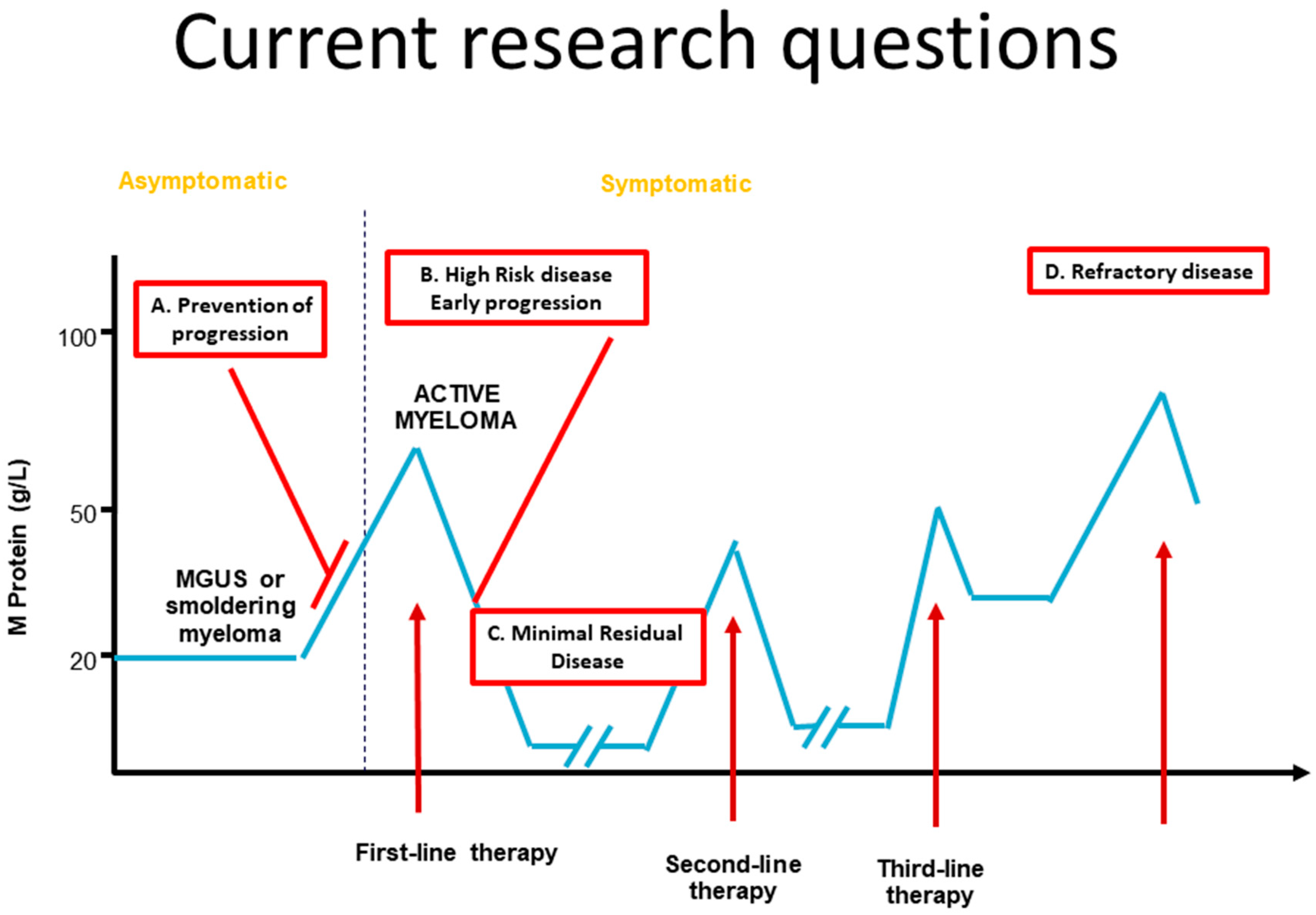
The Road to a Cure: Emerging Treatments for Multiple Myeloma
Funding
Conflicts of Interest
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.; Gay, F.; Anderson, C.K. Multiple myeloma. Nat. Rev. Dis. Prim. 2017, 3, 17046. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kikuchi, J. Molecular basis of clonal evolution in multiple myeloma. Int. J. Hematol. 2020, 111, 496–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasielec, J.; Kubicki, T.; Raje, N.; Vij, R.; Reece, D.; Berdeja, J.; Derman, B.A.; A Rosenbaum, C.; Richardson, P.G.; Gurbuxani, S.; et al. Carfilzomib, lenalidomide, and dexamethasone plus transplant in newly diagnosed multiple myeloma. Blood 2020. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Bertamini, L.; Oliva, S.; Boccadoro, M.; Gay, F. Pursuing a Curative Approach in Multiple Myeloma: A Review of New Therapeutic Strategies. Cancers 2019, 11, 2015. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Ishibashi, M.; Sunakawa-Kii, M.; Inokuchi, K. Immunotherapy for Multiple Myeloma. Cancers 2019, 11, 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caers, J.; De Larrea, C.F.; Leleu, X.; Heusschen, R.; Zojer, N.; Decaux, O.; Kastritis, E.; Minnema, M.; Jurczyszyn, A.; Beguin, Y.; et al. The Changing Landscape of Smoldering Multiple Myeloma: A European Perspective. Oncologist 2016, 21, 333–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caers, J.; Garderet, L.; Kortüm, K.M.; O’Dwyer, M.E.; van de Donk, N.W.C.J.; Binder, M.; Dold, S.M.; Gay, F.; Corre, J.; Beguin, Y.; et al. An European Myeloma Network recommendation on tools for diagnosis and monitoring of Multiple Myeloma: What to use and when. Haematolgica 2018. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Tsakirakis, N.; Malandrakis, P.; Vitsos, P.; Metousis, A.; Orologas-Stavrou, N.; Ntanasis-Stathopoulos, I.; Kanellias, N.; Eleutherakis-Papaiakovou, E.; Pothos, P.; et al. Deep Phenotyping Reveals Distinct Immune Signatures Correlating with Prognostication, Treatment Responses, and MRD Status in Multiple Myeloma. Cancers 2020, 12, 3245. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caers, J. The Road to a Cure: Emerging Treatments for Multiple Myeloma. Cancers 2020, 12, 3593. https://doi.org/10.3390/cancers12123593
Caers J. The Road to a Cure: Emerging Treatments for Multiple Myeloma. Cancers. 2020; 12(12):3593. https://doi.org/10.3390/cancers12123593
Chicago/Turabian StyleCaers, Jo. 2020. "The Road to a Cure: Emerging Treatments for Multiple Myeloma" Cancers 12, no. 12: 3593. https://doi.org/10.3390/cancers12123593
APA StyleCaers, J. (2020). The Road to a Cure: Emerging Treatments for Multiple Myeloma. Cancers, 12(12), 3593. https://doi.org/10.3390/cancers12123593




