Role of Human Papillomavirus Infection in Head and Neck Cancer in Italy: The HPV-AHEAD Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. HPV Type Distribution in HPV-Driven HN Sites According to Different Combination of Biomarkers
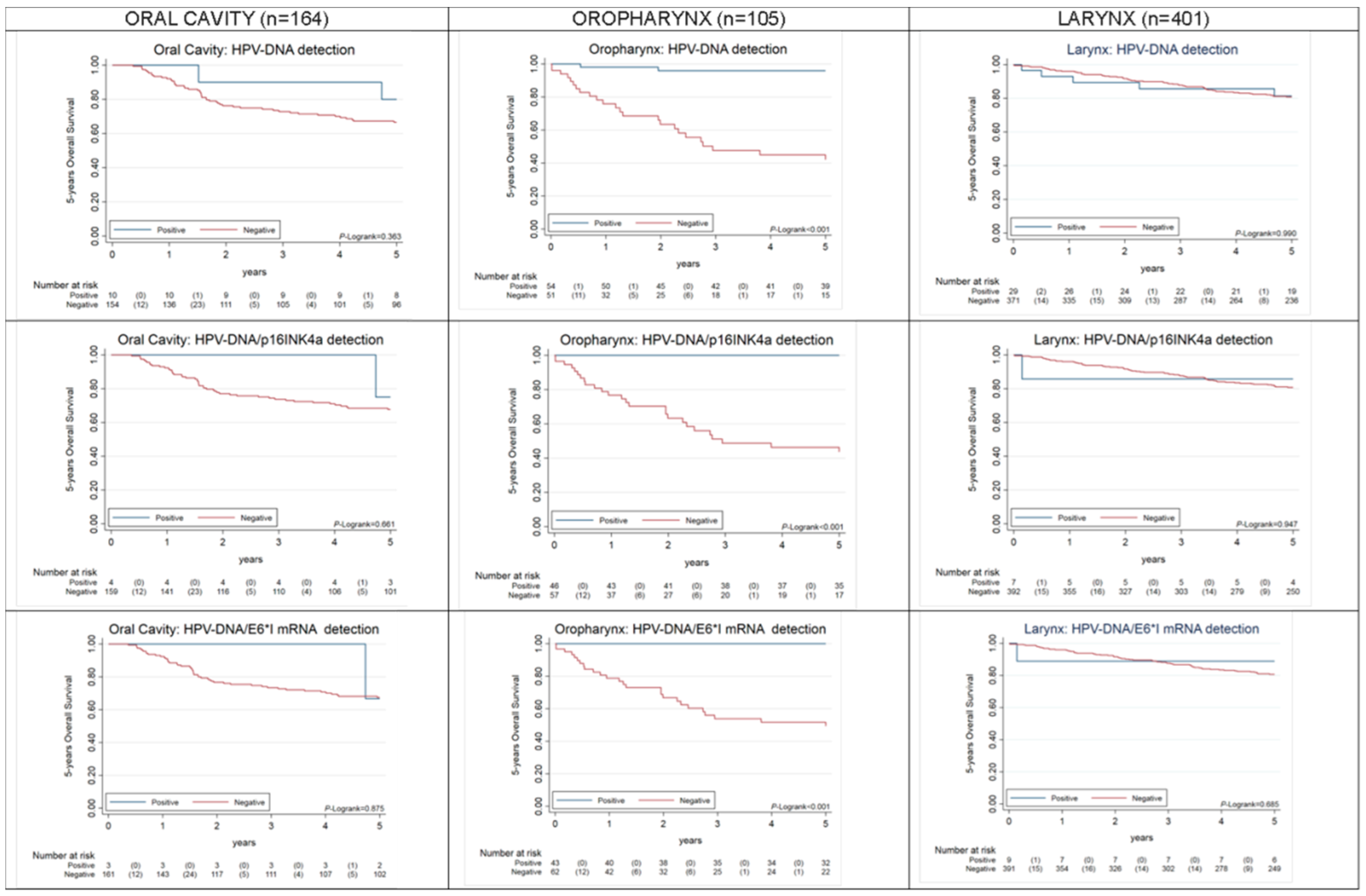
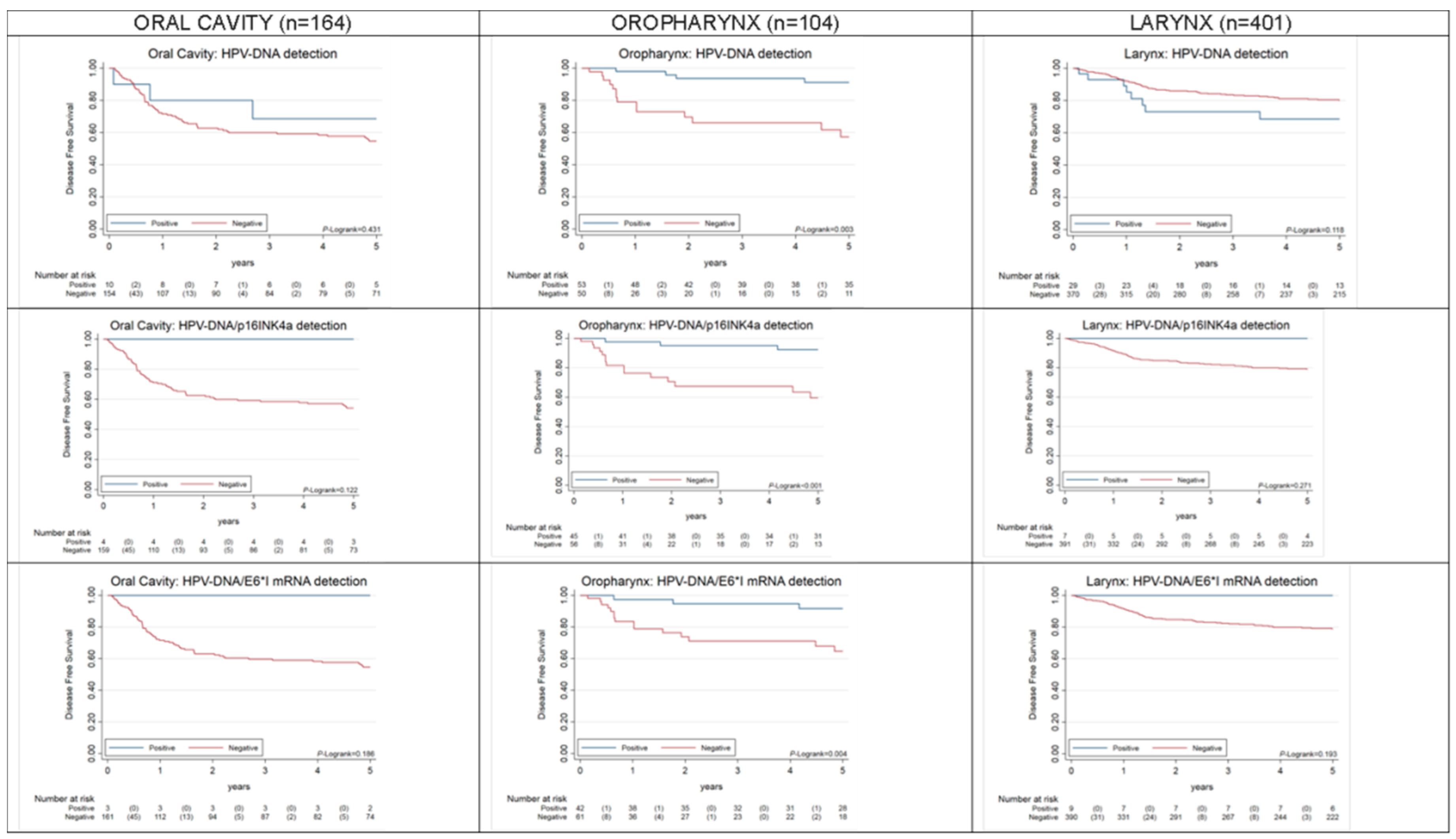
2.2. Overall and Progression-Free Survival of HPV-Driven OPC, OC, LC
3. Discussion
4. Materials and Methods
4.1. Study Design and Samples
4.2. Preparation of the Tissue Sections
4.3. Histological Analysis
4.4. HPV-DNA Genotyping
4.5. HPV E6*I RNA Analysis
4.6. p16INK4a IHC
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Head and Neck Squamous Cell Carcinoma. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/head-and-neck-squamous-cell-carcinoma (accessed on 26 November 2020).
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/graphicisotype?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0#collapse-group-0-4 (accessed on 26 November 2020).
- Boscolo-Rizzo, P.; Zorzi, M.; Del Mistro, A.; Da Mosto, M.C.; Tirelli, G.; Buzzoni, C.; Rugge, M.; Polesel, J.; Guzzinati, S.; AIRTUM Working Group. The evolution of the epidemiological landscape of head and neck cancer in Italy: Is there evidence for an increase in the incidence of potentially HPV-related carcinomas? PLoS ONE 2018, 13, e0192621. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.; Rosenberg, P.S.; Bray, F.; Gillison, M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013, 31, 4550–4559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Torabi, S.J.; Yarbrough, W.G.; Mehra, S.; Osborn, H.A.; Judson, B. Association of Human Papillomavirus Status at Head and Neck Carcinoma Subsites With Overall Survival. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 519–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morbini, P.; Alberizzi, P.; Ferrario, G.; Capello, G.; De Silvestri, A.; Pedrazzoli, P.; Tinelli, C.; Benazzo, M. The evolving landscape of human papillomavirus-related oropharyngeal squamous cell carcinoma at a single institution in Northern Italy. Acta Otorhinolaryngol. 2019, 39, 9–17. [Google Scholar] [CrossRef]
- Castellsagué, X.; Alemany, L.; Quer, M.; Halec, G.; Quirós, B.; Tous, S.; Clavero, O.; Alòs, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; de Sanjosé, S.; Saraiya, M.; Sideri, M.; Palefsky, J.; Lacey, C.; Gillison, M.; Bruni, L.; Ronco, G.; Wentzensen, N.; et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int. J. Cancer 2012, 131, 1969–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Baboci, L.; Holzinger, D.; Boscolo-Rizzo, P.; Tirelli, G.; Spinato, R.; Lupato, V.; Fuson, R.; Schmitt, M.; Michel, A.; Halec, G.; et al. Low prevalence of HPV-driven head and neck squamous cell carcinoma in North-East Italy. Papillomavirus Res. 2016, 2, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Del Mistro, A.; Frayle, H.; Menegaldo, A.; Favaretto, N.; Gori, S.; Nicolai, P.; Spinato, G.; Romeo, S.; Tirelli, G.; da Mosto, M.C.; et al. Age-independent increasing prevalence of Human Papillomavirus-driven oropharyngeal carcinomas in North-East Italy. Sci. Rep. 2020, 10, 9320. [Google Scholar] [CrossRef]
- Husain, Z.A.; Chen, T.; Corso, C.D.; Wang, Z.; Park, H.; Judson, B.; Yarbrough, W.; Deshpande, H.; Mehra, S.; Kuo, P.; et al. A Comparison of Prognostic Ability of Staging Systems for Human Papillomavirus-Related Oropharyngeal Squamous Cell Carcinoma. JAMA Oncol. 2017, 3, 358–365. [Google Scholar] [CrossRef]
- Zhan, K.Y.; Puram, S.V.; Li, M.M.; Silverman, D.A.; Agrawal, A.A.; Ozer, E.; Old, M.O.; Carrau, R.L.; Rocco, J.W.; Higgins, K.M.; et al. National treatment trends in human papillomavirus-positive oropharyngeal squamous cell carcinoma. Cancer 2020, 126, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Marziliano, A.; Teckie, S.; Diefenbach, M.A. Alcohol-related head and neck cancer: Summary of the literature. Head Neck 2020, 42, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Pham, Y.; Ward, M.C.; Houston, N.; Reddy, C.A.; Joshi, N.P.; Greskovich, J.F., Jr.; Woody, N.M.; Chute, D.J.; Lamarre, E.D.; et al. Impact of active smoking on outcomes in HPV+ oropharyngeal cancer. Head Neck 2020, 42, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillison, M.L.; Alemany, L.; Snijders, P.J.; Chaturvedi, A.; Steinberg, B.M.; Schwartz, S.; Castellsagué, X. Human papillomavirus and diseases of the upper airway: Head and neck cancer and respiratory papillomatosis. Vaccine 2012, 30, F34–F54. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Halec, G.; Schmitt, M.; Dondog, B.; Sharkhuu, E.; Wentzensen, N.; Gheit, T.; Tommasino, M.; Kommoss, F.; Bosch, F.X.; Franceschi, S.; et al. Biological activity of probable/possible high-risk human papillomavirus types in cervical cancer. Int. J. Cancer 2013, 132, 63–71. [Google Scholar] [CrossRef]
- Jung, A.C.; Briolat, J.; Millon, R.; de Reyniès, A.; Rickman, D.; Thomas, E.; Abecassis, J.; Clavel, C.; Wasylyk, B. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int. J. Cancer 2010, 126, 1882–1894. [Google Scholar] [CrossRef]
- Holzinger, D.; Schmitt, M.; Dyckhoff, G.; Benner, A.; Pawlita, M.; Bosch, F.X. Viral RNA patterns and high viral load reliably define oropharynx carcinomas with active HPV16 involvement. Cancer Res. 2012, 72, 4993–5003. [Google Scholar] [CrossRef] [Green Version]
- Bussu, F.; Ragin, C.; Boscolo-Rizzo, P.; Rizzo, D.; Gallus, R.; Delogu, G.; Morbini, P.; Tommasino, M. HPV as a marker for molecular characterization in head and neck oncology: Looking for a standardization of clinical use and of detection method(s) in clinical practice. Head Neck 2019, 41, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S., Jr.; Beadle, B.; Bishop, J.A.; Chernock, R.D.; Colasacco, C.; Lacchetti, C.; Moncur, J.T.; Rocco, J.W.; Schwartz, M.R.; Seethala, R.R.; et al. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch. Pathol. Lab. Med. 2018, 142, 559–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mena, M.; Taberna, M.; Tous, S.; Marquez, S.; Clavero, O.; Quiros, B.; Lloveras, B.; Alejo, M.; Leon, X.; Quer, M.; et al. Double positivity for HPV-DNA/p16 is the biomarker with strongest diagnostic accuracy and prognostic value for human papillomavirus related oropharyngeal cancer patients. Oral. Oncol. 2018, 78, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigge, E.S.; Arbyn, M.; von Knebel Doeberitz, M.; Reuschenbach, M. Diagnostic accuracy of p16 immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int. J. Cancer 2017, 140, 1186–1198. [Google Scholar] [CrossRef] [Green Version]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Dok, R.; Nuyts, S. HPV Positive Head and Neck Cancers: Molecular Pathogenesis and Evolving Treatment Strategies. Cancers 2016, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Role of Human Papillomavirus Infection and Other Co-Factors in the Aetiology of Head and Neck Cancer in Europe and INDIA (HPV-AHEAD). Available online: http://hpv-ahead.iarc.fr/ (accessed on 26 November 2020).
- Gheit, T.; Anantharaman, D.; Holzinger, D.; Alemany, L.; Tous, S.; Lucas, E.; Prabhu, P.R.; Pawlita, M.; Ridder, R.; Rehm, S.; et al. Role of mucosal high-risk human papillomavirus types in head and neck cancers in central India. Int. J. Cancer 2017, 141, 143–151. [Google Scholar] [CrossRef]
- Mena, M.; Lloveras, B.; Tous, S.; Bogers, J.; Maffini, F.; Gangane, N.; Kumar, R.V.; Somanathan, T.; Lucas, E.; Anantharaman, D.; et al. Development and validation of a protocol for optimizing the use of paraffin blocks in molecular epidemiological studies: The example from the HPV-AHEAD study. PLoS ONE 2017, 12, e0184520. [Google Scholar] [CrossRef] [Green Version]
- Taberna, M.; Mena, M.; Pavón, M.A.; Alemany, L.; Gillison, M.L.; Mesía, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coordes, A.; Lenz, K.; Qian, X.; Lenarz, M.; Kaufmann, A.M.; Albers, A.E. Meta-analysis of survival in patients with HNSCC discriminates risk depending on combined HPV and p16 status. Eur. Arch. Otorhinolaryngol. 2016, 273, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Gheit, T.; Vaccarella, S.; Schmitt, M.; Pawlita, M.; Franceschi, S.; Sankaranarayanan, R.; Sylla, B.S.; Tommasino, M.; Gangane, N. Prevalence of human papillomavirus types in cervical and oral cancers in central India. Vaccine 2009, 27, 636–639. [Google Scholar] [CrossRef]
- Gheit, T.; Landi, S.; Gemignani, F.; Snijders, P.J.; Vaccarella, S.; Franceschi, S.; Canzian, F.; Tommasino, M. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J. Clin. Microbiol. 2006, 44, 2025–2031. [Google Scholar] [CrossRef] [Green Version]
- Halec, G.; Schmitt, M.; Egger, S.; Abnet, C.C.; Babb, C.; Dawsey, S.M.; Flechtenmacher, C.; Gheit, T.; Hale, M.; Holzinger, D.; et al. Mucosal alpha-papillomaviruses are not associated with esophageal squamous cell carcinomas: Lack of mechanistic evidence from South Africa, China and Iran and from a world-wide meta-analysis. Int. J. Cancer 2016, 139, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, M.; Dondog, B.; Waterboer, T.; Pawlita, M.; Tommasino, M.; Gheit, T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J. Clin. Microbiol. 2010, 48, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Alemany, L.; Saunier, M.; Alvarado-Cabrero, I.; Quirós, B.; Salmeron, J.; Shin, H.R.; Pirog, E.C.; Guimerà, N.; Hernandez-Suarez, G.; Felix, A.; et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int. J. Cancer 2015, 136, 98–107. [Google Scholar] [CrossRef] [Green Version]
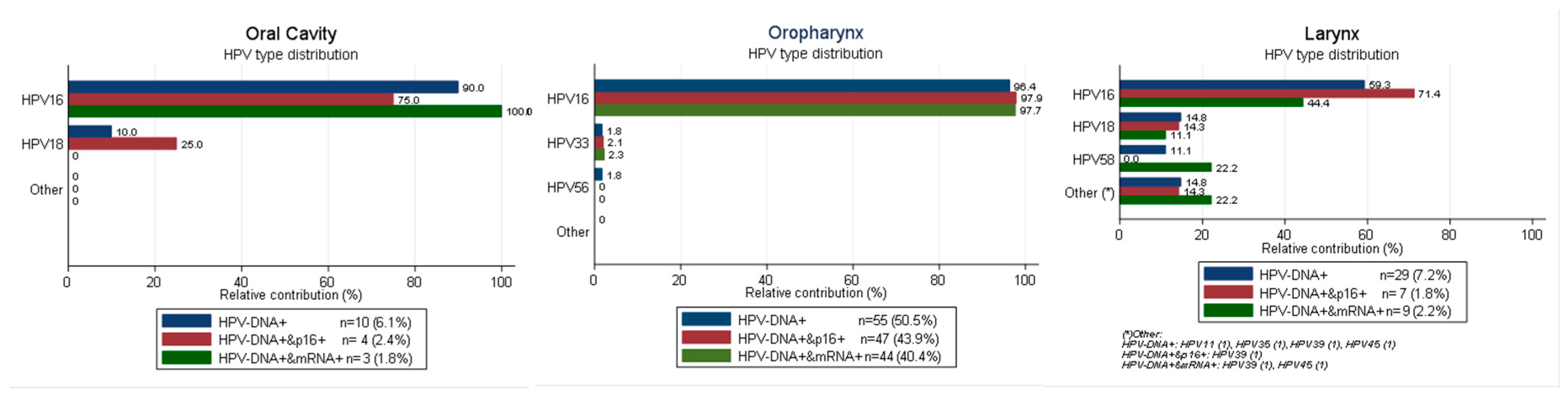
| Total HNC (n = 675) | Oral Cavity (OC) | Oropharynx (OPC) | Larynx (LC) | |||
|---|---|---|---|---|---|---|
| n = 165 (23.7%) a | n = 109 (15.7%) a | n = 401 (57.6%) a | ||||
| n | %b | n | %b | n | %b | |
| HPV markers positivity | ||||||
| HPV-DNA+ | 10/165 | 6.1% | 55/109 | 50.5% | 29/401 | 7.2% |
| p16INK4a+ in HPV-DNA+ cases | 4/9 | 44.4% | 47/53 | 88.7% | 7/28 | 25.0% |
| p16INK4a+ in HPV-DNA- cases | 3/20 | 15.0% | 0/8 | 0.0% | 1/26 | 3.8% |
| E6*I mRNA+ in HPV-DNA+ cases | 3/10 | 30.0% | 44/55 | 80.0% | 9/29 | 31.0% |
| E6*I mRNA+ in HPV-DNA- cases | 0/21 | 0.0% | 0/8 | 0.0% | 0/33 | 0.0% |
| HPV-DNA+ AND p16INK4a+ | 4/164 | 2.4% | 47/107 | 43.9% | 7/400 | 1.8% |
| HPV-DNA+ AND E6*I mRNA+ | 3/165 | 1.8% | 44/109 | 40.4% | 9/401 | 2.2% |
| HPV-DNA+ AND [E6*I mRNA+ OR p16INK4a+] | 4/164 | 2.4% | 47/107 | 43.9% | 9/400 | 2.3% |
| HPV-DNA+ AND E6*I mRNA+ AND p16INK4a+ | 3/164 | 1.8% | 42/107 | 39.3% | 6/400 | 1.5% |
| Covariate | OPC | Crude HR | OC | Crude HR | LC | Crude HR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases/Deaths | HR | 95% CI | p-Value | Cases/Deaths | HR | 95% CI | p-Value | Cases/Deaths | HR | 95% CI | p-Value | |||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||||||
| HPV-DNA | 105/26 | 0.000 | 164/51 | 0.322 | 401/69 | 0.990 | ||||||||||
| Other | 51/24 | Ref. | 154/49 | Ref. | 372/64 | Ref. | ||||||||||
| HPV-DNA+ | 54/2 | 0.05 | 0.01 | 0.22 | 10/2 | 0.52 | 0.13 | 2.16 | 29/5 | 1.01 | 0.40 | 2.50 | ||||
| HPV-E6*I mRNA | 105/26 | - | 164/51 | 0.872 | 401/69 | 0.666 | ||||||||||
| Other | 62/26 | 161/50 | Ref. | 392/68 | Ref. | |||||||||||
| HPV-E6*I mRNA+ | 43/0 | - | - | - | 3/1 | 0.85 | 0.12 | 6.18 | 9/1 | 0.67 | 0.09 | 4.80 | ||||
| HPV-DNA and p16 | 103/26 | - | 163/50 | 0.640 | 400/69 | 0.946 | ||||||||||
| Other | 57/26 | 159/49 | Ref. | 393/68 | Ref. | |||||||||||
| HPV-DNA+ and p16+ | 46/0 | - | - | - | 4/1 | 0.64 | 0.09 | 4.67 | 7/1 | 0.93 | 0.13 | 6.73 | ||||
| Age at diagnosis | 105/26 | 1.05 | 1.01 | 1.10 | 0.023 | 164/51 | 1.01 | 0.99 | 1.03 | 0.190 | 401/69 | 1.04 | 1.01 | 1.07 | 0.002 | |
| 17–54 y | 24/3 | Ref. | 0.076 | 71/17 | Ref. | 0.142 | 70/9 | Ref. | 0.205 | |||||||
| 55–62 y | 38/8 | 1.79 | 0.46 | 6.76 | 28/12 | 2.30 | 1.10 | 4.82 | 115/20 | 1.42 | 0.65 | 3.12 | ||||
| 63–70 y | 22/7 | 2.64 | 0.68 | 10.20 | 27/11 | 1.85 | 0.87 | 3.95 | 108/16 | 1.28 | 0.57 | 2.90 | ||||
| 71–94 y | 21/8 | 4.84 | 1.28 | 18.31 | 38/11 | 1.38 | 0.65 | 2.94 | 108/24 | 2.10 | 0.98 | 4.52 | ||||
| Gender | 105/26 | 0.778 | 164/51 | 0.898 | 401/69 | 0.906 | ||||||||||
| Male | 82/21 | Ref. | 99/31 | Ref. | 355/61 | Ref. | ||||||||||
| Female | 23/5 | 0.87 | 0.33 | 2.31 | 65/20 | 1.04 | 0.59 | 1.82 | 46/8 | 1.05 | 0.50 | 2.18 | ||||
| Period of diagnosis | 105/26 | 0.87 | 0.76 | 0.99 | 0.028 | 164/51 | 0.98 | 0.90 | 1.07 | 0.725 | 401/69 | 1.00 | 0.92 | 1.08 | 0.914 | |
| 2000–2003 | 38/16 | Ref. | 0.028 | 49/15 | Ref. | 0.447 | 147/24 | Ref. | 0.612 | |||||||
| 2004–2007 | 46/7 | 0.32 | 0.13 | 0.79 | 68/24 | 1.28 | 0.67 | 2.44 | 170/33 | 1.23 | 0.73 | 2.08 | ||||
| 2008–2010 | 21/3 | 0.36 | 0.10 | 1.22 | 47/12 | 0.83 | 0.39 | 1.78 | 84/12 | 0.92 | 0.46 | 1.85 | ||||
| Tobacco use | 105/26 | 0.091 | 164/51 | 0.775 | 401/69 | 0.783 | ||||||||||
| Non-smoker | 21/3 | Ref. | 61/17 | Ref. | 11/2 | Ref. | ||||||||||
| Former smoker | 21/3 | 1.04 | 0.21 | 5.14 | 31/11 | 1.22 | 0.57 | 2.61 | 28/6 | 1.28 | 0.26 | 6.34 | ||||
| Smoker | 51/18 | 2.84 | 0.84 | 9.65 | 63/21 | 1.21 | 0.64 | 2.29 | 361/61 | 0.89 | 0.22 | 3.66 | ||||
| Unknown | 12/2 | 0.99 | 0.17 | 5.95 | 9/2 | 0.65 | 0.15 | 2.83 | 1/0 | - | ||||||
| Alcohol use | 105/26 | 0.276 | 164/51 | 0.089 | 401/69 | 0.166 | ||||||||||
| Non-drinker | 25/4 | Ref. | 66/15 | Ref. | 115/18 | Ref. | ||||||||||
| Former drinker | 3/2 | 4.90 | 0.89 | 26.86 | 3/0 | - | 3/1 | 3.28 | 0.44 | 24.6 | ||||||
| Drinker | 62/17 | 1.97 | 0.66 | 5.87 | 84/33 | 1.80 | 0.98 | 3.32 | 274/50 | 1.19 | 0.69 | 2.04 | ||||
| Unknown | 15/3 | 1.13 | 0.25 | 5.06 | 11/3 | 1.05 | 0.30 | 3.61 | 9/0 | - | ||||||
| Subsite | 105/26 | 0.110 | - | - | ||||||||||||
| Tonsil | 47/9 | Ref. | - | - | - | - | ||||||||||
| BOT | 29/6 | 1.16 | 0.41 | 3.26 | - | - | - | - | ||||||||
| Other oropharynx | 29/11 | 2.50 | 1.03 | 6.03 | - | - | - | - | ||||||||
| - | 164/51 | 0.437 | 401/69 | 0.024 | ||||||||||||
| Proximal to oropharynx | - | - | 49/13 | Ref. | 97/23 | Ref. | ||||||||||
| Distal to oropharynx | - | - | 115/38 | 1.28 | 0.68 | 2.39 | 304/46 | 0.55 | 0.33 | 0.91 | ||||||
| Stage (7th edition TNM) | 105/26 | 0.852 | 164/51 | 0.040 | 401/69 | 0.000 | ||||||||||
| I + II | 24/6 | Ref. | 74/17 | Ref. | 256/30 | Ref. | ||||||||||
| III | 22/5 | 0.80 | 0.24 | 2.61 | 28/9 | 1.58 | 0.70 | 3.55 | 77/13 | 1.53 | 0.80 | 2.94 | ||||
| IVa + IVb | 59/15 | 1.06 | 0.41 | 2.73 | 62/25 | 2.19 | 1.18 | 4.07 | 68/26 | 4.05 | 2.39 | 6.85 | ||||
| cN | 105/26 | 0.209 | 164/51 | 0.007 | 401/69 | 0.000 | ||||||||||
| 0 | 37/12 | Ref. | 94/24 | Ref. | 339/46 | Ref. | ||||||||||
| 1 | 22/4 | 0.52 | 0.17 | 1.61 | 37/11 | 1.24 | 0.61 | 2.54 | 16/4 | 2.17 | 0.78 | 6.04 | ||||
| 2 | 39/7 | 0.52 | 0.20 | 1.32 | 33/16 | 2.89 | 1.53 | 5.45 | 41/15 | 3.53 | 1.97 | 6.33 | ||||
| 3 | 7/3 | 1.93 | 0.54 | 6.89 | 0 | 5/4 | 7.64 | 2.74 | 21.28 | |||||||
| Treatment | 105/26 | 0.204 | 164/51 | 0.000 | 401/69 | 0.025 | ||||||||||
| Only surgery | 22/8 | Ref. | 85/17 | Ref. | 267/37 | Ref. | ||||||||||
| Surgery + others | 41/11 | 0.53 | 0.21 | 1.33 | 71/29 | 2.54 | 1.39 | 4.62 | 107/27 | 2.03 | 1.24 | 3.34 | ||||
| Conservative | 37/6 | 0.36 | 0.13 | 1.05 | 3/1 | 1.87 | 0.25 | 14.04 | 19/3 | 1.71 | 0.53 | 5.56 | ||||
| Unknown | 5/1 | 1.96 | 0.23 | 16.51 | 5/4 | 25.57 | 8.17 | 80.10 | 8/2 | 3.93 | 0.95 | 16.32 | ||||
| Positive margins | 77/18 | 0.299 | 159/49 | 0.041 | 353/68 | 0.039 | ||||||||||
| No | 60/16 | Ref. | 144/42 | Ref. | 294/43 | Ref. | ||||||||||
| Yes | 17/2 | 0.49 | 0.11 | 2.14 | 15/7 | 2.54 | 1.14 | 5.68 | 59/15 | 1.92 | 1.07 | 3.46 | ||||
| Time of follow-up | ||||||||||||||||
| Median (years) | 5.08 | 9.05 | 6.83 | |||||||||||||
| (Min–Max) | (0.02–18.71) | (0.02–19.05) | (0.00–18.51) | |||||||||||||
| Time since dead | ||||||||||||||||
| Median (years) | 1.97 | 1.97 | 3.43 | |||||||||||||
| (Min–Max) | (0.02–16.21) | (0.02–16.21) | (0.01–16.40) | |||||||||||||
| Covariate | OPC | Crude HR | OC | Crude HR | LC | Crude HR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases/Rec. | HR | 95% CI | p-Value | Cases/Rec. | HR | 95% CI | p-Value | Cases/Rec. | HR | 95% CI | p-Value | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||
| HPV-DNA | 105/20 | 0.000 | 164/70 | 0.402 | 401/75 | 0.152 | |||||||||
| Other | 51/15 | Ref. | 154/67 | Ref. | 372/67 | Ref. | |||||||||
| HPV-DNA+ | 54/5 | 0.16 | 0.05 | 0.50 | 10/3 | 0.63 | 0.20 | 2.01 | 29/8 | 1.78 | 0.85 | 3.71 | |||
| HPV-E6*I mRNA | 105/20 | 0.003 | 164/70 | 401/75 | |||||||||||
| Other | 62/16 | Ref. | 161/70 | 392/75 | |||||||||||
| HPV-E6*I mRNA+ | 43/4 | 0.19 | 0.06 | 0.67 | 3/0 | - | 9/0 | - | |||||||
| HPV-DNA and p16 | 103/20 | 0.001 | 163/70 | - | 400/75 | - | |||||||||
| Other | 57/16 | Ref. | 159/70 | 393/75 | |||||||||||
| HPV-DNA+ and p16+ | 46/4 | 0.15 | 0.04 | 0.52 | 4/0 | - | 7/0 | - | |||||||
| Age at diagnosis | 105/20 | 1.04 | 0.99 | 1.10 | 0.107 | 164/70 | 1.02 | 1.00 | 1.03 | 0.013 | 401/75 | 1.03 | 1.01 | 1.05 | 0.017 |
| 17–54 y | 24/2 | Ref. | 0.097 | 71/25 | Ref. | 0.079 | 70/10 | Ref. | 0.202 | ||||||
| 55–62 y | 38/7 | 2.21 | 0.45 | 10.94 | 28/16 | 2.08 | 1.11 | 3.90 | 115/18 | 1.16 | 0.54 | 2.52 | |||
| 63–70 y | 22/8 | 5.45 | 1.16 | 25.70 | 27/10 | 1.10 | 0.53 | 2.29 | 108/25 | 1.85 | 0.88 | 3.86 | |||
| 71–94 y | 21/3 | 2.04 | 0.29 | 14.51 | 38/19 | 1.78 | 0.98 | 3.23 | 108/22 | 1.77 | 0.84 | 3.73 | |||
| Gender | 105/20 | 0.364 | 164/70 | 0.404 | 401/75 | 0.867 | |||||||||
| Male | 82/13 | Ref. | 99/40 | Ref. | 355/67 | Ref. | |||||||||
| Female | 23/7 | 1.65 | 0.59 | 4.62 | 65/30 | 1.22 | 0.76 | 1.97 | 46/8 | 0.94 | 0.45 | 1.96 | |||
| Period of diagnosis | 105/20 | 0.92 | 0.79 | 1.07 | 0.270 | 164/70 | 1.01 | 0.94 | 1.09 | 0.698 | 401/75 | 1.02 | 0.94 | 1.10 | 0.636 |
| 2000–2003 | 38/9 | Ref. | 0.312 | 49/21 | Ref. | 0.986 | 147/26 | Ref. | 0.799 | ||||||
| 2004–2007 | 46/6 | 0.45 | 0.16 | 1.27 | 68/28 | 1.05 | 0.59 | 1.84 | 170/31 | 1.06 | 0.63 | 1.79 | |||
| 2008–2010 | 21/5 | 0.65 | 0.18 | 2.40 | 47/21 | 1.04 | 0.57 | 1.90 | 84/18 | 1.23 | 0.67 | 2.27 | |||
| Tobacco use | 105/20 | 0.168 | 164/70 | 0.845 | 401/75 | 0.395 | |||||||||
| Non-smoker | 21/5 | Ref. | 61/27 | Ref. | 11/2 | Ref. | |||||||||
| Former smoker | 21/1 | 0.29 | 0.03 | 2.80 | 31/11 | 0.76 | 0.38 | 1.54 | 28/7 | 1.62 | 0.34 | 7.81 | |||
| Smoker | 51/12 | 1.69 | 0.48 | 6.00 | 63/27 | 0.93 | 0.55 | 1.59 | 361/66 | 0.98 | 0.24 | 4.01 | |||
| Unknown | 12/2 | 0.82 | 0.14 | 4.92 | 9/5 | 1.16 | 0.45 | 3.02 | 1/0 | - | |||||
| Alcohol use | 105/20 | 0.388 | 164/70 | 0.829 | 401/75 | 0.062 | |||||||||
| Non-drinker | 25/5 | Ref. | 66/26 | Ref. | 115/20 | Ref. | |||||||||
| Former drinker | 3/1 | 2.12 | 0.25 | 18.30 | 3/2 | 1.84 | 0.44 | 7.76 | 3/0 | - | |||||
| Drinker | 62/13 | 0.99 | 0.34 | 2.86 | 84/37 | 1.18 | 0.71 | 1.95 | 274/55 | 1.16 | 0.69 | 1.93 | |||
| Unknown | 15/1 | 0.27 | 0.03 | 2.28 | 11/5 | 1.04 | 0.40 | 2.71 | 9/0 | - | |||||
| Subsite | 105/20 | 0.549 | |||||||||||||
| Tonsil | 47/10 | Ref. | |||||||||||||
| BOT | 29/4 | 0.81 | 0.24 | 2.68 | |||||||||||
| Other oropharynx | 29/6 | 1.59 | 0.55 | 4.59 | |||||||||||
| Subsite | 164/70 | 0.258 | 401/75 | 0.012 | |||||||||||
| Proximal to Opx | 49/17 | Ref. | 97/26 | Ref. | |||||||||||
| Distal to Opx | 115/53 | 1.36 | 0.79 | 2.35 | 304/49 | 0.54 | 0.33 | 0.87 | |||||||
| Stage (7th edition TNM) | 105/20 | 0.497 | 164/70 | 0.473 | 401/75 | 0.001 | |||||||||
| I + II | 24/7 | Ref. | 74/33 | Ref. | 256/36 | Ref. | |||||||||
| III | 22/4 | 0.60 | 0.17 | 2.14 | 28/9 | 0.67 | 0.32 | 1.41 | 77/16 | 1.47 | 0.81 | 2.69 | |||
| IVa + IVb | 59/9 | 0.52 | 0.18 | 1.51 | 62/28 | 1.04 | 0.63 | 1.71 | 68/23 | 2.94 | 1.74 | 4.97 | |||
| cN | 105/20 | 0.111 | 164/70 | 0.033 | 401/75 | 0.001 | |||||||||
| 0 | 37/8 | Ref. | 94/40 | Ref. | 339/50 | Ref. | |||||||||
| 1 | 22/4 | 0.90 | 0.26 | 3.08 | 37/11 | 0.64 | 0.33 | 1.25 | 16/7 | 3.18 | 1.36 | 7.042 | |||
| 2 | 39/5 | 0.48 | 0.14 | 1.65 | 33/19 | 1.68 | 0.97 | 2.91 | 41/16 | 3.44 | 1.96 | 6.05 | |||
| 3 | 7/3 | 3.83 | 0.97 | 14.98 | 0 | 5/2 | 3.95 | 0.96 | 16.26 | ||||||
| Treatment | 105/20 | 0.090 | 164/70 | 0.699 | 401/75 | 0.052 | |||||||||
| Only surgery | 22/6 | Ref. | 85/35 | Ref. | 267/45 | Ref. | |||||||||
| Surgery + others | 41/5 | 0.23 | 0.06 | 0.80 | 71/32 | 1.09 | 0.67 | 1.76 | 107/24 | 1.47 | 0.89 | 2.42 | |||
| Conservative treatment | 37/9 | 0.65 | 0.22 | 1.86 | 3/1 | 0.72 | 0.10 | 5.26 | 19/6 | 3.00 | 1.27 | 7.05 | |||
| Unknown | 5/0 | - | 5/2 | 2.50 | 0.59 | 10.51 | 8/0 | - | |||||||
| Positive margins | 77/13 | 0.189 | 159/69 | 0.014 | 353/65 | 0.039 | |||||||||
| No | 60/12 | Ref. | 144/59 | Ref. | 294/49 | Ref. | |||||||||
| Yes | 17/1 | 0.31 | 0.04 | 2.41 | 15/10 | 2.58 | 1.31 | 5.06 | 59/16 | 1.87 | 1.07 | 3.31 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliabue, M.; Mena, M.; Maffini, F.; Gheit, T.; Quirós Blasco, B.; Holzinger, D.; Tous, S.; Scelsi, D.; Riva, D.; Grosso, E.; et al. Role of Human Papillomavirus Infection in Head and Neck Cancer in Italy: The HPV-AHEAD Study. Cancers 2020, 12, 3567. https://doi.org/10.3390/cancers12123567
Tagliabue M, Mena M, Maffini F, Gheit T, Quirós Blasco B, Holzinger D, Tous S, Scelsi D, Riva D, Grosso E, et al. Role of Human Papillomavirus Infection in Head and Neck Cancer in Italy: The HPV-AHEAD Study. Cancers. 2020; 12(12):3567. https://doi.org/10.3390/cancers12123567
Chicago/Turabian StyleTagliabue, Marta, Marisa Mena, Fausto Maffini, Tarik Gheit, Beatriz Quirós Blasco, Dana Holzinger, Sara Tous, Daniele Scelsi, Debora Riva, Enrica Grosso, and et al. 2020. "Role of Human Papillomavirus Infection in Head and Neck Cancer in Italy: The HPV-AHEAD Study" Cancers 12, no. 12: 3567. https://doi.org/10.3390/cancers12123567






