Integrative p53, micro-RNA and Cathepsin Protease Co-Regulatory Expression Networks in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
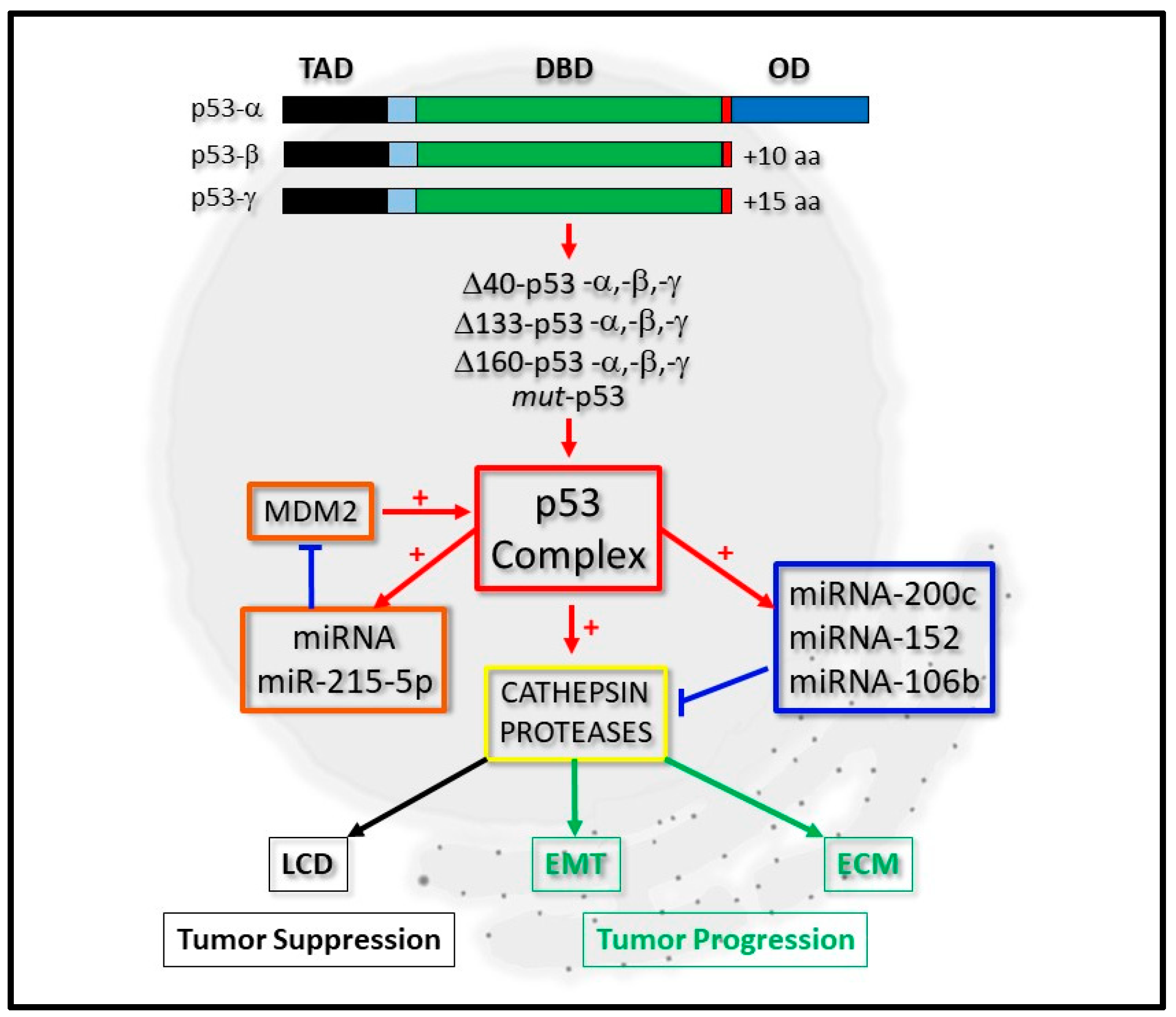
2. The Biochemical Significance of the p53 Isoform Proteins
3. p53, micro-RNA Regulation and Cathepsin Proteases: A Developing Network
3.1. miRNA-200c and Cathepsin Regulation
3.2. miRNA-152-3p and Cathepsin Regulation
3.3. miRNA-106b and Cathepsin Regulation
4. miRNA-200c, -152, -106b Expression and Cancer Progression: A Clinical Perspective
4.1. miRNA-200c Expression
4.2. miRNA-152 Expression
4.3. miRNA-106b Expression
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meek, D.W. Regulation of the p53 response and its relationship to cancer. Biochem. J. 2015, 469, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Schumacher, B. P53 in the DNA-damage-repair process. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, S.; Rossi, A.; Di Daniele, N.; Melino, G.; Annicchiarico-Petruzzelli, M. DNA repair and aging: The impact of the p53 family. Aging 2015, 7, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, M.J.; Mukhopadhyay, S.; Hoofnagle, M.; Chabasse, C.; Sarkar, R. Tumor suppressor protein p53 negatively regulates ischemia-induced angiogenesis and arteriogenesis. J. Vasc. Surg. 2018, 68, 222S–233S. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Iwakuma, T. Non-canonical cell death induced by p53. Int. J. Mol. Sci 2016, 17, 2068. [Google Scholar] [CrossRef]
- Wang, X.; Simpson, E.R.; Brown, K.A. P53: Protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 2015, 75, 5001–5007. [Google Scholar] [CrossRef]
- Chen, J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Avantaggiati, M.L.; Ogryzko, V.; Gardner, K.; Giordano, A.; Levine, A.S.; Kelly, K. Recruitment of p300/cbp in p53-dependent signal pathways. Cell 1997, 89, 1175–1184. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. Tp53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Flaman, J.M.; Waridel, F.; Estreicher, A.; Vannier, A.; Limacher, J.M.; Gilbert, D.; Iggo, R.; Frebourg, T. The human tumour suppressor gene p53 is alternatively spliced in normal cells. Oncogene 1996, 12, 813–818. [Google Scholar]
- Marcel, V.; Dichtel-Danjoy, M.L.; Sagne, C.; Hafsi, H.; Ma, D.; Ortiz-Cuaran, S.; Olivier, M.; Hall, J. Biological functions of p53 isoforms through evolution: Lessons from animal and cellular models. Cell Death Differ. 2011, 18, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Shalgi, R.; Fodstad, O.; Pilpel, Y.; Ju, J. Differentially regulated micro-rnas and actively translated messenger rna transcripts by tumor suppressor p53 in colon cancer. Clin. Cancer Res. 2006, 12, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; Da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microrna target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I.; Yamagata, K.; Sugimoto, K.; Iwamoto, T.; Kato, S.; Miyazono, K. Modulation of microrna processing by p53. Nature 2009, 460, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Goeman, F.; Strano, S.; Blandino, G. Micrornas as key effectors in the p53 network. Int. Rev. Cell Mol. Biol. 2017, 333, 51–90. [Google Scholar] [CrossRef]
- Hermeking, H. Micrornas in the p53 network: Micromanagement of tumour suppression. Nat. Rev. Cancer 2012, 12, 613–626. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Zhao, Y.; Feng, Z. Microrna control of p53. J. Cell Biochem. 2017, 118, 7–14. [Google Scholar] [CrossRef]
- Singh, A.; Bhattacharyya, N.; Srivastava, A.; Pruett, N.; Ripley, R.T.; Schrump, D.S.; Hoang, C.D. Microrna-215-5p treatment suppresses mesothelioma progression via the mdm2-p53-signaling axis. Mol. Ther. 2019, 27, 1665–1680. [Google Scholar] [CrossRef]
- Ong, A.L.C.; Ramasamy, T.S. Role of sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res. Rev. 2018, 43, 64–80. [Google Scholar] [CrossRef]
- Soond, S.M.; Kozhevnikova, M.V.; Zamyatnin, A.A., Jr. Patchiness’ and basic cancer research: Unravelling the proteases. Cell Cycle 2019, 18, 1687–1701. [Google Scholar] [CrossRef]
- Soond, S.M.; Kozhevnikova, M.V.; Townsend, P.A.; Zamyatnin, J.A.A. Cysteine Cathepsin Protease Inhibition: An update on its Diagnostic, Prognostic and Therapeutic Potential in Cancer. Pharmaceuticals 2019, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S.; Saftig, P.; Peters, C.; El-Deiry, W.S. Potential role for Cathepsin D in p53-dependent tumor suppression and chemosensitivity. Oncogene 1998, 16, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.-S.; Derocq, D.; Laurent-Matha, V.; Montcourrier, P.; Sebti, S.S.; Orsetti, B.; Theillet, C.; Gongora, C.; Pattingre, S.; Ibing, E.; et al. Nuclear cathepsin D enhances TRPS1 transcriptional repressor function to regulate cell cycle progression and transformation in human breast cancer cells. Oncotarget 2015, 6, 28084–28103. [Google Scholar] [CrossRef] [PubMed]
- Katara, R.; Mir, R.A.; Shukla, A.A.; Tiwari, A.; Singh, N.; Chauhan, S.S. Wild type p53-dependent transcriptional upregulation of cathepsin L expression is mediated by C/EBPα in human glioblastoma cells. Biol. Chem. 2010, 391, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Kim, S.O.; Han, J. Susceptibility of Lysosomes to Rupture Is a Determinant for Plasma Membrane Disruption in Tumor Necrosis Factor Alpha-Induced Cell Death. Mol. Cell. Biol. 2003, 23, 665–676. [Google Scholar] [CrossRef]
- Fehrenbacher, N.; Bastholm, L.; Kirkegaard-Sørensen, T.; Rafn, B.; Bøttzauw, T.; Nielsen, C.; Weber, E.; Shirasawa, S.; Kallunki, T.; Jäättelä, M. Sensitization to the Lysosomal Cell Death Pathway by Oncogene-Induced Down-regulation of Lysosome-Associated Membrane Proteins 1 and 2. Cancer Res. 2008, 68, 6623–6633. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y.; Chen, W.; Wang, C.; Liu, X.; He, W.; Xu, H.; Cao, X. Adaptor Protein LAPF Recruits Phosphorylated p53 to Lysosomes and Triggers Lysosomal Destabilization in Apoptosis. Cancer Res. 2007, 67, 11176–11185. [Google Scholar] [CrossRef]
- Boya, P.; Andreau, K.; Poncet, D.; Zamzami, N.; Perfettini, J.-L.; Metivier, D.; Ojcius, D.M.; Jäättelaä, M.; Kroemer, G. Lysosomal Membrane Permeabilization Induces Cell Death in a Mitochondrion-dependent Fashion. J. Exp. Med. 2003, 197, 1323–1334. [Google Scholar] [CrossRef]
- Bourdon, J.-C.; Fernandes, K.; Murray-Zmijewski, F.; Liu, G.; Diot, A.; Xirodimas, D.P.; Saville, M.K.; Lane, D.P. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005, 19, 2122–2137. [Google Scholar] [CrossRef]
- Courtois, S.; Verhaegh, G.; North, S.; Luciani, M.-G.; Lassus, P.; Hibner, U.; Oren, M.; Hainaut, P. ΔN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene 2002, 21, 6722–6728. [Google Scholar] [CrossRef]
- Ghosh, A.; Stewart, D.; Matlashewski, G. Regulation of Human p53 Activity and Cell Localization by Alternative Splicing. Mol. Cell. Biol. 2004, 24, 7987–7997. [Google Scholar] [CrossRef] [PubMed]
- Marcel, V.; Perrier, S.; Aoubala, M.; Ageorges, S.; Groves, M.J.; Diot, A.; Fernandes, K.; Tauro, S.; Bourdon, J.-C. Δ160p53 is a novel N-terminal p53 isoform encoded by Δ133p53 transcript. FEBS Lett. 2010, 584, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; E Ruley, H. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993, 7, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Stephen, C.W.; Luciani, M.G.; Fåhraeus, R. p53 stability and activity is regulated by Mdm2-mediated induction of alternative p53 translation products. Nat. Cell Biol. 2002, 4, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Bulavin, D.V.; Saito, S.; Hollander, M.C.; Sakaguchi, K.; Anderson, C.W.; Appella, E.; Jr, A.J.F. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999, 18, 6845–6854. [Google Scholar] [CrossRef] [PubMed]
- D’Orazi, G.; Cecchinelli, B.; Bruno, T.; Manni, I.; Higashimoto, Y.; Saito, S.; Gostissa, M.; Coen, S.; Marchetti, A.; Del Sal, G.; et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 2002, 4, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Liu, H.; Miki, Y. Protein Kinase C δ Regulates Ser46Phosphorylation of p53 Tumor Suppressor in the Apoptotic Response to DNA Damage. J. Biol. Chem. 2005, 281, 5734–5740. [Google Scholar] [CrossRef]
- Taira, N.; Nihira, K.; Yamaguchi, T.; Miki, Y.; Yoshida, K. DYRK2 Is Targeted to the Nucleus and Controls p53 via Ser46 Phosphorylation in the Apoptotic Response to DNA Damage. Mol. Cell 2007, 25, 725–738. [Google Scholar] [CrossRef]
- Graupner, V.; Schulze-Osthoff, K.; Essmann, F.; Jänicke, R.U. Functional characterization of p53? and p53?, two isoforms of the tumor suppressor p53. Cell Cycle 2009, 8, 1238–1248. [Google Scholar] [CrossRef]
- Brosh, R.; Rotter, V. When mutants gain new powers: News from the mutant p53 field. Nat. Rev. Cancer 2009, 9, 701–713. [Google Scholar] [CrossRef]
- Strano, S.; Dell’Orso, S.; Di Agostino, S.; Fontemaggi, G.; Sacchi, A.; Blandino, G. Mutant p53: An oncogenic transcription factor. Oncogene 2007, 26, 2212–2219. [Google Scholar] [CrossRef]
- Bargonetti, J.; Prives, C. Gain-of-function mutant p53: History and speculation. J. Mol. Cell Biol. 2019, 11, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Chao, C.-H.; Xia, W.; Yang, J.-Y.; Xiong, Y.; Li, C.-W.; Yu, W.-H.; Rehman, S.K.; Hsu, J.L.; Lee, H.-H.; et al. p53 regulates epithelial–mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 2011, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Sasaki, Y.; Kobashi, K.; Takeda, K.; Nakagaki, T.; Idogawa, M.; Tokino, T. CRKL oncogene is downregulated by p53 through miR-200s. Cancer Sci. 2015, 106, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Mezhal, F.; El Hasasna, H.; A Nair, V.; Aravind, S.R.; Ayad, M.S.; El-Serafi, A.; Abdel-Rahman, W.M. The role of p53-microRNA 200-Moesin axis in invasion and drug resistance of breast cancer cells. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Soond, S.M.; Kozhevnikova, M.V.; Frolova, A.S.; Savvateeva, L.V.; Plotnikov, E.Y.; Townsend, P.A.; Han, Y.-P.; Zamyatnin, A.A. Lost or Forgotten: The nuclear cathepsin protein isoforms in cancer. Cancer Lett. 2019, 462, 43–50. [Google Scholar] [CrossRef]
- Gocheva, V.; Joyce, J.A. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle 2007, 6, 60–64. [Google Scholar] [CrossRef]
- E Koblinski, J.; Ahram, M.; Sloane, B.F. Unraveling the role of proteases in cancer. Clin. Chim. Acta 2000, 291, 113–135. [Google Scholar] [CrossRef]
- Cirman, T.; Orešić, K.; Mazovec, G.D.; Turk, V.; Reed, J.C.; Myers, R.M.; Salvesen, G.S.; Turk, B. Selective Disruption of Lysosomes in HeLa Cells Triggers Apoptosis Mediated by Cleavage of Bid by Multiple Papain-like Lysosomal Cathepsins. J. Biol. Chem. 2003, 279, 3578–3587. [Google Scholar] [CrossRef]
- Duncan, E.M.; Muratore-Schroeder, T.L.; Cook, R.G.; Garcia, B.A.; Shabanowitz, J.; Hunt, D.F.; Allis, C.D. Cathepsin L Proteolytically Processes Histone H3 During Mouse Embryonic Stem Cell Differentiation. Cell 2008, 135, 284–294. [Google Scholar] [CrossRef]
- Adams-Cioaba, M.A.; Krupa, J.C.; Xu, C.; Mort, J.S.; Min, J. Structural basis for the recognition and cleavage of histone H3 by cathepsin L. Nat. Commun. 2011, 2, 197. [Google Scholar] [CrossRef] [PubMed]
- Goulet, B.; Baruch, A.; Moon, N.-S.; Poirier, M.; Sansregret, L.L.; Erickson, A.; Bogyo, M.; Nepveu, A. A Cathepsin L Isoform that Is Devoid of a Signal Peptide Localizes to the Nucleus in S Phase and Processes the CDP/Cux Transcription Factor. Mol. Cell 2004, 14, 207–219. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Xiong, Y.; Wang, W.; Fei, Y.; Tan, C.; Liang, Z. K-ras mutation promotes ionizing radiation-induced invasion and migration of lung cancer in part via the Cathepsin L/CUX1 pathway. Exp. Cell Res. 2018, 362, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.J.; Dougan, J.; Jones, J.; Smith, B.N.; Randle, D.; Henderson, V.; A Odero-Marah, V. Targeting the Nuclear Cathepsin L CCAAT Displacement Protein/Cut Homeobox Transcription Factor-Epithelial Mesenchymal Transition Pathway in Prostate and Breast Cancer Cells with the Z-FY-CHO Inhibitor. Mol. Cell. Biol. 2016, 37. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-W.; Lian, W.-S.; Chen, Y.-S.; Kuo, C.-W.; Ke, H.-C.; Hsieh, C.-K.; Wang, S.-Y.; Ko, J.-Y.; Wang, F.-S. MicroRNA-29a Counteracts Glucocorticoid Induction of Bone Loss through Repressing TNFSF13b Modulation of Osteoclastogenesis. Int. J. Mol. Sci. 2019, 20, 5141. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Rao, C.-L.; Tang, M.-L.; Zhang, Y.; Lu, X.-X.; Chen, J.-G.; Mao, C.; Deng, L.; Li, Q.; Mao, X.-H. Rab32 GTPase, as a direct target of miR-30b/c, controls the intracellular survival of Burkholderia pseudomallei by regulating phagosome maturation. PLoS Pathog. 2019, 15. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, K.; Yao, T.; Zhu, H.; Xu, Y.; Ye, H.; Chen, Z.; Lv, J.; Shen, S.; Ma, J. MicroRNA-25-3p regulates osteoclasts through nuclear factor I X. Biochem. Biophys. Res. Commun. 2019, 522, 74–80. [Google Scholar] [CrossRef]
- Ho, K.-H.; Cheng, C.-H.; Chou, C.-M.; Chen, P.-H.; Liu, A.-J.; Lin, C.-W.; Shih, C.-M.; Chen, K.-C. miR-140 targeting CTSB signaling suppresses the mesenchymal transition and enhances temozolomide cytotoxicity in glioblastoma multiforme. Pharmacol. Res. 2019, 147. [Google Scholar] [CrossRef]
- Li, K.; Chen, S.; Cai, P.; Chen, K.; Li, L.; Yang, X.; Yi, J.; Luo, X.; Du, Y.; Zheng, H. MiRNA-483–5p is involved in the pathogenesis of osteoporosis by promoting osteoclast differentiation. Mol. Cell. Probes 2020, 49. [Google Scholar] [CrossRef]
- Dinesh, P.; Kalaiselvan, S.; Sujitha, S.; Rasool, M. miR-506-3p alleviates uncontrolled osteoclastogenesis via repression of RANKL/NFATc1 signaling pathway. J. Cell. Physiol. 2020, 235, 9497–9509. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Han, M.-L.; Xiong, Y.-J.; Wang, L.; Fei, Y.; Shen, X.; Zhu, Y.; Liang, Z.-Q. A miRNA-200c/cathepsin L feedback loop determines paclitaxel resistance in human lung cancer A549 cells in vitro through regulating epithelial–mesenchymal transition. Acta Pharmacol. Sin. 2017, 39, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Okamura, S.; Arakawa, H.; Tanaka, T.; Nakanishi, H.; Ng, C.C.; Taya, Y.; Monden, M.; Nakamura, Y. p53DINP1, a p53-Inducible Gene, Regulates p53-Dependent Apoptosis. Mol. Cell 2001, 8, 85–94. [Google Scholar] [CrossRef]
- Lu, H.-J.; Yan, J.; Jin, P.-Y.; Zheng, G.-H.; Qin, S.-M.; Wu, D.; Lu, J.; Zheng, Y.-L. MicroRNA-152 inhibits tumor cell growth while inducing apoptosis via the transcriptional repression of cathepsin L in gastrointestinal stromal tumor. Cancer Biomark. 2018, 21, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.; Wojtukiewicz, M.Z.; Ostrowska, H. Cathepsin A activity in primary and metastatic human melanocytic tumors. Arch. Dermatol. Res. 2000, 292, 68–71. [Google Scholar] [CrossRef]
- Ni, S.; Weng, W.; Xu, M.; Wang, Q.; Tan, C.; Sun, H.; Wang, L.; Huang, D.; Du, X.; Sheng, W. miR-106b-5p inhibits the invasion and metastasis of colorectal cancer by targeting CTSA. OncoTargets Ther. 2018, 11, 3835–3845. [Google Scholar] [CrossRef]
- Ljepoja, B.; García-Román, J.; Sommer, A.-K.; Fröhlich, T.; Arnold, G.J.; Wagner, E.; Roidl, A. A proteomic analysis of an in vitro knock-out of miR-200c. Sci. Rep. 2018, 8, 6927. [Google Scholar] [CrossRef]
- Mutlu, M.; Raza, U.; Saatci, Ö.; Eyüpoğlu, E.; Yurdusev, E.; Sahin, Ö. miR-200c: A versatile watchdog in cancer progression, EMT, and drug resistance. J. Mol. Med. 2016, 94, 629–644. [Google Scholar] [CrossRef]
- Humphries, B.; Yang, C. The microRNA-200 family: Small molecules with novel roles in cancer development, progression and therapy. Oncotarget 2015, 6, 6472–6498. [Google Scholar] [CrossRef]
- Muralidhar, G.G.; Barbolina, M.V. The miR-200 Family: Versatile Players in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2015, 16, 16833–16847. [Google Scholar] [CrossRef]
- Perdigão-Henriques, R.; Petrocca, F.; Altschuler, G.; Thomas, M.P.; Le, M.T.N.; Tan, S.M.; Hide, W.; Lieberman, J. miR-200 promotes the mesenchymal to epithelial transition by suppressing multiple members of the Zeb2 and Snail1 transcriptional repressor complexes. Oncogene 2015, 35, 158–172. [Google Scholar] [CrossRef]
- Dykxhoorn, D.M.; Wu, Y.; Xie, H.; Yu, F.; Lal, A.; Petrocca, F.; Martinvalet, D.; Song, E.; Lim, B.; Lieberman, J. miR-200 Enhances Mouse Breast Cancer Cell Colonization to Form Distant Metastases. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Leonora, B.; Hamar, P.; Guo, C.; Basar, E.; Perdigão-Henriques, R.; Balaj, L.; Lieberman, J. miR-200–containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Investig. 2014, 124, 5109–5128. [Google Scholar] [CrossRef]
- Bracken, C.P.; Gregory, P.A.; Kolesnikoff, N.; Bert, A.G.; Wang, J.; Shannon, M.F.; Goodall, G.J. A Double-Negative Feedback Loop between ZEB1-SIP1 and the microRNA-200 Family Regulates Epithelial-Mesenchymal Transition. Cancer Res. 2008, 68, 7846–7854. [Google Scholar] [CrossRef] [PubMed]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nag, A.; Mandal, C.C. A Comprehensive Review on miR-200c, A Promising Cancer Biomarker with Therapeutic Potential. Curr. Drug Targets 2015, 16, 1381–1403. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, D.; Duan, H.; Shen, B.; Guo, N. Metastasis-related miRNAs, active players in breast cancer invasion, and metastasis. Cancer Metastasis Rev. 2010, 29, 785–799. [Google Scholar] [CrossRef]
- Brabletz, S.; Brabletz, T. The ZEB/miR-200 feedback loop—A motor of cellular plasticity in development and cancer? EMBO Rep. 2010, 11, 670–677. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Park, S.-M.; Gaur, A.B.; Lengyel, E.; E Peter, M. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The miR-200 Family Inhibits Epithelial-Mesenchymal Transition and Cancer Cell Migration by Direct Targeting of E-cadherin Transcriptional RepressorsZEB1andZEB2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bracken, C.P.; Bert, A.G.; Goodall, G.J. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle 2008, 7, 3112–3117. [Google Scholar] [CrossRef] [PubMed]
- Tryndyak, V.P.; Beland, F.A.; Pogribny, I.P. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int. J. Cancer 2010, 126, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.J.; Sarraferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Formentini, A.; Chien, M.; Weir, D.B.; Russo, J.J.; Ju, J.; Kornmann, M.; Ju, J. Prognostic Values of microRNAs in Colorectal Cancer. Biomark. Insights 2006, 1, 113–121. [Google Scholar] [CrossRef]
- Fei, Y.; Xiong, Y.; Shen, X.; Zhao, Y.; Zhu, Y.; Wang, L.; Liang, Z. Cathepsin L promotes ionizing radiation-induced U251 glioma cell migration and invasion through regulating the GSK-3β/CUX1 pathway. Cell. Signal. 2018, 44, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Q.; Wang, W.-J.; Li, J.; Yang, N.; Chen, G.; Wang, Z.; Liang, Z.-Q. Cathepsin L suppression increases the radiosensitivity of human glioma U251 cells via G2/M cell cycle arrest and DNA damage. Acta Pharmacol. Sin. 2015, 36, 1113–1125. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, Y.; Ding, X.; Wang, L.; Zhao, Y.; Fei, Y.; Zhu, Y.; Shen, X.; Tan, C.; Liang, Z. Cathepsin L activated by mutant p53 and Egr-1 promotes ionizing radiation-induced EMT in human NSCLC. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef]
- Prislei, S.; Martinelli, E.; Mariani, M.; Raspaglio, G.; Sieber, S.; Ferrandina, G.; Shahabi, S.; Scambia, G.; Ferlini, C. MiR-200c and HuR in ovarian cancer. BMC Cancer 2013, 13, 72. [Google Scholar] [CrossRef]
- Wang, G.Y.; Scheiber, M.N.; Neumann, C.; Calin, G.; Zhou, D. MicroRNA Regulation of Ionizing Radiation-Induced Premature Senescence. Int. J. Radiat. Oncol. 2011, 81, 839–848. [Google Scholar] [CrossRef]
- Zhang, B.-L.; Dong, F.-L.; Guo, T.-W.; Gu, X.-H.; Huang, L.-Y.; Gao, D.-S. MiRNAs Mediate GDNF-Induced Proliferation and Migration of Glioma Cells. Cell. Physiol. Biochem. 2017, 44, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Knösel, T.; Chen, Y.; Hotovy, S.; Settmacher, U.; Altendorf-Hofmann, A.; Petersen, I. Loss of desmocollin 1-3 and homeobox genes PITX1 and CDX2 are associated with tumor progression and survival in colorectal carcinoma. Int. J. Color. Dis. 2012, 27, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-K.; Gu, W.; Li, E.-M.; Wu, Z.-Y.; Shen, Z.-Y.; Shen, J.-H.; Wu, J.-Y.; Pan, F.; Lv, Z.; Xu, X.-E.; et al. Reduced membranous and ectopic cytoplasmic expression of DSC2 in esophageal squamous cell carcinoma: An independent prognostic factor. Hum. Pathol. 2010, 41, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Widodo; Djati, M.S.; Rifa’i, M. Role of MicroRNAs in carcinogenesis that potential for biomarker of endometrial cancer. Ann. Med. Surg. 2016, 7, 9–13. [Google Scholar] [CrossRef]
- Stumpel, D.J.P.M.; Schotte, D.; Lange-Turenhout, E.A.M.; A Schneider, P.; Seslija, L.; De Menezes, R.X.; E Marquez, V.; Pieters, R.; Boer, M.L.D.; Stam, R.W. Hypermethylation of specific microRNA genes in MLL-rearranged infant acute lymphoblastic leukemia: Major matters at a micro scale. Leukemia 2011, 25, 429–439. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Wang, Z.-N.; Yue, Z.; Xu, H.-M.; Xing, C.; Liu, Z. Altered Expression of MiR-148a and MiR-152 in Gastrointestinal Cancers and Its Clinical Significance. J. Gastrointest. Surg. 2010, 14, 1170–1179. [Google Scholar] [CrossRef]
- Braconi, C.; Huang, N.; Patel, T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology 2010, 51, 881–890. [Google Scholar] [CrossRef]
- Kan, T.; Sato, F.; Ito, T.; Matsumura, N.; David, S.; Cheng, Y.; Agarwal, R.; Paun, B.C.; Jin, Z.; Olaru, A.V.; et al. The miR-106b-25 Polycistron, Activated by Genomic Amplification, Functions as an Oncogene by Suppressing p21 and Bim. Gastroenterology 2009, 136, 1689–1700. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Yu, J.; Han, T.S.; Park, S.-Y.; Namkoong, B.; Kim, D.H.; Hur, K.; Yoo, M.-W.; Lee, H.-J.; Yang, H.-K.; et al. Functional links between clustered microRNAs: Suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009, 37, 1672–1681. [Google Scholar] [CrossRef]
- Poliseno, L.; Salmena, L.; Riccardi, L.; Fornari, A.; Song, M.S.; Hobbs, R.M.; Sportoletti, P.; Varmeh, S.; Egia, A.; Fedele, G.; et al. Identification of the miR-106b 25 MicroRNA Cluster as a Proto-Oncogenic PTEN-Targeting Intron That Cooperates with Its Host Gene MCM7 in Transformation. Sci. Signal. 2010, 3. [Google Scholar] [CrossRef]
- Li, Y.; Tan, W.; Neo, T.W.; Aung, M.O.; Wasser, S.; Lim, S.G.; Tan, T.M. Role of themiR-106b-25microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009, 100, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Verboon, L.J.; Obulkasim, A.; De Rooij, J.D.; Katsman-Kuipers, J.E.; Sonneveld, E.; Baruchel, A.; Trka, J.; Reinhardt, D.; Pieters, R.; Cloos, J.; et al. MicroRNA-106b~25 cluster is upregulated in relapsed MLL-rearranged pediatric acute myeloid leukemia. Oncotarget 2016, 7, 48412–48422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-N.; Bai, J.-X.; Zhou, Q.; Yan, B.; Qin, W.-W.; Jia, L.-T.; Meng, Y.-L.; Jin, B.-Q.; Yao, L.-B.; Wang, T.; et al. TSA Suppresses miR-106b-93-25 Cluster Expression through Downregulation of MYC and Inhibits Proliferation and Induces Apoptosis in Human EMC. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Tamilzhalagan, S.; Rathinam, D.; Ganesan, K. Amplified 7q21-22 geneMCM7and its intronic miR-25 suppressCOL1A2associated genes to sustain intestinal gastric cancer features. Mol. Carcinog. 2017, 56, 1590–1602. [Google Scholar] [CrossRef]
- Zhu, D.-X.; Zhu, W.; Fang, C.; Fan, L.; Zou, Z.-J.; Wang, Y.-H.; Liu, P.; Hong, M.; Miao, K.-R.; Liu, P.; et al. miR-181a/b significantly enhances drug sensitivity in chronic lymphocytic leukemia cells via targeting multiple anti-apoptosis genes. Carcinogenesis 2012, 33, 1294–1301. [Google Scholar] [CrossRef]
- Suh, S.-S.; Yoo, J.Y.; Nuovo, G.J.; Jeon, Y.-J.; Kim, S.; Lee, T.J.; Kim, T.; Bakàcs, A.; Alder, H.; Kaur, B.; et al. MicroRNAs/TP53 feedback circuitry in glioblastoma multiforme. Proc. Natl. Acad. Sci. USA 2012, 109, 5316–5321. [Google Scholar] [CrossRef]
- Petrocca, F.; Visone, R.; Onelli, M.R.; Shah, M.H.; Nicoloso, M.S.; De Martino, I.; Iliopoulos, D.; Pilozzi, E.; Liu, C.-G.; Negrini, M.; et al. E2F1-Regulated MicroRNAs Impair TGFβ-Dependent Cell-Cycle Arrest and Apoptosis in Gastric Cancer. Cancer Cell 2008, 13, 272–286. [Google Scholar] [CrossRef]
- Razumilava, N.; Bronk, S.F.; Smoot, R.L.; Fingas, C.D.; Werneburg, N.W.; Roberts, L.R.; Mott, J.L. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology 2011, 55, 465–475. [Google Scholar] [CrossRef]
- Ivanovska, I.; Ball, A.S.; Diaz, R.L.; Magnus, J.F.; Kibukawa, M.; Schelter, J.M.; Kobayashi, S.V.; Lim, L.; Burchard, J.; Jackson, A.L.; et al. MicroRNAs in the miR-106b Family Regulate p21/CDKN1A and Promote Cell Cycle Progression. Mol. Cell. Biol. 2008, 28, 2167–2174. [Google Scholar] [CrossRef]
- Xu, N.; Wang, Z.; Liu, M.; Zhu, H.; Zhang, W.; He, S.; Hu, C.; Quan, L.; Bai, J.; Xu, N. Suppression of p21 by c-Myc through members of miR-17 family at the post-transcriptional level. Int. J. Oncol. 2010, 37, 1315–1321. [Google Scholar] [CrossRef]
- Brosh, R.; Shalgi, R.; Liran, A.; Landan, G.; Korotayev, K.; Nguyen, G.H.; Enerly, E.; Johnsen, H.; Buganim, Y.; Solomon, H.; et al. p53-repressed miRNAs are involved with E2F in a feed-forward loop promoting proliferation. Mol. Syst. Biol. 2008, 4, 229. [Google Scholar] [CrossRef]
- Ou, Y.-H.; Chung, P.-H.; Hsu, F.-F.; Sun, T.-P.; Chang, W.-Y.; Shieh, S.-Y. The candidate tumor suppressor BTG3 is a transcriptional target of p53 that inhibits E2F1. EMBO J. 2007, 26, 3968–3980. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; He, J.; Huang, C.; Chen, L.; Tao, D.; Wu, X.; Wang, M.; Luo, G.; Xiao, X.; Zeng, F.; et al. miR-106b-5p targets tumor suppressor gene SETD2 to inactive its function in clear cell renal cell carcinoma. Oncotarget 2015, 6, 4066–4079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Weng, T.; Gou, D.; Chen, Z.; Chintagari, N.R.; Liu, L. Identification of rat lung-specific microRNAs by microRNA microarray: Valuable discoveries for the facilitation of lung research. BMC Genom. 2007, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ceppi, P.; Mudduluru, G.; Kumarswamy, R.; Rapa, I.; Scagliotti, G.V.; Papotti, M.; Allgayer, H. Loss of miR-200c Expression Induces an Aggressive, Invasive, and Chemoresistant Phenotype in Non-Small Cell Lung Cancer. Mol. Cancer Res. 2010, 8, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Cittelly, D.M.; Dimitrova, I.; Howe, E.N.; Cochrane, D.R.; Jean, A.; Spoelstra, N.S.; Post, M.D.; Lu, X.; Broaddus, R.R.; Spillman, M.A.; et al. Restoration of miR-200c to Ovarian Cancer Reduces Tumor Burden and Increases Sensitivity to Paclitaxel. Mol. Cancer Ther. 2012, 11, 2556–2565. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, S.; Wu, H.; Zhang, L.; Dai, X.; Hu, J.; Xue, J.; Liu, T.; Liang, Y.; Wu, G. MiR-200c Increases the Radiosensitivity of Non-Small-Cell Lung Cancer Cell Line A549 by Targeting VEGF-VEGFR2 Pathway. PLoS ONE 2013, 8, e78344. [Google Scholar] [CrossRef]
- Cortez, M.A.; Valdecanas, D.; Zhang, X.; Zhan, Y.; Bhardwaj, V.; A Calin, G.; Komaki, R.; Giri, D.K.; Quini, C.C.; Wolfe, T.; et al. Therapeutic Delivery of miR-200c Enhances Radiosensitivity in Lung Cancer. Mol. Ther. 2014, 22, 1494–1503. [Google Scholar] [CrossRef]
- Kopp, F.; Wagner, E.; Roidl, A. The proto-oncogene KRAS is targeted by miR-200c. Oncotarget 2013, 5, 185–195. [Google Scholar] [CrossRef]
- Li, J.; Tan, Q.; Yan, M.; Liu, L.; Lin, H.; Zhao, F.; Bao, G.; Kong, H.; Ge, C.; Zhang, F.; et al. miRNA-200c inhibits invasion and metastasis of human non-small cell lung cancer by directly targeting ubiquitin specific peptidase 25. Mol. Cancer 2014, 13, 166. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, F.; Guo, Y.; Huang, J.; Xie, Y.; Yue, S.; Chen, M.; Jiang, H.; Li, M. miR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibites cell migration via targeting ZEB1. Biomed. Pharmacother. 2017, 85, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Sui, M.; Zhang, L.; Sun, P.; Geng, D.; Zhang, W.; Wang, X.; Li, J. MicroRNA-200c inhibits the metastasis of non-small cell lung cancer cells by targeting ZEB2, an epithelial-mesenchymal transition regulator. Mol. Med. Rep. 2016, 13, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Tejero, R.; Navarro, A.; Campayo, M.; Viñolas, N.; Marrades, R.M.; Cordeiro, A.; Ruíz-Martínez, M.; Santasusagna, S.; Molins, L.; Ramirez, J.; et al. miR-141 and miR-200c as Markers of Overall Survival in Early Stage Non-Small Cell Lung Cancer Adenocarcinoma. PLoS ONE 2014, 9, e101899. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Dong, D.-S.; Pei, L. Synergistic antitumor activity of resveratrol and miR-200c in human lung cancer. Oncol. Rep. 2014, 31, 2293–2297. [Google Scholar] [CrossRef]
- Kim, M.K.; Jung, S.B.; Kim, J.-S.; Roh, M.S.; Lee, J.H.; Lee, E.H.; Lee, H.W. Expression of microRNA miR-126 and miR-200c is associated with prognosis in patients with non-small cell lung cancer. Virchows Archiv. 2014, 465, 463–471. [Google Scholar] [CrossRef]
- Shao, Y.; Geng, Y.; Gu, W.; Huang, J.; Pei, H.; Jiang, J. Prognostic Role of Tissue and Circulating MicroRNA-200c in Malignant Tumors: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2015, 35, 1188–1200. [Google Scholar] [CrossRef]
- Teng, Y.; Su, X.; Zhang, X.; Zhang, Y.; Li, C.; Niu, W.; Liu, C.; Qu, K. miRNA-200a/c as potential biomarker in epithelial ovarian cancer (EOC): Evidence based on miRNA meta-signature and clinical investigations. Oncotarget 2016, 7, 81621–81633. [Google Scholar] [CrossRef]
- Si, L.; Tian, H.; Yue, W.; Li, L.; Li, S.; Gao, C.; Qi, L. Potential use of microRNA-200c as a prognostic marker in non-small cell lung cancer. Oncol. Lett. 2017, 14, 4325–4330. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.-C.; Chen, L.-Q.; Xu, L.-Y.; Li, E.-M.; Zhang, J.-J. Predictive biomarkers for response of esophageal cancer to chemo(radio)therapy: A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 460–472. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, C.; Guan, H.; Kang, W.; Yu, C. Prognostic role of microRNAs in human gastrointestinal cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 46611–46623. [Google Scholar] [CrossRef]
- Shi, M.; Mu, Y.; Zhang, H.; Liu, M.; Wan, J.; Qin, X.; Li, C. MicroRNA-200 and microRNA-30 family as prognostic molecular signatures in ovarian cancer. Medicine 2018, 97. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, D.; Kong, Q.; Gao, W.; Sun, J. Function of miR-152 as a Tumor Suppressor in Human Breast Cancer by Targeting PIK3CA. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xie, Z.; Li, B. miR-152 functions as a tumor suppressor in colorectal cancer by targeting PIK3R3. Tumor Biol. 2016, 37, 10075–10084. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, M.; Lu, M.M.; Qu, L.Y.; Xu, S.G.; Li, Y.Z.; Wang, M.Y.; Zhu, H.F.; Zhang, Z.Y.; He, G.Y.; et al. EPAS1 targeting by miR-152-3p in Paclitaxel-resistant Breast Cancer. J. Cancer 2020, 11, 5822–5830. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Kan, X.; Wang, B.; Du, H.; Long, Y.; Wu, H.; Tao, K.; Wang, G.; Bao, L.; Li, F.; et al. miR-152 suppresses gastric cancer cell proliferation and motility by targeting CD151. Tumor Biol. 2014, 35, 11367–11373. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, Y.; Yin, Y.; Li, Q.; He, J.; Jing, Y.; Qi, Y.-T.; Xu, Q.; Li, W.; Lu, B.; et al. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J. Mol. Cell Biol. 2012, 5, 3–13. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Xie, G.; Yin, Y.; Zhao, E.; Tao, K.; Li, R. MicroRNA-152 regulates immune response via targeting B7-H1 in gastric carcinoma. Oncotarget 2017, 8, 28125–28134. [Google Scholar] [CrossRef]
- Yin, T.; Liu, M.-M.; Jin, R.-T.; Kong, J.; Wang, S.-H.; Sun, W.-B. miR-152-3p Modulates hepatic carcinogenesis by targeting cyclin-dependent kinase 8. Pathol. Res. Pract. 2019, 215, 152406. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Lin, J. MiR-152-3p promotes the development of chronic myeloid leukemia by inhibiting p27. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 8789–8796. [Google Scholar]
- Wang, M.; Wu, Q.; Fang, M.; Huang, W.; Zhu, H. miR-152-3p Sensitizes Glioblastoma Cells Towards Cisplatin Via Regulation of SOS1. OncoTargets Ther. 2019, 12, 9513–9525. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, Z.; Man, D.; Ruan, H.; Huang, S. miR-152-3p Affects the Progression of Colon Cancer via the KLF4/IFITM3 Axis. Comput. Math. Methods Med. 2020, 2020, 8209504. [Google Scholar] [CrossRef] [PubMed]
- Sanfiorenzo, C.; Ilie, M.; Belaid, A.; Barlesi, F.; Mouroux, J.; Marquette, C.-H.; Brest, P.; Hofman, P. Two Panels of Plasma MicroRNAs as Non-Invasive Biomarkers for Prediction of Recurrence in Resectable NSCLC. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Zhang, X.; Ji, M.; Yu, Y.; Chen, C.; Xiong, Y.; Liu, Y.; Sun, Y.; Tan, C.; Zhang, H.; et al. MiR-152-5p as a microRNA passenger strand special functions in human gastric cancer cells. Int. J. Biol. Sci. 2018, 14, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Matin, F.; BioResource, A.P.C.; Jeet, V.; Moya, L.; Selth, L.A.; Chambers, S.; Clements, J.A.; Batra, J. A Plasma Biomarker Panel of Four MicroRNAs for the Diagnosis of Prostate Cancer. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Zou, H.; Chen, R.; Dou, Y.; Sheng, S.; Dai, S.; Ai, J.; Melson, J.; Kittles, R.A.; et al. Evaluation of Plasma miR-21 and miR-152 as Diagnostic Biomarkers for Common Types of Human Cancers. J. Cancer 2016, 7, 490–499. [Google Scholar] [CrossRef]
- Li, X.; Zou, W.; Wang, Y.; Liao, Z.; Li, L.; Zhai, Y.; Zhang, L.; Gu, S.; Zhao, X.H. Plasma-based microRNA signatures in early diagnosis of breast cancer. Mol. Genet. Genom. Med. 2020, 8. [Google Scholar] [CrossRef]
- Cai, K.; Wang, Y.; Bao, X. MiR-106b promotes cell proliferation via targeting RB in laryngeal carcinoma. J. Exp. Clin. Cancer Res. 2011, 30, 73. [Google Scholar] [CrossRef]
- Zhai, Z.; Wu, F.; Chuang, A.Y.; Kwon, J.H. miR-106b Fine Tunes ATG16L1 Expression and Autophagic Activity in Intestinal Epithelial HCT116 Cells. Inflamm. Bowel Dis. 2013, 19, 2295–2301. [Google Scholar] [CrossRef]
- Lu, C.; Chen, J.; Xu, H.; Zhou, X.; He, Q.; Li, Y.; Jiang, G.; Shan, Y.; Xue, B.; Zhao, R.; et al. MIR106B and MIR93 Prevent Removal of Bacteria from Epithelial Cells by Disrupting ATG16L1-Mediated Autophagy. Gastroenterology 2014, 146, 188–199. [Google Scholar] [CrossRef]
- Guo, J.; Miao, Y.; Xiao, B.; Huan, R.; Jiang, Z.; Meng, D.; Wang, Y. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J. Gastroenterol. Hepatol. 2009, 24, 652–657. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Y.; Liu, Y.; Zhou, M.; Lu, Y.; Yuan, L.; Zhang, C.; Hong, M.; Wang, S.; Li, X. MiR-106b induces cell radioresistance via the PTEN/PI3K/AKT pathways and p21 in colorectal cancer. J. Transl. Med. 2015, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Sun, P.; Leng, K.; Xu, Y.; Mei, L.; Han, P.; Zhang, B.; Yao, K.; Li, C.; et al. Colorectal cancer-derived exosomal miR-106b-3p promotes metastasis by down-regulating DLC-1 expression. Clin. Sci. 2020, 134, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chen, Q.; Lu, Y.; Wei, F.; Chen, C.; Tang, G.; Huang, H. MiR-106b-5p Regulates the Migration and Invasion of Colorectal Cancer Cells by Targeting FAT4. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhang, X.Y.; Zhang, B.F.; Yang, C.Q.; Chen, X.M.; Gao, H.J. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. J. Dig. Dis. 2010, 11, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Nagahara, M.; Sato, T.; Mimori, K.; Sudo, T.; Tanaka, F.; Shibata, K.; Ishii, H.; Sugihara, K.; Doki, Y.; et al. Microarray Analysis of Colorectal Cancer Stromal Tissue Reveals Upregulation of Two Oncogenic miRNA Clusters. Clin. Cancer Res. 2012, 18, 3054–3070. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Lang, F.; Liu, Y.-X.; Yang, C.-Q.; Gao, H. In situ hybridization analysis of the expression of miR-106b in colonic cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 786–792. [Google Scholar]
- Zhang, G.-J.; Li, J.-S.; Zhou, H.; Xiao, H.-X.; Li, Y.; Zhou, T. MicroRNA-106b promotes colorectal cancer cell migration and invasion by directly targeting DLC1. J. Exp. Clin. Cancer Res. 2015, 34, 1–11. [Google Scholar] [CrossRef]
- Gu, L.; Li, H.; Chen, L.; Ma, X.; Gao, Y.; Li, X.; Zhang, Y.; Fan, Y.; Zhang, X. MicroRNAs as prognostic molecular signatures in renal cell carcinoma: A systematic review and meta-analysis. Oncotarget 2015, 6, 32545–32560. [Google Scholar] [CrossRef]
- Liu, D.; Wan, X.; Shan, X.; Fan, R.; Zha, W. Drugging the “undruggable” microRNAs. Cell. Mol. Life Sci. 2020, 1–11. [Google Scholar] [CrossRef]
- Guerra, E.; Cimadamore, A.; Simeone, P.; Vacca, G.; Lattanzio, R.; Botti, G.; Gatta, V.; D’Aurora, M.; Simionati, B.; Piantelli, M.; et al. p53, cathepsin D, Bcl-2 are joint prognostic indicators of breast cancer metastatic spreading. BMC Cancer 2016, 16, 649. [Google Scholar] [CrossRef]
| p53 Isoform | Amino Acids | Protein (kD) | Reference |
|---|---|---|---|
| p53-α | 1-393 | 53 | [33] |
| p53-β | 1-331+10 | 47 | [10] |
| p53-γ | 1-331+15 | 48 | [29] |
| Δ40-p53-α | 40-393 | 47 | [30,31] |
| Δ40-p53-β | 40-331+10 | 42 | [29] |
| Δ40-p53-γ | 40-331+15 | 42 | [29] |
| Δ133-p53-α | 133-393 | 35 | [29] |
| Δ133-p53-β | 133-331+10 | 29 | [29] |
| Δ133-p53-γ | 133-331+15 | 29 | [29] |
| Δ160-p53-α | 161-393 | 31 | [32] |
| Δ160-p53-β | 161-331+10 | 26 | [11,32] |
| Δ160-p53-γ | 161-331+15 | 26 | [11,32] |
| Micro-RNA | Cathepsin | p53 Isoform | Cell Type | Reference |
|---|---|---|---|---|
| miRNA-200c | L | WT-p53-α | A549 Lung | [43,61] |
| miRNA-152 | L | WT-p53-α | Gastrointestinal | [62,63] |
| miRNA-106b | A | WT-p53-α | Colorectal | [64,65] |
| miRNA-140 | B | - | Glioblastoma | [58] |
| miRNA-30 | D | - | Macrophage | [56] |
| miRNA-25-3p | K | - | Osteoblast | [57] |
| miRNA-483-5p | K | - | PBMC | [59] |
| miRNA-506-3p | K | - | Macrophage | [60] |
| miRNA-29a | K | - | Osteoblast | [55] |
| micro-RNA | Target | Negative Effect | SensitizingAgent | Cell Type | Reference |
|---|---|---|---|---|---|
| 200c (+) | VEGF, VEGFR2 | Angiogenesis, Cell Migration | Radiation | A549 | [117] |
| 200c (+) | PRDX2, SENS1, GABPA/Nrf2 | Oxidative Response | Radiation | A549, H460, H1299 | [118] |
| 200c (+) | K-Ras | Proliferation, Cell cycle | − | Lung and BC cell lines | [119] |
| 200c (+) | USP25 | Cell Migration EMT | − | NSCLC cell lines | [120] |
| 200c (−) | ZEB1 | Cell Migration | Gefitinib | PC-9-ZD | [121] |
| 200c (−) | ZEB2 | EMT | − | A-549 | [122] |
| 200c (+) | Possibly E-cadherin | Cell Migration | − | H23, A549, HCC-44 | [123] |
| 200c (+) | Possibly RECK | Proliferation | Reservatol | H-460 | [124] |
| micro-RNA | Cancer Type | Source | Cohort Size | Diagnostic | Prognosis | Reference |
|---|---|---|---|---|---|---|
| 200c (+) | NSCLC | Tissue | 155 | − | Reduced | [123] |
| 200c (+) | NSCLC | Tissue | 72 | − | Reduced | [125] |
| 200c (−) | varied | Tissue/Blood | 18 studies | − | Poor OS and PFS | [126] |
| 200c (+) | EOC | Tissue/Plasma | 14 studies | + | + | [127] |
| 200c (+) | NSCLC | Tissue | 110 | − | Reduced | [128] |
| 200c (−) | EC | Tissue | 46 studies | − | + | [129] |
| 200c (+/−) | GIC | Tissue/Blood | 60 studies | − | + | [130] |
| 200c (+) | OC | Tissue/Blood | 15 studies | − | + | [131] |
| micro-RNA | Target | Negative Effect | Sensitizing Agent | Cell Type | Reference |
|---|---|---|---|---|---|
| 152 (−) | PIK3CA | Cell Proliferation | − | HCC1806 | [132] |
| 152 (−) | PIK3R3 | Cell Proliferation Migration | − | CRC cell lines | [133] |
| 152 (−) | EPAS | Apoptosis | Paclitaxel | BC cell lines | [134] |
| 152 (−) | CD151 | Proliferation Migration | − | GC Tissues | [135] |
| 152 (−) | IGF-1R | Proliferation Angiogenesis | − | BC Tissues | [136] |
| 152 (−) | IRS1 | Proliferation Angiogenesis | − | BC Tissues | [136] |
| 152 (−) | B7-H1 | T-cell Proliferation | − | GC cell lines | [137] |
| 152 (−) | CDK8 | Proliferation Apoptosis | − | HCC cell lines | [138] |
| 152 (+) | p27 | Proliferation | − | BM cells, K562 | [139] |
| 152 (−) | SOS1 | Proliferation Apoptosis | Cisplatin | GBM cell lines | [140] |
| 152 (+) | KLF4 | Proliferation | − | CC cell lines | [141] |
| micro-RNA | Cancer Type | Source | Cohort Size | Diagnostic | Prognosis | Reference |
|---|---|---|---|---|---|---|
| 152 (−) | CRC | Tissue | 28 | +/− | − | [133] |
| 152 (−) | BC invasive | Tissue | 30 | − | Poor | [134] |
| 152 (−) | GC | Tissues | 42 | − | − | [137] |
| 152 (+) | CML | Bone Marrow | 40 | - | - | [137] |
| 152 (−) | HCC | Tissue | 89 | − | +/− | [138] |
| 152 (−) | Stage I-IIIA NSCLC | Plasma | 52 | − | Reduced DFS | [142] |
| 152 (−) | PC, lung, CRC, BC | Plasma | 204 | + | − | [145] |
| 152 (−) | BC stage I-II | Plasma | 106 | + | − | [146] |
| micro-RNA | Target | Positive Effect | Sensitizing Agent | Cell Type | Reference |
|---|---|---|---|---|---|
| 106b (+) | RB | Reduced Cell Arrest | − | Laryngeal carcinoma HEP2G+T1U212 | [147] |
| 106b (+) | ATG16L1 | Decreased Autophagy | − | CD | [148,149] |
| 106b (+) | PTEN | Tumor Initiation Stemness | Radiation | CRC cell lines | [151] |
| 106b (+) | p21 (indirectly) | Tumor Initiation Stemness | Radiation | CRC cell lines | [151] |
| 106b (+) | DLC-1 | EMT | − | CRC TissuesCRC cell lines | [152] |
| 106b (+) | FAT4 | Viability Angiogenesis Migration | − | CRC TissuesCRC cell lines | [153] |
| micro-RNA | Cancer Type | Source | Cohort Size | Diagnostic | Prognosis | Reference |
|---|---|---|---|---|---|---|
| 106b (+) Exo | CRC | Serum | 80 | + | − | [152] |
| 106b (−) | CC | Tissue | 180 | − | Long OS * | [156] |
| 106b (+) | Metastatic CRC | Tissue | 95 | − | Short OS/DFS | [157] |
| 106b (−) | RCCC | Tissue | 27 studies | − | Poor | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soond, S.M.; Kozhevnikova, M.V.; Townsend, P.A.; Zamyatnin, A.A., Jr. Integrative p53, micro-RNA and Cathepsin Protease Co-Regulatory Expression Networks in Cancer. Cancers 2020, 12, 3454. https://doi.org/10.3390/cancers12113454
Soond SM, Kozhevnikova MV, Townsend PA, Zamyatnin AA Jr. Integrative p53, micro-RNA and Cathepsin Protease Co-Regulatory Expression Networks in Cancer. Cancers. 2020; 12(11):3454. https://doi.org/10.3390/cancers12113454
Chicago/Turabian StyleSoond, Surinder M., Maria V. Kozhevnikova, Paul A. Townsend, and Andrey A. Zamyatnin, Jr. 2020. "Integrative p53, micro-RNA and Cathepsin Protease Co-Regulatory Expression Networks in Cancer" Cancers 12, no. 11: 3454. https://doi.org/10.3390/cancers12113454
APA StyleSoond, S. M., Kozhevnikova, M. V., Townsend, P. A., & Zamyatnin, A. A., Jr. (2020). Integrative p53, micro-RNA and Cathepsin Protease Co-Regulatory Expression Networks in Cancer. Cancers, 12(11), 3454. https://doi.org/10.3390/cancers12113454







