression of Msi-1 or ALDH1 was an independent predictor of poor prognosis (n = 100) | [100] |
| MUC5AC | IHC | Reduced expression associated with decreased OS (n = 108) | [101] |
| ENT1 | IHC | Low expression correlated with shorter median survival and lower OS (n = 214) | [102] |
| phospho-mTOR | IHC | High expression associated with poor prognosis in advanced GBC (n = 128) | [103] |
| lncRNA-LET | RT-qPCR | Low expression correlated with reduced metastasis free survival and OS (n = 128) | [104] |
| lncRNA-PAGBC | RT–qPCR | High expression associated with reduced OS (n = 77) | [105] |
| lncRNA-MEG3 | RT-qPCR | Low levels correlated with a shorter OS (n = 50) | [106] |
| lncRNA-LINC01694 | RT-qPCR | High expression correlated with a decreased OS (n = 40). | [107] |
| lncRNA-PVT1 | ISH | High expression correlated with worse OS (n = 66). | [108] |
| serum VEGF-C | ELISA | High levels correlated with a shorter mean survival (n = 31 and 51 in each study). | [109,110] |
| serum IL-6 | ECLIA | Low levels correlated with a better 5-year overall survival rate in a subgroup of patients with hepatic side tumors (n = 69). | [111] |
| preoperative NLR | Hemogram | High levels associated with lower median survival periods in GBC with hepatic involvement (n = 84). | [112] |
| Biomarker | Measurement Technique | Scenario of Relevance and Relevance in Clinical Practice | Reference |
|---|---|---|---|
| Thymidine phosphorilase | IHC | Response to capecitabine/worse prognosis Relevance + | [128] |
| ERCC1/XRCC1 | IHC | Response to gemcitabine/prognosis Relevance + | [129] |
| Cytidine deaminase | Sequencing; SNPs identification | Response to gemcitabine Relevance + | [139] |
| Circulating angiogenesis and inflammatory markers (i.e., VEGFR2) | ELISA | Prognosis on CisGem-treated patients. Relevance + | [140] |
| DNA Damage Repair deficiency | Sequencing/IHC | Response/prognosis in platinum-treated patients Relevance ++ | [31,147,148,149,150,151] |
| TMB high/MMR deficiency/MSI high | Sequencing/IHC | Response to immunotherapy Relevance +++ | [148,183,186,187,189] |
| HER2 | Mutations (sequencing) | Precision medicine strategies (HER2 inhibitors) Relevance ++ | [176,177] |
| BRAF mutations, RNF43 mutations, TRK-fusions | Mutations/fusions (sequencing) | Tumor agnostic precision medicine strategies Relevance +++ | [161,162,163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, P.; Lamarca, A.; Díaz, J.; Carrera, E.; Roa, J.C.; on behalf of the European-Latin American ESCALON Consortium. Current and New Biomarkers for Early Detection, Prognostic Stratification, and Management of Gallbladder Cancer Patients. Cancers 2020, 12, 3670. https://doi.org/10.3390/cancers12123670
García P, Lamarca A, Díaz J, Carrera E, Roa JC, on behalf of the European-Latin American ESCALON Consortium. Current and New Biomarkers for Early Detection, Prognostic Stratification, and Management of Gallbladder Cancer Patients. Cancers. 2020; 12(12):3670. https://doi.org/10.3390/cancers12123670
Chicago/Turabian StyleGarcía, Patricia, Angela Lamarca, Javier Díaz, Enrique Carrera, Juan Carlos Roa, and on behalf of the European-Latin American ESCALON Consortium. 2020. "Current and New Biomarkers for Early Detection, Prognostic Stratification, and Management of Gallbladder Cancer Patients" Cancers 12, no. 12: 3670. https://doi.org/10.3390/cancers12123670
APA StyleGarcía, P., Lamarca, A., Díaz, J., Carrera, E., Roa, J. C., & on behalf of the European-Latin American ESCALON Consortium. (2020). Current and New Biomarkers for Early Detection, Prognostic Stratification, and Management of Gallbladder Cancer Patients. Cancers, 12(12), 3670. https://doi.org/10.3390/cancers12123670






