Continuous 14 Day Infusional Ifosfamide for Management of Soft-Tissue and Bone Sarcoma: A Single Centre Retrospective Cohort Analysis
Abstract
Simple Summary
Abstract
1. Introduction
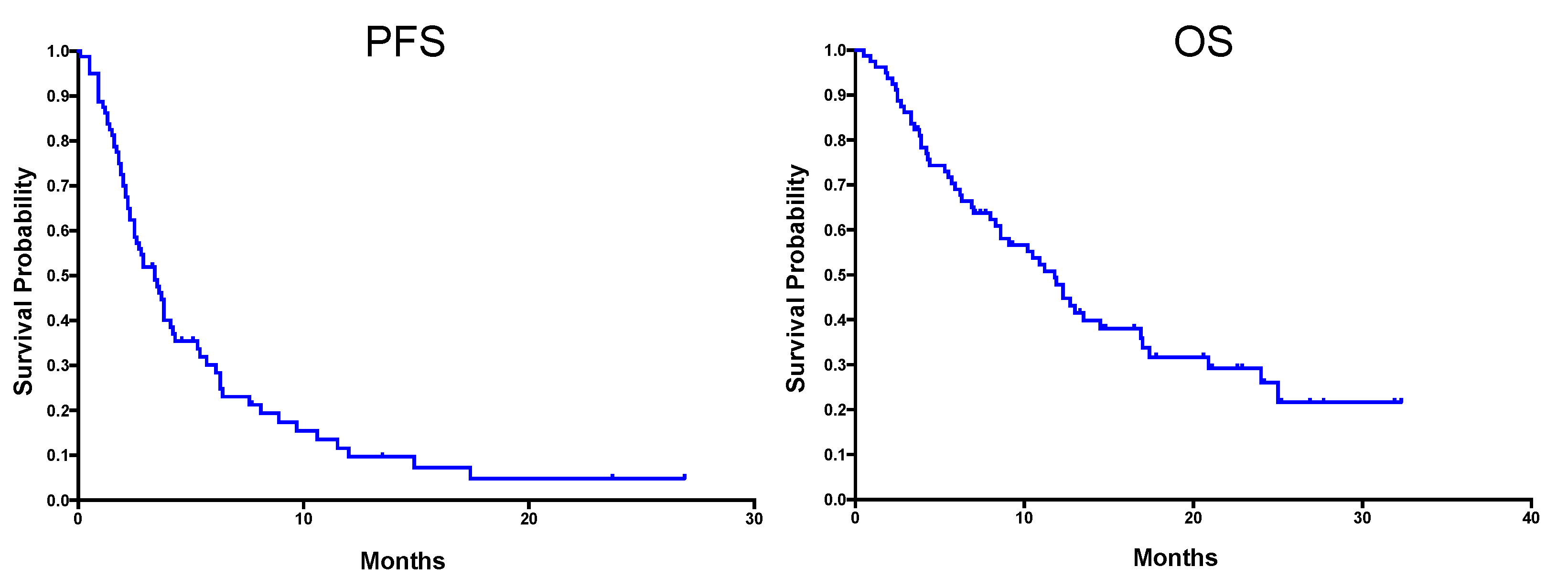
2. Results
2.1. Patient Characteristics
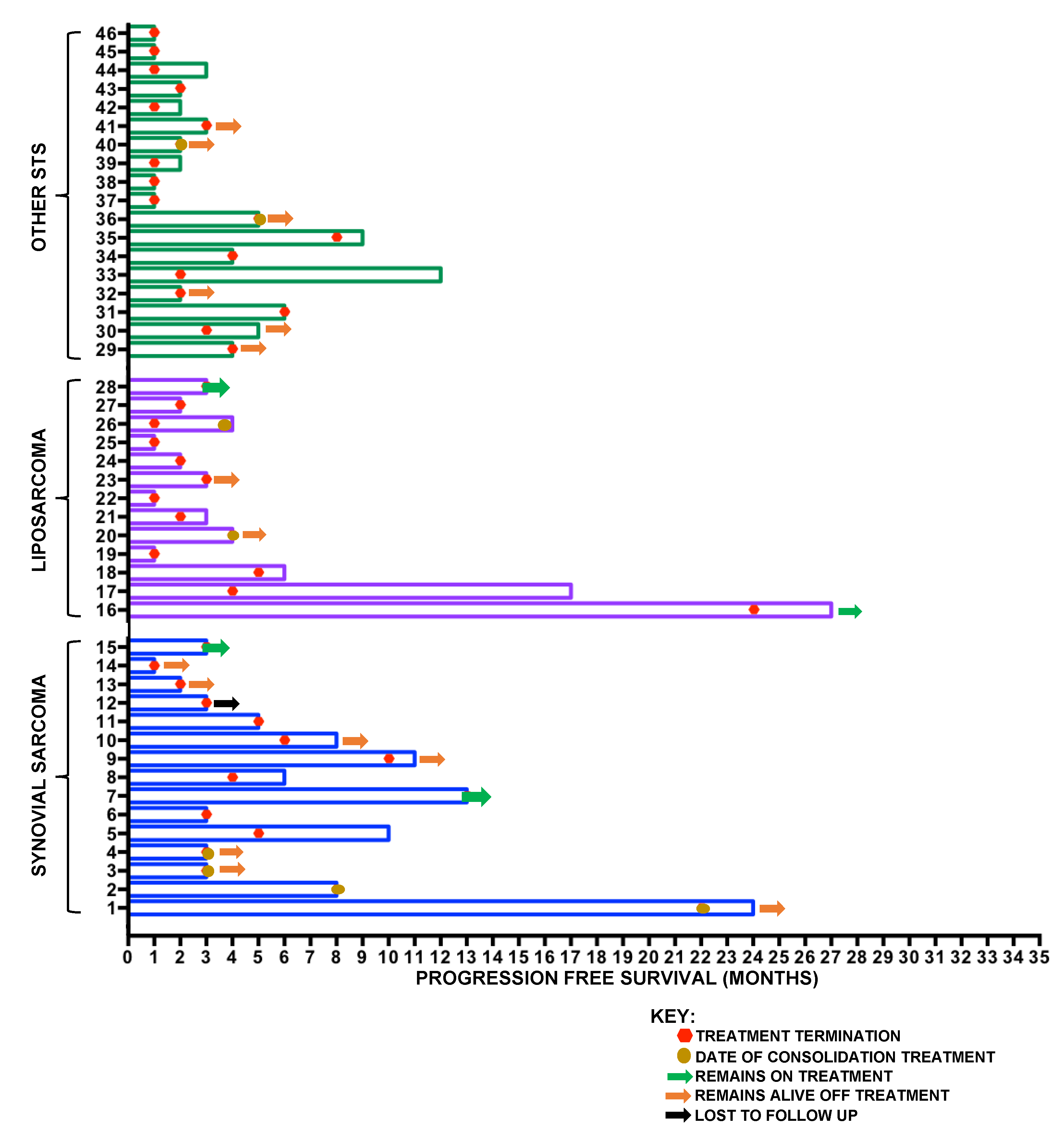
2.2. Soft-Tissue Sarcoma
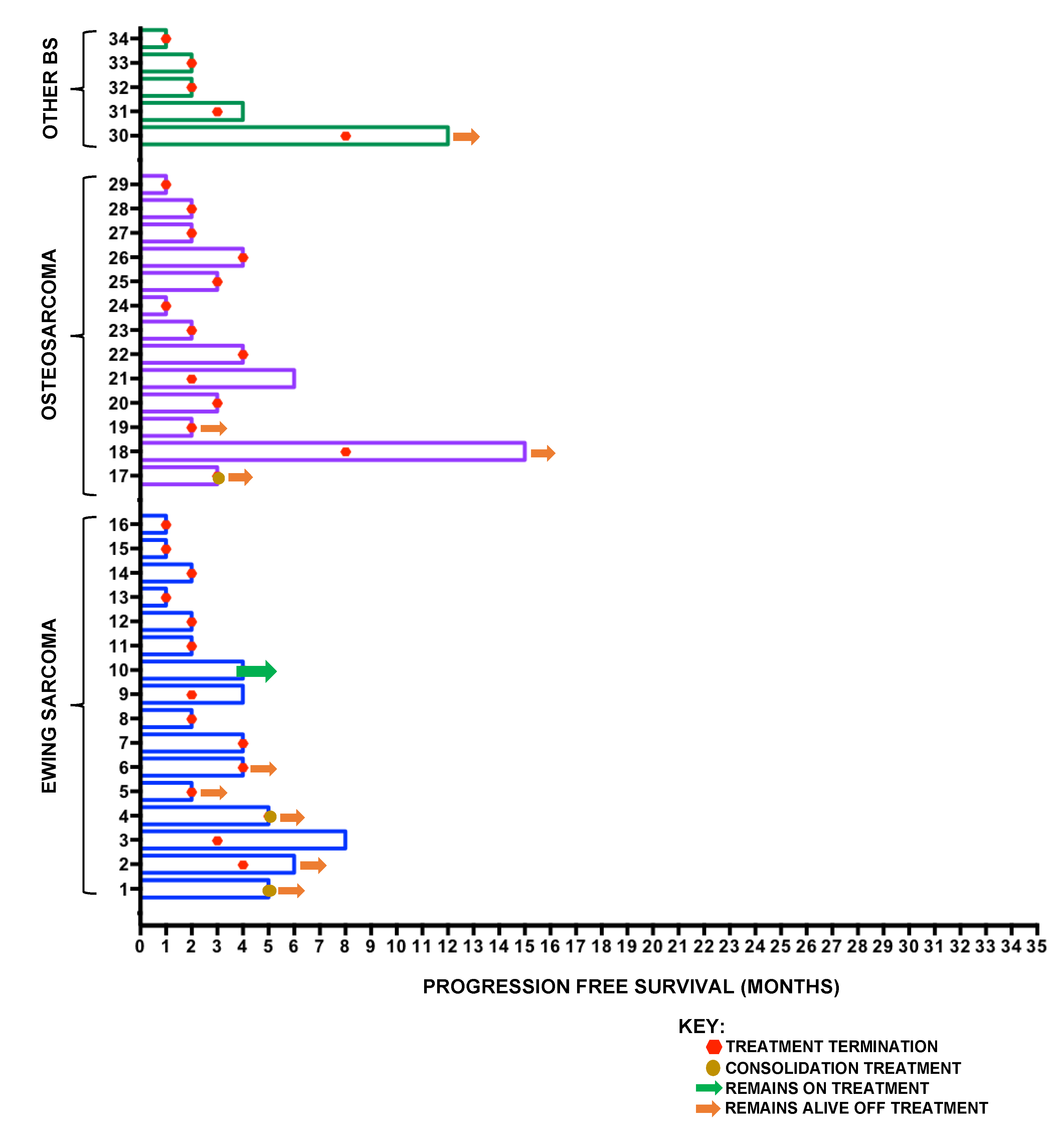
2.3. Bone Sarcoma
2.4. Consolidation Treatment after Infusional Ifosfamide
2.5. Toxicity Analysis
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tascilar, M.; Loos, W.J.; Seynaeve, C.; Verweij, J.; Sleijfer, S. The Pharmacologic Basis of Ifosfamide Use in Adult Patients with Advanced Soft Tissue Sarcomas. Oncologist 2007, 12, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B. First-line treatment in advanced or metastatic disease: One size fits all or adapted to specific histiotypes? Curr. Opin. Oncol. 2016, 28, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Gaspar, N.; Hawkins, D.S.; Dirksen, U.; Lewis, I.J.; Ferrari, S.; Le Deley, M.-C.; Kovar, H.; Grimer, R.; Whelan, J.; Claude, L.; et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J. Clin. Oncol. 2015, 33, 3036–3046. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.S.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Cerny, T.; Castiglione, M.; Brunner, K.; Kupfer, A.; Martinelli, G.; Lind, M. Ifosfamide by continuous infusion to prevent encephalopathy. Lancet 1990, 335, 175. [Google Scholar] [CrossRef]
- Palumbo, R.; Palmeri, S.; Antimi, M.; Gatti, C.; Raffo, P.; Villani, G.; Toma, S. Phase II study of continuous-infusion high-dose ifosfamide in advanced and/or metastatic pretreated soft tissue sarcomas. Ann. Oncol. 1997, 8, 1159–1162. [Google Scholar] [CrossRef]
- Gharote, M.A. Is continuous infusion of high-dose ifosfamide, a safe option? Drug review. Int. J. Mol. Immuno Oncol. 2020, 5, 62–66. [Google Scholar] [CrossRef]
- Perego, G.; Gregis, F.; Rossi, L.; Mazzoleni, M.; Nozza, S.; Nozza, R.; Gatti, V.P. Continuous-infusion and outpatient setting: A chance for patients, a challenge for hospital pharmacists. J. Oncol. Pharm. Pract. 2020. [Google Scholar] [CrossRef]
- Schoenike, S.E.; Dana, W.J. Ifosfamide and mesna. Clin. Pharm. 1990, 9, 179–191. [Google Scholar]
- Sprangers, B.; Lapman, S. The growing pains of ifosfamide. Clin. Kidney J. 2020, 13, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Lorigan, P.C.; Verweij, J.; Papai, Z.; Rodenhuis, S.; Le Cesne, A.; Leahy, M.G.; Radford, J.A.; Van Glabbeke, M.M.; Kirkpatrick, A.; Hogendoorn, P.C.W.; et al. Phase III Trial of Two Investigational Schedules of Ifosfamide Compared With Standard-Dose Doxorubicin in Advanced or Metastatic Soft Tissue Sarcoma: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J. Clin. Oncol. 2007, 25, 3144–3150. [Google Scholar] [CrossRef]
- Martin-Liberal, J.; Alam, S.; Constantinidou, A.; Fisher, C.; Khabra, K.; Messiou, C.; Olmos, D.; Mitchell, S.; Al-Muderis, O.; Miah, A.; et al. Clinical Activity and Tolerability of a 14-Day Infusional Ifosfamide Schedule in Soft-Tissue Sarcoma. Sarcoma 2013, 2013, 868973. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, R.; Bertulli, R.; Marrari, A.; Fumagalli, E.; Pilotti, S.; Morosi, C.; Messina, A.; Tos, A.P.D.; Gronchi, A.; Casali, P.G. High-dose continuous-infusion ifosfamide in advanced well-differentiated/dedifferentiated liposarcoma. Clin. Sarcoma Res. 2014, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chang, M.H.; Baek, K.K.; Han, B.; Lim, T.; Lee, J.; Park, J.O. High-Dose Ifosfamide as Second- or Third-Line Chemotherapy in Refractory Bone and Soft Tissue Sarcoma Patients. Oncology 2011, 80, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Sankhala, K.K.; Mukherjee, A.; Narasimha, V.; Chmielowski, B.; Quon, D.V.; Chua, V.; Chawla, S.P. 14-day continuous infusion ifosfamide in advanced refractory sarcomas. J. Clin. Oncol. 2014, 32, 10596. [Google Scholar] [CrossRef]
- Noujaim, J.; Constantinidou, A.; Messiou, C.; Thway, K.; Miah, A.; Benson, C.; Judson, I.; Jones, R.L. Successful Ifosfamide Rechallenge in Soft-Tissue Sarcoma. Am. J. Clin. Oncol. 2015, 41, 147–151. [Google Scholar] [CrossRef]
- Crago, A.M.; Singer, S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr. Opin. Oncol. 2011, 23, 373–378. [Google Scholar] [CrossRef]
- Spillane, A.J.; Fisher, C.; Thomas, J.M. Myxoid liposarcoma—Frequency and the natural history of nonpulmonary soft tissue metastases. Ann. Surg. Oncol. 1999, 6, 389–394. [Google Scholar] [CrossRef]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of Computed Tomography and Positron Emission Tomography in Patients With Metastatic Gastrointestinal Stromal Tumor Treated at a Single Institution With Imatinib Mesylate: Proposal of New Computed Tomography Response Criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Collini, P.; Messina, A.; Morosi, C.; Barisella, M.; Bertulli, R.; Piovesan, C.; Dileo, P.; Torri, V.; Gronchi, A.; et al. High-Grade Soft-Tissue Sarcomas: Tumor Response Assessment—Pilot Study to Assess the Correlation between Radiologic and Pathologic Response by Using RECIST and Choi Criteria. Radiology 2009, 251, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Livingston, J.A.; Patel, S.R.; Benjamin, R. Chemotherapy for Bone Sarcoma in Adults. J. Oncol. Pract. 2016, 12, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Casanova, M.; Luksch, R.; Podda, M.; Favini, F.; Cefalo, G.; Massimino, M.; Ferrari, A. Prolonged 14-day continuous infusion of high-dose ifosfamide with an external portable pump: Feasibility and efficacy in refractory pediatric sarcoma. Pediatr. Blood Cancer 2010, 55, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.M.; McTiernan, A.M.; Michelagnoli, M.P.; Gabbie, S.; Daw, S.; Whelan, J.S. High-dose ifosfamide in relapsed or progressive Ewing’s sarcoma. In Proceedings of the Sarcoma Meeting Stuttgart, Stuttgart, Germany, 14–17 June 2005; Volume 9, p. 108, Sarcoma, March/June 2005. [Google Scholar]
- Ferrari, S.; Del Prever, A.B.; Palmerini, E.; Staals, E.; Berta, M.; Balladelli, A.; Picci, P.; Fagioli, F.; Bacci, G.; Vanel, D. Response to high-dose ifosfamide in patients with advanced/recurrent Ewing sarcoma. Pediatr. Blood Cancer 2009, 52, 581–584. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.G.; Kirton, L.; Khan, M.; Fenwick, N.; Dirksen, U.; Gaspar, N.; Kanerva, J.; Kuehne, T.; Longhi, A.; Luksch, R.; et al. Results of the second interim assessment of rEECur, an international randomized controlled trial of chemotherapy for the treatment of recurrent and primary refractory Ewing sarcoma (RR-ES). J. Clin. Oncol. 2020, 38, 11502. [Google Scholar] [CrossRef]
- Brain EC, G.; Mita, A.; Soulie, P.; Errihani, H.; Bessard, A.H.; Chaouche, M.; Alexandre, J.; Cvitkovic, E.; Jasmin, C.; Misset, J.L. 6-day continuous infusion of high-dose ifosfamide with bone marrow growth factors in advanced refractory malignancies. J. Cancer Res. Clin. Oncol. 1997, 123, 227–231. [Google Scholar] [CrossRef]
- Palmerini, E.; Setola, E.; Grignani, G.; D’Ambrosio, L.; Comandone, A.; Righi, A.; Longhi, A.; Cesari, M.; Paioli, A.; Hakim, R.; et al. High Dose Ifosfamide in Relapsed and Unresectable High-Grade Osteosarcoma Patients: A Retrospective Series. Cells 2020, 9, 2389. [Google Scholar] [CrossRef]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef]
- Authority, H.R. Defining Research—National Research Ethics Service Guidance to Help You Decide If Your Project Requires Review by a Research Ethics Committee. April 2013. Available online: http://www.hra-decisiontools.org.uk (accessed on 16 November 2020).


| Demographic | Soft-Tissue Sarcoma (STS) | Bone Sarcoma (BS) |
|---|---|---|
| Number of Patients | 46 | 34 |
| Histology | Synovial sarcoma—15 Liposarcoma *—13 Spindle cell sarcoma—4 MPNST—3 Epithelioid sarcoma—3 Other **—8 | Ewing Sarcoma—16 Osteosarcoma—13 Mesenchymal chondrosarcoma—3 De-differentiated chondrosarcoma—1 High-grade spindle cell sarcoma, NOS—1 |
| Median Age in Years (range) | 43 (18–72) | 23 (12–64) |
| % Male | 48% | 62% |
| Number of Metastatic Sites | 1 (0–2) | 1 (0–2) |
| Median Previous Chemotherapy Lines (range) | 1 (0–4) | 2 (0–5) |
| Prior Ifosfamide | 24 (52%) | 23 (68%) |
| Pt | Diagnosis | Recurrence | Cycles of Inf-Ifos | Radiological Response | Treatment | Current Status * | Overall Survival * |
|---|---|---|---|---|---|---|---|
| 1 | ES | Isolated Bone | 6 | Radiological/Metabolic Improvement | Surgery | AWD | 28 m |
| 2 | ES | Local | 5 | Radiological/Metabolic Improvement | RT | AWD | 24 m |
| 3 | Osteosarcoma | Mediastinal | 4 | Radiological/Metabolic Improvement | RT + Surgery | NED | 21 m |
| 4 | SS | Lung | 8 | Radiological Improvement | RT | AWD/Lost to Follow Up | 24 m |
| 5 | SS | Lung | 22 | Radiological Improvement | Surgery | AWD | 32 m |
| 6 | SS | Local | 3 | Radiological Improvement | RT + Surgery | Further RT and Now NED | 24 m |
| 7 | SS | Local | 3 | Stable Disease | Surgery | NED | 23 m |
| 8 | De-diff LPS | Local | 4 | Stable Disease | RT | NED | 12 m |
| 9 | MPNST | Local | 5 | Stable Disease | RT + Surgery | NED | 13 m |
| Adverse Event | Grade 2 N (%) | Grade ¾ N (%) |
|---|---|---|
| Haematological | ||
| All | 17 (21%) | 45 (56%) |
| Anaemia | 13 (16%) | 8 (10%) |
| Thrombocytopenia | 2 (2%) | 12 (15%) |
| Neutropenia | 2 (2%) | 25 (31%) |
| Non-Haematological | ||
| All | 32 (40%) | 27 (34%) |
| Fatigue | 7 (9%) | 2 (3%) |
| Nausea/Vomiting | 10 (13%) | 10 (13%) |
| Infection | 5 (6%) | 7 (9%) |
| Encephalopathy/Confusion | 3 (4%) | 3 (4%) |
| Haematuria | 3 (4%) | Nil |
| AKI | Nil | 2 (3%) |
| Electrolyte Disturbance | 1 (1%) | 2 (3%) |
| Other * | 3 (4%) | 1 (1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, T.J.; Milic, M.; McDerra, J.; McTiernan, A.; Ahmed, M.; Karavasilis, V.; Michelagnoli, M.; Windsor, R.; Seddon, B.; Whelan, J.; et al. Continuous 14 Day Infusional Ifosfamide for Management of Soft-Tissue and Bone Sarcoma: A Single Centre Retrospective Cohort Analysis. Cancers 2020, 12, 3408. https://doi.org/10.3390/cancers12113408
Carter TJ, Milic M, McDerra J, McTiernan A, Ahmed M, Karavasilis V, Michelagnoli M, Windsor R, Seddon B, Whelan J, et al. Continuous 14 Day Infusional Ifosfamide for Management of Soft-Tissue and Bone Sarcoma: A Single Centre Retrospective Cohort Analysis. Cancers. 2020; 12(11):3408. https://doi.org/10.3390/cancers12113408
Chicago/Turabian StyleCarter, Thomas J., Marina Milic, Joanna McDerra, Anne McTiernan, Mahbubl Ahmed, Vasilios Karavasilis, Maria Michelagnoli, Rachael Windsor, Beatrice Seddon, Jeremy Whelan, and et al. 2020. "Continuous 14 Day Infusional Ifosfamide for Management of Soft-Tissue and Bone Sarcoma: A Single Centre Retrospective Cohort Analysis" Cancers 12, no. 11: 3408. https://doi.org/10.3390/cancers12113408
APA StyleCarter, T. J., Milic, M., McDerra, J., McTiernan, A., Ahmed, M., Karavasilis, V., Michelagnoli, M., Windsor, R., Seddon, B., Whelan, J., Dileo, P., & Strauss, S. J. (2020). Continuous 14 Day Infusional Ifosfamide for Management of Soft-Tissue and Bone Sarcoma: A Single Centre Retrospective Cohort Analysis. Cancers, 12(11), 3408. https://doi.org/10.3390/cancers12113408







