Tumor BRCA Testing in High Grade Serous Carcinoma: Mutation Rates and Optimal Tissue Requirements
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
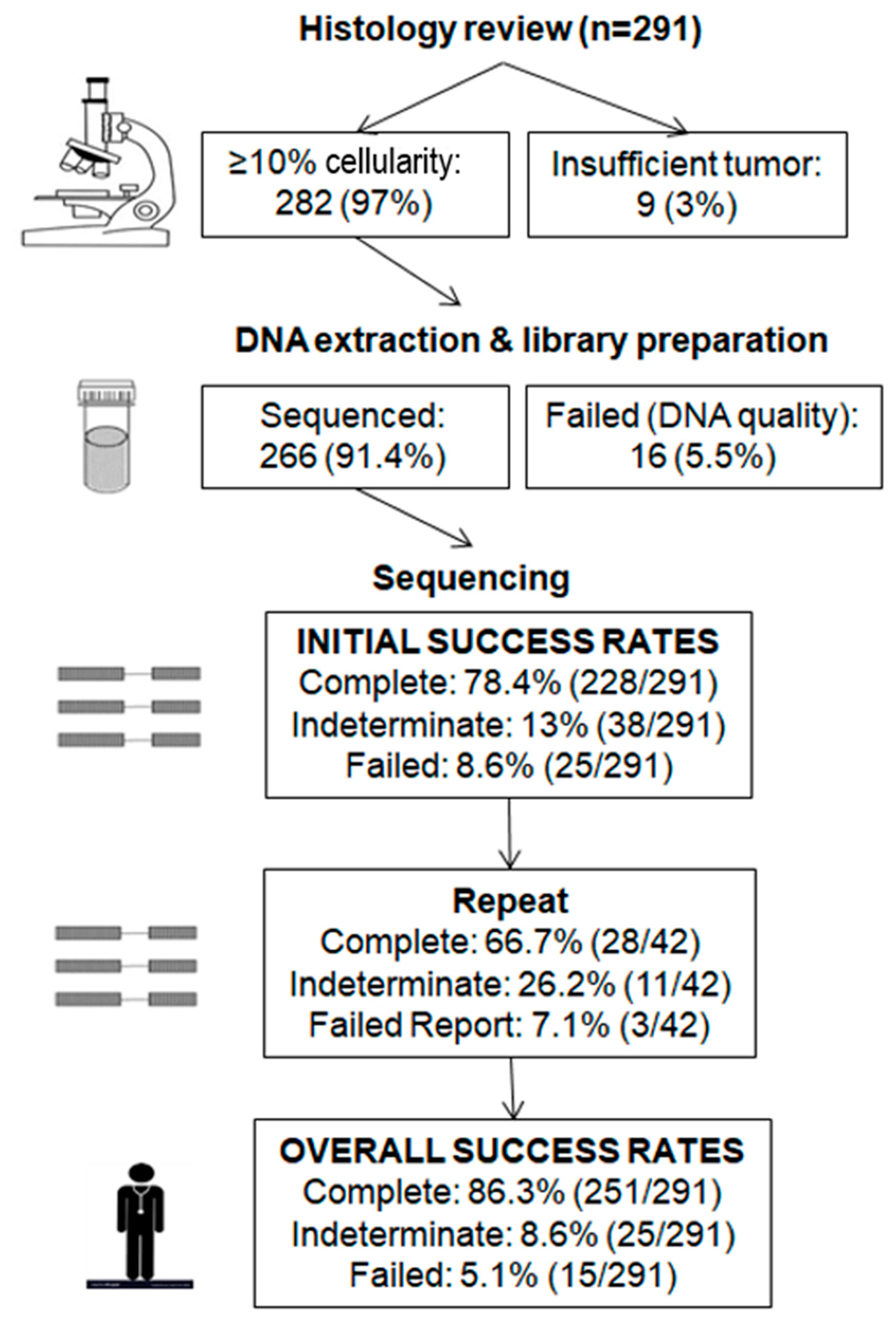
2.1. Overview of Clinical Data
2.2. Initial Testing
2.3. Repeat Testing
2.4. Predictors of Successful Tumor BRCA Analysis
2.5. Histologic Analysis
2.6. Clinically Significant BRCA Mutations
2.7. Loss of heterozygosity (LOH) Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population and Reference Laboratory
4.2. Tissue Samples and DNA Extraction
4.3. Library Preparation and Analysis
4.4. Bioinformatic Analysis
4.5. Reporting and Variant Interpretation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilks, C.B.; Ionescu, D.N.; Kalloger, S.E.; Kobel, M.; Irving, J.; Clarke, B.; Santos, J.; Le, N.; Moravan, V.; Swenerton, K.; et al. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum. Pathol. 2008, 39, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Gockley, A.; Melamed, A.; Bregar, A.J.; Clemmer, J.T.; Birrer, M.; Schorge, J.O.; Del Carmen, M.G.; Rauh-Hain, J.A. Outcomes of Women with High-Grade and Low-Grade Advanced-Stage Serous Epithelial Ovarian Cancer. Obstet. Gynecol. 2017, 129, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women with Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.; Marchetti, C.; De Leo, R.; Musella, A.; Capoluongo, E.; Paris, I.; Benedetti Panici, P.; Scambia, G.; Fagotti, A. BRCA mutational status, initial disease presentation, and clinical outcome in high-grade serous advanced ovarian cancer: A multicenter study. Am. J. Obstet. Gynecol. 2017, 217, 334.e1–334.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Royer, R.; Li, S.; McLaughlin, J.R.; Rosen, B.; Risch, H.A.; Fan, I.; Bradley, L.; Shaw, P.A.; Narod, S.A. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol. Oncol. 2011, 121, 353–357. [Google Scholar] [CrossRef]
- Mafficini, A.; Simbolo, M.; Parisi, A.; Rusev, B.; Luchini, C.; Cataldo, I.; Piazzola, E.; Sperandio, N.; Turri, G.; Franchi, M.; et al. BRCA somatic and germline mutation detection in paraffin embedded ovarian cancers by next-generation sequencing. Oncotarget 2016, 7, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- McAlpine, J.N.; Porter, H.; Kobel, M.; Nelson, B.H.; Prentice, L.M.; Kalloger, S.E.; Senz, J.; Milne, K.; Ding, J.; Shah, S.P.; et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod. Pathol. 2012, 25, 740–750. [Google Scholar] [CrossRef] [Green Version]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, B.T.; Timms, K.M.; Carey, M.S.; Gutin, A.; Meyer, L.A.; Flake, D.D., 2nd; Abkevich, V.; Potter, J.; Pruss, D.; Glenn, P.; et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J. Clin. Oncol. 2010, 28, 3570–3576. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Khan, S.; Sun, Y.; Hess, K.; Shmulevich, I.; Sood, A.K.; Zhang, W. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011, 306, 1557–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.M.; Ruano, D.; van Eijk, R.; van der Stoep, N.; Nielsen, M.; Wijnen, J.T.; Ter Haar, N.T.; Baalbergen, A.; Bos, M.; Kagie, M.J.; et al. Validation and Implementation of BRCA1/2 Variant Screening in Ovarian Tumor Tissue. J. Mol. Diagn. 2018, 20, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.R.; Fakkert, I.E.; de Hullu, J.A.; van Altena, A.M.; Sie, A.S.; Ouchene, H.; Willems, R.W.; Nagtegaal, I.D.; Jongmans, M.C.J.; Mensenkamp, A.R.; et al. Universal Tumor DNA BRCA1/2 Testing of Ovarian Cancer: Prescreening PARPi Treatment and Genetic Predisposition. J. Natl. Cancer Inst. 2020, 112, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wooster, R.; Neuhausen, S.L.; Mangion, J.; Quirk, Y.; Ford, D.; Collins, N.; Nguyen, K.; Seal, S.; Tran, T.; Averill, D.; et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science 1994, 265, 2088–2090. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.S.; Ostermeyer, E.A.; Szabo, C.I.; Dowd, P.; Lynch, E.D.; Rowell, S.E.; King, M.C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat. Genet. 1994, 8, 399–404. [Google Scholar] [CrossRef]
- Chapman, J.R.; Taylor, M.R.; Boulton, S.J. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef] [Green Version]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Stoppa-Lyonnet, D. The biological effects and clinical implications of BRCA mutations: Where do we go from here? Eur. J. Hum. Genet. 2016, 24 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- Ritterhouse, L.L.; Nowak, J.A.; Strickland, K.C.; Garcia, E.P.; Jia, Y.; Lindeman, N.I.; Macconaill, L.E.; Konstantinopoulos, P.A.; Matulonis, U.A.; Liu, J.; et al. Morphologic correlates of molecular alterations in extrauterine Mullerian carcinomas. Mod. Pathol. 2016, 29, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Alsop, K.; Fereday, S.; Meldrum, C.; deFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.C.; Lheureux, S.; Karakasis, K.; Burnier, J.V.; Bruce, J.P.; Clouthier, D.L.; Danesh, A.; Quevedo, R.; Dowar, M.; Hanna, Y.; et al. Landscape of genomic alterations in high-grade serous ovarian cancer from exceptional long- and short-term survivors. Genome Med. 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisay, M.; Edessa, D. PARP inhibitors as potential therapeutic agents for various cancers: Focus on niraparib and its first global approval for maintenance therapy of gynecologic cancers. Gynecol. Oncol. Res. Pract. 2017, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, J.; George, A.; Banerjee, S. The current status of PARP inhibitors in ovarian cancer. Tumori J. 2016, 102, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Wang, P.Y.; Wang, Y.T.; Yang, G.F.; Zhang, A.; Miao, Z.H. An Update on Poly(ADP-ribose)polymerase-1 (PARP-1) Inhibitors: Opportunities and Challenges in Cancer Therapy. J. Med. Chem. 2016, 59, 9575–9598. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Caruso, D.; Strudel, M.; Tomao, S.; Tomao, F. Update on Poly-ADP-ribose polymerase inhibition for ovarian cancer treatment. J. Transl. Med. 2016, 14, 267. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- Dougherty, B.A.; Lai, Z.; Hodgson, D.R.; Orr, M.C.M.; Hawryluk, M.; Sun, J.; Yelensky, R.; Spencer, S.K.; Robertson, J.D.; Ho, T.W.; et al. Biological and clinical evidence for somatic mutations in BRCA1 and BRCA2 as predictive markers for olaparib response in high-grade serous ovarian cancers in the maintenance setting. Oncotarget 2017, 8, 43653–43661. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Capoluongo, E.; Ellison, G.; Lopez-Guerrero, J.A.; Penault-Llorca, F.; Ligtenberg, M.J.L.; Banerjee, S.; Singer, C.; Friedman, E.; Markiefka, B.; Schirmacher, P.; et al. Guidance Statement On BRCA1/2 Tumor Testing in Ovarian Cancer Patients. Semin. Oncol. 2017, 44, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.J. New challenges for BRCA testing: A view from the diagnostic laboratory. Eur J. Hum. Genet. 2016, 24 (Suppl. 1), S10–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.Q.; Li, J.; Tan, A.Y.; Vedururu, R.; Pang, J.M.; Do, H.; Ellul, J.; Doig, K.; Bell, A.; MacArthur, G.A.; et al. Sequence artefacts in a prospective series of formalin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med. Genom. 2014, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koczkowska, M.; Zuk, M.; Gorczynski, A.; Ratajska, M.; Lewandowska, M.; Biernat, W.; Limon, J.; Wasag, B. Detection of somatic BRCA1/2 mutations in ovarian cancer—Next-generation sequencing analysis of 100 cases. Cancer Med. 2016, 5, 1640–1646. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, C.; Tomao, F.; Betella, I.; Rappa, A.; Calvello, M.; Bonanni, B.; Bernard, L.; Peccatori, F.; Colombo, N.; Viale, G.; et al. Tumor BRCA Test for Patients with Epithelial Ovarian Cancer: The Role of Molecular Pathology in the Era of PARP Inhibitor Therapy. Cancers (Basel) 2019, 11, 1641. [Google Scholar] [CrossRef] [Green Version]
- Lerner-Ellis, J.; Mighton, C.; Lazaro, C.; Watkins, N.; Di Gioacchino, V.; Wong, A.; Chang, M.C.; Charames, G.S. Multigene panel testing for hereditary breast and ovarian cancer in the province of Ontario. J. Cancer Res. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Finch, A.; Bacopulos, S.; Rosen, B.; Fan, I.; Bradley, L.; Risch, H.; McLaughlin, J.R.; Lerner-Ellis, J.; Narod, S.A. Preventing ovarian cancer through genetic testing: A population-based study. Clin. Genet. 2014, 86, 496–499. [Google Scholar] [CrossRef]
- Fujiwara, K. Three ongoing intraperitoneal chemotherapy trials in ovarian cancer. J. Gynecol. Oncol. 2012, 23, 75–77. [Google Scholar] [CrossRef] [Green Version]
- Soslow, R.A.; Han, G.; Park, K.J.; Garg, K.; Olvera, N.; Spriggs, D.R.; Kauff, N.D.; Levine, D.A. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod. Pathol. 2012, 25, 625–636. [Google Scholar] [CrossRef]
- Reyes, M.C.; Arnold, A.G.; Kauff, N.D.; Levine, D.A.; Soslow, R.A. Invasion patterns of metastatic high-grade serous carcinoma of ovary or fallopian tube associated with BRCA deficiency. Mod. Pathol. 2014, 27, 1405–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledermann, J.A.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016, 17, 1579–1589. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmana, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.N.; Wubbenhorst, B.; Wenz, B.M.; De Sloover, D.; Pluta, J.; Emery, L.; Barrett, A.; Kraya, A.A.; Anastopoulos, I.N.; Yu, S.; et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat. Commun. 2017, 8, 319. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [Green Version]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Drew, Y. The development of PARP inhibitors in ovarian cancer: From bench to bedside. Br. J. Cancer 2015, 113 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Husain, A.; He, G.; Venkatraman, E.S.; Spriggs, D.R. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II). Cancer Res. 1998, 58, 1120–1123. [Google Scholar]
- Sakai, W.; Swisher, E.M.; Jacquemont, C.; Chandramohan, K.V.; Couch, F.J.; Langdon, S.P.; Wurz, K.; Higgins, J.; Villegas, E.; Taniguchi, T. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009, 69, 6381–6386. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, S.L.; Tian, L.; Kahkonen, M.; Schwartzentruber, J.; Kircher, M.; Majewski, J.; Dyment, D.A.; Innes, A.M.; Boycott, K.M.; Moreau, L.A.; et al. Biallelic mutations in BRCA1 cause a new Fanconi anemia subtype. Cancer Discov. 2015, 5, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weren, R.D.; Mensenkamp, A.R.; Simons, M.; Eijkelenboom, A.; Sie, A.S.; Ouchene, H.; van Asseldonk, M.; Gomez-Garcia, E.B.; Blok, M.J.; de Hullu, J.A.; et al. Novel BRCA1 and BRCA2 Tumor Test as Basis for Treatment Decisions and Referral for Genetic Counselling of Patients with Ovarian Carcinomas. Hum. Mutat. 2017, 38, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018, 2, PO.17.00286. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Davies, L.; Huntsman, D.; Ruas, M.; Fuks, F.; Bye, J.; Chin, S.F.; Milner, J.; Brown, L.A.; Hsu, F.; Gilks, B.; et al. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell 2003, 115, 523–535. [Google Scholar] [CrossRef] [Green Version]
- Hollis, R.L.; Churchman, M.; Michie, C.O.; Rye, T.; Knight, L.; McCavigan, A.; Perren, T.; Williams, A.R.W.; McCluggage, W.G.; Kaplan, R.S.; et al. High EMSY expression defines a BRCA-like subgroup of high-grade serous ovarian carcinoma with prolonged survival and hypersensitivity to platinum. Cancer 2019, 125, 2772–2781. [Google Scholar] [CrossRef]
- Liu, J.F.; Barry, W.T.; Birrer, M.; Lee, J.M.; Buckanovich, R.J.; Fleming, G.F.; Rimel, B.; Buss, M.K.; Nattam, S.; Hurteau, J.; et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: A randomised phase 2 study. Lancet Oncol. 2014, 15, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Nishimura, M.; Onoe, T.; Sakai, H.; Urakawa, Y.; Onda, T.; Yaegashi, N. PARP inhibitors for BRCA wild typse ovarian cancer; gene alterations, homologous recombination deficiency and combination therapy. Jpn J. Clin. Oncol. 2019, 49, 703–707. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]


| Tissue Type | No (%) |
|---|---|
| Ovary | 120 (41.2) |
| Fallopian tube | 32 (11.0) |
| Adnexa | 32 (11.0) |
| Omentum | 58 (20.0) |
| Other | 49 (16.8) |
| Post neoadjuvant chemotherapy | |
| Yes | 85 (29.2) |
| No | 206 (70.8) |
| Clinical–Pathologic Variables | Complete | Failed | Indeterminate | Total | p-Value (Pearson Chi-Square) | |
|---|---|---|---|---|---|---|
| Sample type | Surgical | 206 (81.4%) | 15 (5.9%) | 32 (12.7%) | 253 (87%) | 0.001 |
| Biopsy | 20 (57.1%) | 9 (25.7%) | 6 (17.2%) | 35 (12%) | ||
| Cytology | 2 (66.7%) | 1 (33.3%) | 0 | 3 (1%) | ||
| Tumor cellularity | ≥50% | 185 (81.9%) | 9 (4.0%) | 32 (14.1%) | 226 (77.7%) | <0.0001 |
| >10% to <50% | 34 (73.9%) | 7 (15.2%) | 5 (10.9%) | 46 (15.8%) | ||
| ≤10% | 9 (47.4%) | 9 (47.4%) * | 1 (5.2%) | 19 (6.5%) | ||
| Tumor size | ≥10 mm | 206 (79.5%) | 17 (6.6%) | 36 (13.9%) | 259 (89%) | <0.0001 |
| 5–9 mm | 13 (92.9%) | 1 (7.1%) | 0 | 14 (4.8%) | ||
| ≤4 mm | 9 (50.0%) | 7 (38.9%) | 2 (11.1%) | 18 (6.2%) | ||
| Chemotherapy | Yes | 64 (75.3%) | 9 (10.6%) | 12 (14.1%) | 85 (29.2%) | 0.669 |
| No | 164 (79.6%) | 16 (7.8%) | 26 (12.6%) | 206 (70.8%) | ||
| Age of tissue block, years | <3 | 111 (75.5%) | 13 (8.8%) | 23 (15.7%) | 147 (50.5%) | 0.396 |
| ≥3 | 117 (81.3%) | 12 (8.3%) | 15 (10.4%) | 144 (49.5%) | ||
| Total | 228 (78.4%) | 25 (8.6%) | 38 (13.0%) | 291 (100%) | ||
| Histologic Features | Mutation Total | BRCA1 Mutation | BRCA2 Mutation | No Mutation | Total |
|---|---|---|---|---|---|
| SET features | |||||
| S | 13 (38.2%) | 9 (45%) | 4 (28.6%) | 35 (25.4%) | 48 (27.9%) |
| E | 11 (32.4%) | 7 (35%) | 4 (28.6%) | 21 (15.2%) | 32 (18.6%) |
| T | 4 (11.8%) | 0 | 4 (28.6%) | 2 (1.4%) | 6 (3.5%) |
| All 3 features | 2 (5.9%) | 0 | 2 (14.3%) | 2 (1.4%) | 4 (2.3%) |
| Any 2 of 3 features | 7 (20.6%) | 7 (35%) | 0 | 19 (13.8%) | 26 (15.1%) |
| Any 1 of 3 features | 2 (5.9%) | 2 (10%) | 0 | 14 (10.1%) | 16 (9.3%) |
| Necrosis | 27 (79.4%) | 18 (90%) | 9 (64.3%) | 93 (67.4%) | 120 (69.8%) |
| Grade 3 nuclei | 15 (44.1%) | 11 (55%) | 4 (28.6%) | 79 (57.2%) | 94 (54.6%) |
| TILs | 5 (14.7%) | 2 (10%) | 3 (21.4%) | 14 (10.1%) | 19 (11%) |
| Metastasis with pushing or micropapillary pattern | 3 (8.8%) | 2 (10%) | 1 (7.1%) | 4 (2.9%) | 7 (4.1%) |
| Total | 34 (19.8%) | 20 (11.6%) | 14 (8.2%) | 138 (80.2%) | 172 (100%) |
| No | Exon | Variant | Allele Frequency | Cellularity, % | Amino Acid Change | Mutation Type | Mutation Effect | LOH Status |
|---|---|---|---|---|---|---|---|---|
| BRCA1 | ||||||||
| 1 | 22–23 | c.5333-?_5467+?del | Unknown | 70 | p.(Asp1778_His1822del) | Deletion | Frameshift | None |
| 2 | 18 | c.5141_5144del | 0.642 | 80 | p.(Val1714fs) | Deletion | Frameshift | BRCA2 only |
| 3 | 11 | c.3607C>T | 0.541 | 80 | p.(Arg1203*) | Substitution | Stop codon | BRCA2 only |
| 4 | 15 | c.4485-10_4491del | 0.362 | 80 | p.(Arg1495fs) | Deletion | Frameshift | None |
| 5 | 19 | c.5154G>A | 0.050 | 90 | p.(Trp1718*) | Substitution | Stop codon | BRCA1 and BRCA2 |
| 6 | 11 | c.1387_1390delinsGAAAG | 0.855 | 80 | p.(Lys463Glufs*17) | Indel | Frameshift | BRCA1 and BRCA2 |
| 7 | 11 | c.2263G>T | 0.399 | 60 | p.(Glu755*) | Substitution | Stop codon | BRCA1 and BRCA2 |
| 8 | 11 | c.3476del | 0.704 | 80 | p.(Ile1159fs) | Deletion | Frameshift | BRCA1 and BRCA2 |
| 9 | 5 | c.212+3A>G | 0.802 | 70 | p.? | Substitution | Splicing | BRCA1 and BRCA2 |
| 10 | 11 | c.1961del | 0.631 | 80 | p.(Lys654fs) | Deletion | Frameshift | BRCA2 only |
| 11 | 24 | c.5497G>A | 0.927 | 80 | p.(Val1833Met) | Substitution | Missense | BRCA1 and BRCA2 |
| 12 | 11 | c.2365del | 0.642 | 70 | p.(Ser789Alafs*3) | Deletion | Frameshift | BRCA2 only |
| 13 | 11 | c.2827A>T | 0.304 | 80 | p.(Lys943*) | Substitution | Stop codon | BRCA1 only |
| 14 | 11 | c.3225_3226del | 0.685 | 80 | p.(Asn1075fs) | Deletion | Frameshift | BRCA1 and BRCA2 |
| 15 | 11 | c.2188G>T | 0.800 | 80 | p.(Glu730*) | Substitution | Stop codon | BRCA1 and BRCA2 |
| 16 | 2 | c.3G>A | 0.632 | 70 | p.(Met1?) | Substitution | Missense | None |
| 17 | 16 | c.4689C>G | 0.802 | 60 | p.(Tyr1563*) | Substitution | Stop codon | None |
| 18 | 14 | c.4484G>T | 0.686 | 40 | p.(Arg1495Met) | Substitution | Splicing | BRCA1 only |
| 19 | 24 | c.5468-2A>G | 0.503 | 20 | p.? | Substitution | Splicing | BRCA2 only |
| 20 | 11 | c.2269del | 0.173 | 10 | p.(Val757fs) | Deletion | Frameshift | None |
| 21 | 11 | c.3481_3491del | 0.585 | 70 | p.(Glu1161fs) | Deletion | Frameshift | BRCA2 only |
| 22 | 21 | c.5324T>G | 0.628 | 80 | p.(Met1775Arg) | Substitution | Missense | BRCA1 and BRCA2 |
| 23 | 14 | c.4372C>T | 0.154 | 70 | p.(Gln1458*) | Substitution | Stop codon | None |
| 24 | 9 | c.593+1G>A | 0.051 | 80 | p.? | Substitution | Splicing | BRCA1 only |
| 25 | 11 | c.1603G>T | 0.715 | 80 | p.(Gly535*) | Substitution | Stop codon | BRCA1 only |
| 26 | 11 | c.1390_1391insG | 0.674 | 80 | p.(Thr464Serfs*16) | Insertion | Frameshift | BRCA1 only |
| 27 | 11 | c.1504_1508del | 0.743 | 80 | p.(Leu502Alafs*2) | Deletion | Frameshift | BRCA1 and BRCA2 |
| 28 | 16 | c.4689C>G | 0.818 | 70 | p.(Tyr1563*) | Substitution | Stop codon | BRCA1 and BRCA2 |
| 29 | 19 | c.5193+1G>T | 0.614 | 90 | p.? | Substitution | Splicing | BRCA1 only |
| 30 | 21 | c.5296del | 0.391 | 90 | p.(Ile1766Serfs*27) | Deletion | Frameshift | BRCA1 only |
| BRCA2 | ||||||||
| 31 | 11 | c.4321G>T | 0.616 | 60 | p.(Glu1441*) | Substitution | Stop codon | BRCA2 only |
| 32 | 11 | c.5238dupT | 0.681 | 50 | p.(Asn1747fs) | Duplication | Frameshift | BRCA2 only |
| 33 | 11 | c.5073dup | 0.848 | 80 | p.(Trp1692fs) | Duplication | Frameshift | BRCA1 and BRCA2 |
| 34 | 9 | c.755_758del | 0.711 | 80 | p.(Asp252fs) | Deletion | Frameshift | BRCA1 and BRCA2 |
| 35 | 23 | c.8954-1G>A | 0.863 | 70 | p.? | Substitution | Splicing | None |
| 36 | 9 | c.712G>T | 0.072 | 50 | p.(Glu238*) | Substitution | Stop codon | None |
| 37 | 8 | c.658_659del | 0.495 | 40 | p.(Val220fs) | Deletion | Frameshift | BRCA1 and BRCA2 |
| 38 | 11 | c.6808_6836del | 0.320 | 40 | p.(Gly2270fs) | Deletion | Frameshift | BRCA2 only |
| 39 | 21 | c.8732del | 0.097 | 80 | p.(Ala2911Glufs*16) | Deletion | Frameshift | BRCA1 only |
| 40 | 11 | c.3339del | 0.819 | 80 | p.(Glu1113Aspfs*6) | Deletion | Frameshift | BRCA2 only |
| 41 | 8 | c.632-1G>A | 0.129 | 80 | p.? | Substitution | Splicing | BRCA1 only |
| 42 | 11 | c.6267_6269delinsC | 0.848 | 40 | p.(His2090fs) | Indel | Frameshift | BRCA1 and BRCA2 |
| 43 | 11 | c.4631del | 0.869 | 80 | p.(Asn1544fs) | Deletion | Frameshift | BRCA2 only |
| 44 | 11 | c.3545_3546delTT | 0.813 | 40 | p.(Phe1182fs) | Deletion | Frameshift | None |
| 45 | 24 | c.9154C>T | 0.341 | 80 | p.(Arg3052Trp) | Substitution | Missense | BRCA1 and BRCA2 |
| 46 | 10 | c.1859_1865del | 0.645 | 90 | p.(Phe620*) | Deletion | Frameshift | BRCA2 only |
| 47 | 11 | c.5351dup | 0.293 | 80 | p.(Asn1784Lysfs*3) | Duplication | Frameshift | BRCA2 only |
| 48 | 11 | c.3187C>T | 0.057 | 20 | p.(Gln1063*) | Substitution | Stop codon | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turashvili, G.; Lazaro, C.; Ying, S.; Charames, G.; Wong, A.; Hamilton, K.; Yee, D.; Agro, E.; Chang, M.; Pollett, A.; et al. Tumor BRCA Testing in High Grade Serous Carcinoma: Mutation Rates and Optimal Tissue Requirements. Cancers 2020, 12, 3468. https://doi.org/10.3390/cancers12113468
Turashvili G, Lazaro C, Ying S, Charames G, Wong A, Hamilton K, Yee D, Agro E, Chang M, Pollett A, et al. Tumor BRCA Testing in High Grade Serous Carcinoma: Mutation Rates and Optimal Tissue Requirements. Cancers. 2020; 12(11):3468. https://doi.org/10.3390/cancers12113468
Chicago/Turabian StyleTurashvili, Gulisa, Conxi Lazaro, Shengjie Ying, George Charames, Andrew Wong, Krista Hamilton, Denise Yee, Evangeline Agro, Martin Chang, Aaron Pollett, and et al. 2020. "Tumor BRCA Testing in High Grade Serous Carcinoma: Mutation Rates and Optimal Tissue Requirements" Cancers 12, no. 11: 3468. https://doi.org/10.3390/cancers12113468
APA StyleTurashvili, G., Lazaro, C., Ying, S., Charames, G., Wong, A., Hamilton, K., Yee, D., Agro, E., Chang, M., Pollett, A., & Lerner-Ellis, J. (2020). Tumor BRCA Testing in High Grade Serous Carcinoma: Mutation Rates and Optimal Tissue Requirements. Cancers, 12(11), 3468. https://doi.org/10.3390/cancers12113468







