Oncolytic Adenovirus in Cancer Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Adenovirus Cell Entry, Replication, and Immunogenicity
3. Current Concepts of Tumor-Selective Replicating Adenoviruses
4. Translational Efforts and Clinical Development of Oncolytic Adenoviruses
5. Armed Oncolytic Adenoviruses
5.1. Arming with Transgenes to Amplify Tumor Lysis
5.2. Arming with Matrix-Modifying Genes to Enhance Intratumoral Virus Spreading
5.3. Arming with Antiangiogenic Transgenes
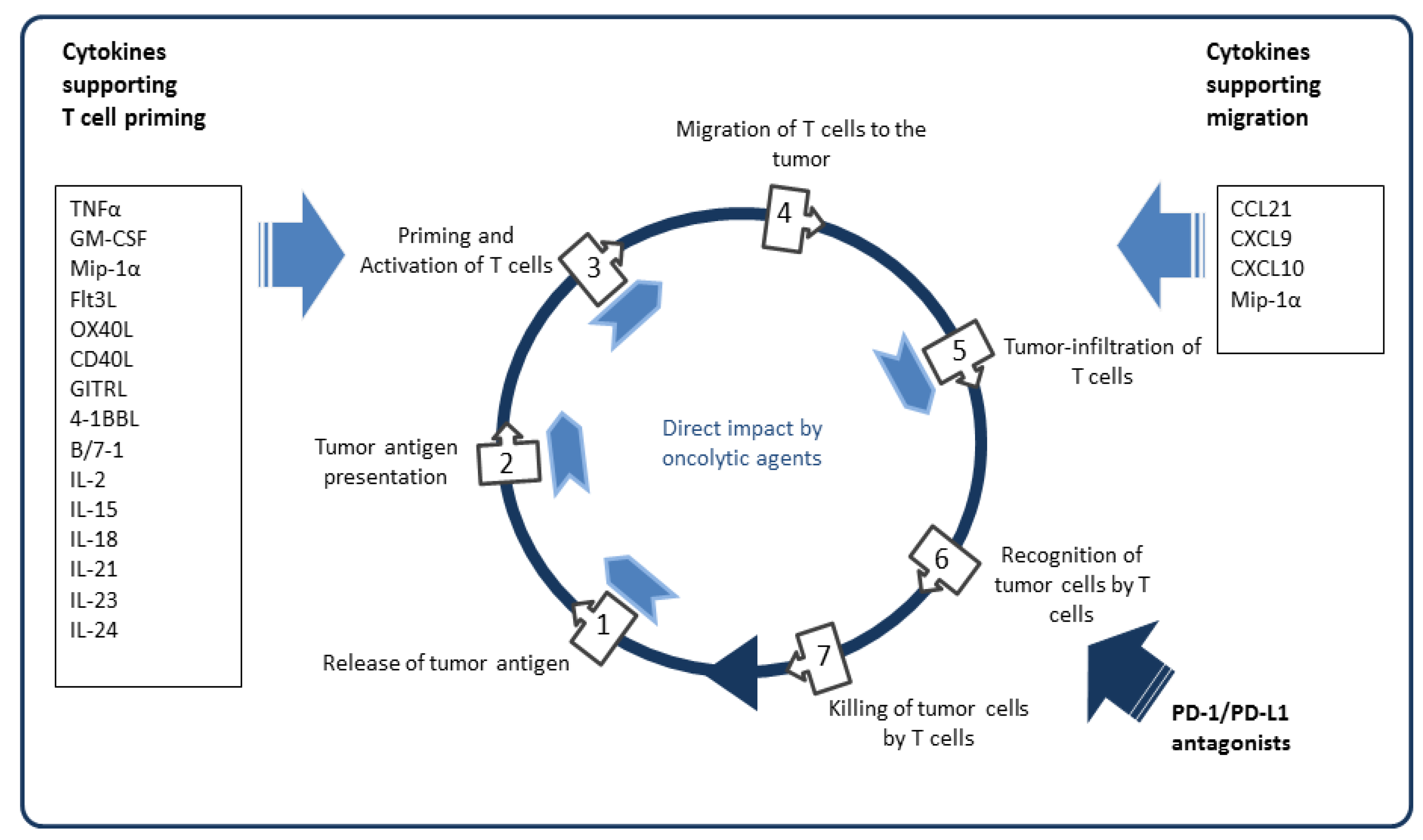
5.4. Arming with Immunostimulatory Cytokines and Chemokines
5.5. Immunological Arming to Improve Antigen Presentation
5.6. Arming with Transgenes Addressing T Cell Costimulation or Immune Checkpoints
5.7. Arming with T Cell Engager Proteins
6. Oncolytic Adenoviruses in Multi-Stage Immunotherapies
7. Conclusions
Funding
Conflicts of Interest
References
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.R.; Kirn, D.H.; Williams, A.; Heise, C.; Horn, S.; Muna, M.; Ng, L.; Nye, J.A.; Sampson-Johannes, A.; Fattaey, A.; et al. An Adenovirus Mutant That Replicates Selectively in p53-Deficient Human Tumor Cells. Science 1996, 274, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Galanis, E.; Kirn, D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007, 4, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, R.J.; Harrington, K.J.; Pandha, H.S.; Vile, R.G.; Melcher, A.A.; Errington, F. Oncolytic viruses: A novel form of immunotherapy. Expert Rev. Anticancer Ther. 2008, 8, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Salazar Arenas, S.; Sirima, S.B.; Takoudjou Dzomo, G.R.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A review of 65 years of human adenovirus seroprevalence. Expert Rev. Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef]
- Wold, W.; Toth, K. Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr. Gene Ther. 2014, 13, 421–433. [Google Scholar] [CrossRef]
- Shaw, A.R.; Suzuki, M. Immunology of Adenoviral Vectors in Cancer Therapy. Mol. Ther. Methods Clin. Dev. 2019, 15, 418–429. [Google Scholar] [CrossRef]
- Abou El Hassan, M.A.I.; van der Meulen-Muileman, I.; Abbas, S.; Kruyt, F.A.E. Conditionally Replicating Adenoviruses Kill Tumor Cells via a Basic Apoptotic Machinery-Independent Mechanism That Resembles Necrosis-Like Programmed Cell Death. J. Virol. 2004, 78, 12243–12251. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Aoki, H.; Kühnel, F.; Kondo, Y.; Kubicka, S.; Wirth, T.; Iwado, E.; Iwamaru, A.; Fujiwara, K.; Hess, K.R.; et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J. Natl. Cancer Inst. 2006, 98, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Baird, S.K.; Aerts, J.L.; Eddaoudi, A.; Lockley, M.; Lemoine, N.R.; McNeish, I.A. Oncolytic adenoviral mutants induce a novel mode of programmed cell death in ovarian cancer. Oncogene 2008, 27, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, S.; Iannuzzi, C.A.; Passaro, C.; Forte, I.M.; Iannone, R.; Gigantino, V.; Indovina, P.; Botti, G.; Giordano, A.; Formisano, P.; et al. The Oncolytic Virus dl922-947 Triggers Immunogenic Cell Death in Mesothelioma and Reduces Xenograft Growth. Front. Oncol. 2019, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, I.; Ahtiainen, L.; Hirvinen, M.L.; Bramante, S.; Cerullo, V.; Nokisalmi, P.; Hemminki, O.; Diaconu, I.; Pesonen, S.; Koski, A.; et al. Oncolytic Adenovirus With Temozolomide Induces Autophagy and Antitumor Immune Responses in Cancer Patients. Mol. Ther. 2013, 21, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ramachandran, M.; Jin, C.; Quijano-Rubio, C.; Martikainen, M.; Yu, D.; Essand, M. Characterization of virus-mediated immunogenic cancer cell death and the consequences for oncolytic virus-based immunotherapy of cancer. Cell Death Dis. 2020, 11, 48. [Google Scholar] [CrossRef]
- Boozari, B.; Mundt, B.; Woller, N.; Struver, N.; Gurlevik, E.; Schache, P.; Kloos, A.; Knocke, S.; Manns, M.P.; Wirth, T.C.; et al. Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut 2010, 59, 1416–1426. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Li, H.; Du, X.; Liu, M.; Huang, Q.; Wang, Y.; Wang, S. The Efficacy of Oncolytic Adenovirus Is Mediated by T-cell Responses against Virus and Tumor in Syrian Hamster Model. Clin. Cancer Res. 2017, 23, 239–249. [Google Scholar] [CrossRef]
- Koch, P.; Gatfield, J.; Löber, C.; Hobom, U.; Lenz-Stöppler, C.; Roth, J.; Dobbelstein, M. Efficient replication of adenovirus despite the overexpression of active and nondegradable p53. Cancer Res. 2001, 61, 5941–5947. [Google Scholar]
- O’Shea, C.C.; Johnson, L.; Bagus, B.; Choi, S.; Nicholas, C.; Shen, A.; Boyle, L.; Pandey, K.; Soria, C.; Kunich, J.; et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell 2004, 6, 611–623. [Google Scholar] [CrossRef]
- Ramachandra, M.; Rahman, A.; Zou, A.; Vaillancourt, M.; Howe, J.A.; Antelman, D.; Sugarman, B.; Demers, G.W.; Engler, H.; Johnson, D.; et al. Re-engineering adenovirus regulatory pathways to enhance oncolytic specificity and efficacy. Nat. Biotechnol. 2001, 19, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Gurlevik, E.; Woller, N.; Schache, P.; Malek, N.P.; Wirth, T.C.; Zender, L.; Manns, M.P.; Kubicka, S.; Kuhnel, F. p53-dependent antiviral RNA-interference facilitates tumor-selective viral replication. Nucleic Acids Res. 2009, 37, e84. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.; Hermiston, T.; Johnson, L.; Brooks, G.; Sampson-Johannes, A.; Williams, A.; Hawkins, L.; Kirn, D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000, 6, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.X.; Levin, V.A.; Yung, W.K.A.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Ulasov, I.V.; Tyler, M.A.; Rivera, A.A.; Nettlebeck, D.M.; Douglas, J.T.; Lesniak, M.S. Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy. J. Med. Virol. 2008, 80, 1595–1603. [Google Scholar] [CrossRef]
- Lau, L.; Gray, E.E.; Brunette, R.L.; Stetson, D.B. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 2015, 350, 568–571. [Google Scholar] [CrossRef]
- Spurrell, E.; Gangeswaran, R.; Wang, P.; Cao, F.; Gao, D.; Feng, B.; Wold, W.; Tollefson, A.; Lemoine, N.R.; Wang, Y. STAT1 Interaction with E3-14.7K in Monocytes Affects the Efficacy of Oncolytic Adenovirus. J. Virol. 2014, 88, 2291–2300. [Google Scholar] [CrossRef][Green Version]
- Zeng, X.; Carlin, C.R. Adenovirus early region 3 RIDα protein limits NFκB signaling through stress-activated EGF receptors. PLoS Pathog. 2019, 15, e1008017. [Google Scholar] [CrossRef]
- Cerullo, V.; Diaconu, I.; Romano, V.; Hirvinen, M.; Ugolini, M.; Escutenaire, S.; Holm, S.L.; Kipar, A.; Kanerva, A.; Hemminki, A. An Oncolytic Adenovirus Enhanced for Toll-like Receptor 9 Stimulation Increases Antitumor Immune Responses and Tumor Clearance. Mol. Ther. 2012, 20, 2076–2086. [Google Scholar] [CrossRef]
- Young, A.M.; Archibald, K.M.; Tookman, L.A.; Pool, A.; Dudek, K.; Jones, C.; Williams, S.L.; Pirlo, K.J.; Willis, A.E.; Lockley, M.; et al. Failure of Translation of Human Adenovirus mRNA in Murine Cancer Cells Can be Partially Overcome by L4-100K Expression In Vitro and In Vivo. Mol. Ther. 2012, 20, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Jacobus, E.J.; Taverner, W.K.; Fisher, K.D.; Hemmi, S.; West, K.; Slater, L.; Lilley, F.; Brown, A.; Champion, B.; et al. Expression of human CD46 and trans-complementation by murine adenovirus 1 fails to allow productive infection by a group B oncolytic adenovirus in murine cancer cells. J. Immunother. Cancer 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.L.; Chang, Y.N.; Hay, C.; Golightly, D.; Stewart, D.; Lin, J.; Phipps, S.; Chiang, Y.L. A novel tumor-specific replication-restricted adenoviral vector for gene therapy of hepatocellular carcinoma. Hum. Gene Ther. 1999, 10, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Brough, D.E.; Kovesdi, I.; Kufe, D.W. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J. Clin. Investig. 2000, 106, 763–771. [Google Scholar] [CrossRef][Green Version]
- Jakubczak, J.L.; Ryan, P.; Gorziglia, M.; Clarke, L.; Hawkins, L.K.; Hay, C.; Huang, Y.; Kaloss, M.; Marinov, A.; Phipps, S.; et al. An oncolytic adenovirus selective for retinoblastoma tumor suppressor protein pathway-defective tumors: Dependence on E1A, the E2F-1 promoter, and viral replication for selectivity and efficacy. Cancer Res. 2003, 63, 1490–1499. [Google Scholar]
- Alonso, M.M.; Cascallo, M.; Gomez-Manzano, C.; Jiang, H.; Bekele, B.N.; Perez-Gimenez, A.; Lang, F.F.; Piao, Y.; Alemany, R.; Fueyo, J. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007, 67, 8255–8263. [Google Scholar] [CrossRef]
- Lanson, N.A.; Friedlander, P.L.; Schwarzenberger, P.; Kolls, J.K.; Wang, G. 1114. Replication of an Adenoviral Vector Controlled by the Human Telomerase Reverse Transcriptase Promoter Causes Tumor-Selective Cell Lysis. Mol. Ther. 2003, 7, S429. [Google Scholar] [CrossRef]
- Kawashima, T.; Kagawa, S.; Kobayashi, N.; Shirakiya, Y.; Umeoka, T.; Teraishi, F.; Taki, M.; Kyo, S.; Tanaka, N.; Fujiwara, T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004, 10, 285–292. [Google Scholar] [CrossRef]
- Rojas, J.J.; Cascallo, M.; Guedan, S.; Gros, A.; Martinez-Quintanilla, J.; Hemminki, A.; Alemany, R. A modified E2F-1 promoter improves the efficacy to toxicity ratio of oncolytic adenoviruses. Gene Ther. 2009, 16, 1441–1451. [Google Scholar] [CrossRef]
- Kim, E.; Kim, J.H.; Shin, H.Y.; Lee, H.; Yang, J.M.; Kim, J.; Sohn, J.H.; Kim, H.; Yun, C.O. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum. Gene Ther. 2003, 14, 1415–1428. [Google Scholar] [CrossRef]
- Wirth, T.; Zender, L.; Schulte, B.; Mundt, B.; Plentz, R.; Rudolph, K.L.; Manns, M.; Kubicka, S.; Kühnel, F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003, 63, 3181–3188. [Google Scholar]
- Mantwill, K.; Köhler-Vargas, N.; Bernshausen, A.; Bieler, A.; Lage, H.; Kaszubiak, A.; Surowiak, P.; Dravits, T.; Treiber, U.; Hartung, R.; et al. Inhibition of the Multidrug-Resistant Phenotype by Targeting YB-1 with a Conditionally Oncolytic Adenovirus: Implications for Combinatorial Treatment Regimen with Chemotherapeutic Agents. Cancer Res. 2006, 66, 7195–7202. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, Y.; Hernández-Alcoceba, R.; Aragones, J.; Naranjo-Suárez, S.; Castellanos, M.C.; Esteban, M.A.; Martín-Puig, S.; Landazuri, M.O.; del Peso, L. Specific oncolytic effect of a new hypoxia-inducible factor-dependent replicative adenovirus on von Hippel-Lindau-defective renal cell carcinomas. Cancer Res. 2003, 63, 6877–6884. [Google Scholar] [PubMed]
- Li, Y.; Hong, J.; Oh, J.E.; Yoon, A.R.; Yun, C.O. Potent antitumor effect of tumor microenvironment-targeted oncolytic adenovirus against desmoplastic pancreatic cancer. Int. J. Cancer 2018, 142, 392–413. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.T.; Kim, M.; Sumerel, L.A.; Carey, D.E.; Curiel, D.T. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001, 61, 813–817. [Google Scholar] [PubMed]
- Suzuki, K.; Fueyo, J.; Krasnykh, V.; Reynolds, P.N.; Curiel, D.T.; Alemany, R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin. Cancer Res. 2001, 7, 120–126. [Google Scholar] [PubMed]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Milenova, I.; Wenthe, J.; Ståhle, M.; Leja-Jarblad, J.; Ullenhag, G.; Dimberg, A.; Moreno, R.; Alemany, R.; Loskog, A. Shaping the Tumor Stroma and Sparking Immune Activation by CD40 and 4-1BB Signaling Induced by an Armed Oncolytic Virus. Clin. Cancer Res. 2017, 23, 5846–5857. [Google Scholar] [CrossRef]
- Beatty, M.S.; Curiel, D.T. Adenovirus Strategies for Tissue-Specific Targeting. Adv. Cancer Res. 2012, 115, 39–67. [Google Scholar]
- Kishimoto, H.; Kojima, T.; Watanabe, Y.; Kagawa, S.; Fujiwara, T.; Uno, F.; Teraishi, F.; Kyo, S.; Mizuguchi, H.; Hashimoto, Y.; et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat. Med. 2006, 12, 1213–1219. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Tong, A.W.; Nemunaitis, M.; Senzer, N.; Phadke, A.P.; Bedell, C.; Adams, N.; Zhang, Y.A.; Maples, P.B.; Chen, S.; et al. A Phase I Study of Telomerase-specific Replication Competent Oncolytic Adenovirus (Telomelysin) for Various Solid Tumors. Mol. Ther. 2010, 18, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Kuroda, S.; Kakiuchi, Y.; Kumon, K.; Tsumura, T.; Hashimoto, M.; Morihiro, T.; Kubota, T.; Aoyama, K.; Kikuchi, S.; et al. Immune Modulation by Telomerase-Specific Oncolytic Adenovirus Synergistically Enhances Antitumor Efficacy with Anti-PD1 Antibody. Mol. Ther. 2020, 28, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Alemany, R.; Gomez-Manzano, C.; Fuller, G.N.; Khan, A.; Conrad, C.A.; Liu, T.J.; Jiang, H.; Lemoine, M.G.; Suzuki, K.; et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J. Natl. Cancer Inst. 2003, 95, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Roife, D.; Kang, Y.; Gumin, J.; Rios Perez, M.V.; Li, X.; Pratt, M.; Brekken, R.A.; Fueyo-Margareto, J.; Lang, F.F.; et al. Preclinical Evaluation of Sequential Combination of Oncolytic Adenovirus Delta-24-RGD and Phosphatidylserine-Targeting Antibody in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2017, 16, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, A.; Gimenez-Alejandre, M.; Rojas, J.J.; Moreno, R.; Bazan-Peregrino, M.; Cascallo, M.; Alemany, R. Safety and Efficacy of VCN-01, an Oncolytic Adenovirus Combining Fiber HSG-Binding Domain Replacement with RGD and Hyaluronidase Expression. Clin. Cancer Res. 2015, 21, 1406–1418. [Google Scholar] [CrossRef]
- Guedan, S.; Rojas, J.J.; Gros, A.; Mercade, E.; Cascallo, M.; Alemany, R. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010. [Google Scholar] [CrossRef]
- Pascual-Pasto, G.; Bazan-Peregrino, M.; Olaciregui, N.G.; Restrepo-Perdomo, C.A.; Mato-Berciano, A.; Ottaviani, D.; Weber, K.; Correa, G.; Paco, S.; Vila-Ubach, M.; et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci. Transl. Med. 2019, 11, eaat9321. [Google Scholar] [CrossRef]
- Ramesh, N. CG0070, a Conditionally Replicating Granulocyte Macrophage Colony-Stimulating Factor-Armed Oncolytic Adenovirus for the Treatment of Bladder Cancer. Clin. Cancer Res. 2006, 12, 305–313. [Google Scholar] [CrossRef]
- Packiam, V.T.; Lamm, D.L.; Barocas, D.A.; Trainer, A.; Fand, B.; Davis, R.L.; Clark, W.; Kroeger, M.; Dumbadze, I.; Chamie, K.; et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non–muscle-invasive bladder cancer: Interim results. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 440–447. [Google Scholar] [CrossRef]
- Kuhn, I.; Harden, P.; Bauzon, M.; Chartier, C.; Nye, J.; Thorne, S.; Reid, T.; Ni, S.; Lieber, A.; Fisher, K.; et al. Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLoS ONE 2008, 3, e2409. [Google Scholar] [CrossRef]
- Machiels, J.P.; Salazar, R.; Rottey, S.; Duran, I.; Dirix, L.; Geboes, K.; Wilkinson-Blanc, C.; Pover, G.; Alvis, S.; Champion, B.; et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE). J. Immunother. Cancer 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Wildner, O.; Blaese, R.M.; Morris, J.C. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999, 59, 410–413. [Google Scholar] [PubMed]
- Freytag, S.O.; Khil, M.; Stricker, H.; Peabody, J.; Menon, M.; DePeralta-Venturina, M.; Nafziger, D.; Pegg, J.; Paielli, D.; Brown, S.; et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002, 62, 4968–4976. [Google Scholar] [PubMed]
- Barton, K.N.; Paielli, D.; Zhang, Y.; Koul, S.; Brown, S.L.; Lu, M.; Seely, J.; Kim, J.H.; Freytag, S.O. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol. Ther. 2006, 13, 347–356. [Google Scholar] [CrossRef]
- Freytag, S.O.; Barton, K.N.; Zhang, Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther. 2013, 20, 1131–1139. [Google Scholar] [CrossRef]
- Sova, P.; Ren, X.W.; Ni, S.; Bernt, K.M.; Mi, J.; Kiviat, N.; Lieber, A. A Tumor-Targeted and Conditionally Replicating Oncolytic Adenovirus Vector Expressing TRAIL for Treatment of Liver Metastases. Mol. Ther. 2004, 9, 496–509. [Google Scholar] [CrossRef]
- Fernández-Ulibarri, I.; Hammer, K.; Arndt, M.A.E.; Kaufmann, J.K.; Dorer, D.; Engelhardt, S.; Kontermann, R.E.; Hess, J.; Allgayer, H.; Krauss, J.; et al. Genetic delivery of an immunoRNase by an oncolytic adenovirus enhances anticancer activity. Int. J. Cancer 2015, 136, 2228–2240. [Google Scholar] [CrossRef]
- Wildner, O.; Hoffmann, D.; Jogler, C.; Überla, K. Comparison of HSV-1 thymidine kinase-dependent and -independent inhibition of replication-competent adenoviral vectors by a panel of drugs. Cancer Gene Ther. 2003, 10, 791–802. [Google Scholar] [CrossRef]
- Liikanen, I.; Tahtinen, S.; Guse, K.; Gutmann, T.; Savola, P.; Oksanen, M.; Kanerva, A.; Hemminki, A. Oncolytic Adenovirus Expressing Monoclonal Antibody Trastuzumab for Treatment of HER2-Positive Cancer. Mol. Cancer Ther. 2016, 15, 2259–2269. [Google Scholar] [CrossRef]
- Yang, S.W.; Cody, J.J.; Rivera, A.A.; Waehler, R.; Wang, M.; Kimball, K.J.; Alvarez, R.A.; Siegal, G.P.; Douglas, J.T.; Ponnazhagan, S. Conditionally replicating adenovirus expressing TIMP2 for ovarian cancer therapy. Clin. Cancer Res. 2011, 17, 538–549. [Google Scholar] [CrossRef]
- Lamfers, M.L.M.; Gianni, D.; Tung, C.H.; Idema, S.; Schagen, F.H.E.; Carette, J.E.; Quax, P.H.A.; Van Beusechem, V.W.; Vandertop, W.P.; Dirven, C.M.F.; et al. Tissue inhibitor of metalloproteinase-3 expression from an oncolytic adenovirus inhibits matrix metalloproteinase activity in vivo without affecting antitumor efficacy in malignant glioma. Cancer Res. 2005, 65, 9398–9405. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sauthoff, H.; Huang, Y.; Kutler, D.I.; Bajwa, S.; Rom, W.N.; Hay, J.G. Human matrix metalloproteinase-8 gene delivery increases the oncolytic activity of a replicating adenovirus. Mol. Ther. 2007, 15, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, Y.S.; Kim, H.; Huang, J.H.; Yoon, A.R.; Yun, C.O. Relaxin Expression From Tumor-Targeting Adenoviruses and Its Intratumoral Spread, Apoptosis Induction, and Efficacy. JNCI J. Natl. Cancer Inst. 2006, 98, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.K.; Lee, Y.S.; Yoo, J.Y.; Yoon, A.R.; Kim, H.; Kim, D.S.; Seidler, D.G.; Kim, J.H.; Yun, C.O. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther. 2010, 17, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; Ko, H.Y.; Kang, H.; Hong, J.; Ahn, H.M.; Na, Y.; Kim, H.; Kim, J.S.; Yun, C.O. Relaxin-expressing oncolytic adenovirus induces remodeling of physical and immunological aspects of cold tumor to potentiate PD-1 blockade. J. Immunother. Cancer 2020, 8, e000763. [Google Scholar] [CrossRef]
- Li, G.; Sham, J.; Yang, J.; Su, C.; Xue, H.; Chua, D.; Sun, L.; Zhang, Q.; Cui, Z.; Wu, M.; et al. Potent antitumor efficacy of an E1B 55kDa-deficient adenovirus carrying murineendostatin in hepatocellular carcinoma. Int. J. Cancer 2005, 113, 640–648. [Google Scholar] [CrossRef]
- Xiao, T.; Fan, J.K.; Huang, H.L.; Gu, J.F.; Li, L.Y.; Liu, X.Y. VEGI-armed oncolytic adenovirus inhibits tumor neovascularization and directly induces mitochondria-mediated cancer cell apoptosis. Cell Res. 2010, 20, 367–378. [Google Scholar] [CrossRef]
- Xu, W.; Neill, T.; Yang, Y.; Hu, Z.; Cleveland, E.; Wu, Y.; Hutten, R.; Xiao, X.; Stock, S.R.; Shevrin, D.; et al. The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Ther. 2015, 22, 247–256. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, J.H.; Kwon, Y.G.; Kim, E.C.; Kim, N.K.; Choi, H.J.; Yun, C.O. VEGF-specific Short Hairpin RNA–expressing Oncolytic Adenovirus Elicits Potent Inhibition of Angiogenesis and Tumor Growth. Mol. Ther. 2007, 15, 295–302. [Google Scholar] [CrossRef]
- Kang, Y.A.; Shin, H.C.; Yoo, J.Y.; Kim, J.H.; Kim, J.S.; Yun, C.O. Novel Cancer Antiangiotherapy Using the VEGF Promoter-targeted Artificial Zinc-finger Protein and Oncolytic Adenovirus. Mol. Ther. 2008, 16, 1033–1040. [Google Scholar] [CrossRef]
- Shashkova, E.V.; Spencer, J.F.; Wold, W.S.M.; Doronin, K. Targeting Interferon-α Increases Antitumor Efficacy and Reduces Hepatotoxicity of E1A-mutated Spread-enhanced Oncolytic Adenovirus. Mol. Ther. 2007, 15, 598–607. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, C.J.; Han, J.; Gavrikova, T.; Armstrong, L.; Oliveira, A.R.; Shanley, R.; Vickers, S.M.; Yamamoto, M.; Davydova, J. Oncolytic adenovirus expressing interferon alpha in a syngeneic Syrian hamster model for the treatment of pancreatic cancer. Surgery 2015, 157, 888–898. [Google Scholar] [CrossRef]
- Park, M.Y.; Kim, D.R.; Jung, H.W.; Yoon, H.I.; Lee, J.H.; Lee, C.T. Genetic immunotherapy of lung cancer using conditionally replicating adenovirus and adenovirus-interferon-β. Cancer Gene Ther. 2010, 17, 356–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.T.; Park, K.H.; Yanagisawa, K.; Adachi, Y.; Ohm, J.E.; Nadaf, S.; Dikov, M.M.; Curiel, D.T.; Carbone, D.P. Combination Therapy with Conditionally Replicating Adenovirus and Replication Defective Adenovirus. Cancer Res. 2004, 64, 6660–6665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirvinen, M.; Rajecki, M.; Kapanen, M.; Parviainen, S.; Rouvinen-Lagerström, N.; Diaconu, I.; Nokisalmi, P.; Tenhunen, M.; Hemminki, A.; Cerullo, V. Immunological Effects of a Tumor Necrosis Factor Alpha–Armed Oncolytic Adenovirus. Hum. Gene Ther. 2015, 26, 134–144. [Google Scholar] [CrossRef]
- Havunen, R.; Siurala, M.; Sorsa, S.; Grönberg-Vähä-Koskela, S.; Behr, M.; Tähtinen, S.; Santos, J.M.; Karell, P.; Rusanen, J.; Nettelbeck, D.M.; et al. Oncolytic Adenoviruses Armed with Tumor Necrosis Factor Alpha and Interleukin-2 Enable Successful Adoptive Cell Therapy. Mol. Ther. Oncolytics 2017, 4, 77–86. [Google Scholar] [CrossRef]
- Watanabe, K.; Luo, Y.; Da, T.; Guedan, S.; Ruella, M.; Scholler, J.; Keith, B.; Young, R.M.; Engels, B.; Sorsa, S.; et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Santos, J.M.; Heiniö, C.; Cervera-Carrascon, V.; Quixabeira, D.C.A.; Siurala, M.; Havunen, R.; Butzow, R.; Zafar, S.; de Gruijl, T.; Lassus, H.; et al. Oncolytic adenovirus shapes the ovarian tumor microenvironment for potent tumor-infiltrating lymphocyte tumor reactivity. J. Immunother. Cancer 2020, 8, e000188. [Google Scholar] [CrossRef]
- Bortolanza, S.; Bunuales, M.; Otano, I.; Gonzalez-Aseguinolaza, G.; Ortiz-de-Solorzano, C.; Perez, D.; Prieto, J.; Hernandez-Alcoceba, R. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol. Ther. 2009, 17, 614–622. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.H.; Choi, K.J.; Choi, I.K.; Kim, H.; Cho, S.; Cho, B.C.; Yun, C.O. Enhanced Antitumor Effect of Oncolytic Adenovirus Expressing Interleukin-12 and B7-1 in an Immunocompetent Murine Model. Clin. Cancer Res. 2006, 12, 5859–5868. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, J.; Dong, A.; Zhang, Y.; Zhong, L.; He, L.; Wang, Y.; Zhang, J.; Zhang, Z.; Huiwang, J.; et al. Potent Antitumor Activity of Oncolytic Adenovirus Expressing mda-7/IL-24 for Colorectal Cancer. Hum. Gene Ther. 2005, 16, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xia, Q.; Zhang, R.; Lv, C.; Zhang, W.; Wang, Y.; Cui, Q.; Liu, L.; Cai, R.; Qian, C. Treatment of Cancer with a Novel Dual-Targeted Conditionally Replicative Adenovirus Armed with mda-7/IL-24 Gene. Clin. Cancer Res. 2008, 14, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Post, D.E.; Sandberg, E.M.; Kyle, M.M.; Devi, N.S.; Brat, D.J.; Xu, Z.; Tighiouart, M.; Van Meir, E.G. Targeted Cancer Gene Therapy Using a Hypoxia Inducible Factor–Dependent Oncolytic Adenovirus Armed with Interleukin-4. Cancer Res. 2007, 67, 6872–6881. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.N.; Pei, D.S.; Mao, L.J.; Liu, X.Y.; Sun, F.H.; Zhang, B.F.; Liu, Y.Q.; Liu, J.J.; Li, W.; Han, D. Oncolytic adenovirus expressing interleukin-18 induces significant antitumor effects against melanoma in mice through inhibition of angiogenesis. Cancer Gene Ther. 2010, 17, 28–36. [Google Scholar] [CrossRef]
- Choi, I.K.; Lee, J.S.; Zhang, S.N.; Park, J.; Lee, K.M.; Sonn, C.H.; Yun, C.O. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rβ2 or IL-18Rα. Gene Ther. 2011, 18, 898–909. [Google Scholar] [CrossRef]
- Choi, I.K.; Li, Y.; Oh, E.; Kim, J.; Yun, C.O. Oncolytic Adenovirus Expressing IL-23 and p35 Elicits IFN-γ- and TNF-α-Co-Producing T Cell-Mediated Antitumor Immunity. PLoS ONE 2013, 8, e67512. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Si, C.; Zhu, Y.; Jin, Y.; Zhu, T.; Liu, M.; Liu, G. CCL21/IL21-armed oncolytic adenovirus enhances antitumor activity against TERT-positive tumor cells. Virus Res. 2016, 220, 172–178. [Google Scholar] [CrossRef]
- Ye, J.F.; Lin, Y.Q.; Yu, X.H.; Liu, M.Y.; Li, Y. Immunotherapeutic effects of cytokine-induced killer cells combined with CCL21/IL15 armed oncolytic adenovirus in TERT-positive tumor cells. Int. Immunopharmacol. 2016, 38, 460–467. [Google Scholar] [CrossRef]
- Hu, Z.; Gerseny, H.; Zhang, Z.; Chen, Y.J.; Berg, A.; Zhang, Z.; Stock, S.; Seth, P. Oncolytic Adenovirus Expressing Soluble TGFβ Receptor II-Fc-mediated Inhibition of Established Bone Metastases: A Safe and Effective Systemic Therapeutic Approach for Breast Cancer. Mol. Ther. 2011, 19, 1609–1618. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Peng, D.; Wang, H.; Zhang, X.; Wang, H.; Xiao, F.; Zhu, Y.; Ji, Y.; Gulukota, K.; et al. An Oncolytic Adenovirus Targeting Transforming Growth Factor β Inhibits Protumorigenic Signals and Produces Immune Activation: A Novel Approach to Enhance Anti-PD-1 and Anti-CTLA-4 Therapy. Hum. Gene Ther. 2019, 30, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, V.; Pesonen, S.; Diaconu, I.; Escutenaire, S.; Arstila, P.T.; Ugolini, M.; Nokisalmi, P.; Raki, M.; Laasonen, L.; Särkioja, M.; et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- Koski, A.; Kangasniemi, L.; Escutenaire, S.; Pesonen, S.; Cerullo, V.; Diaconu, I.; Nokisalmi, P.; Raki, M.; Rajecki, M.; Guse, K.; et al. Treatment of Cancer Patients With a Serotype 5/3 Chimeric Oncolytic Adenovirus Expressing GMCSF. Mol. Ther. 2010, 18, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, A.; Nokisalmi, P.; Diaconu, I.; Koski, A.; Cerullo, V.; Liikanen, I.; Tahtinen, S.; Oksanen, M.; Heiskanen, R.; Pesonen, S.; et al. Antiviral and Antitumor T-cell Immunity in Patients Treated with GM-CSF-Coding Oncolytic Adenovirus. Clin. Cancer Res. 2013, 19, 2734–2744. [Google Scholar] [CrossRef]
- Zhang, S.N.; Choi, I.K.; Huang, J.H.; Yoo, J.Y.; Choi, K.J.; Yun, C.O. Optimizing DC Vaccination by Combination With Oncolytic Adenovirus Coexpressing IL-12 and GM-CSF. Mol. Ther. 2011, 19, 1558–1568. [Google Scholar] [CrossRef]
- Serafini, P.; Carbley, R.; Noonan, K.A.; Tan, G.; Bronte, V.; Borrello, I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004. [Google Scholar] [CrossRef]
- Marigo, I.; Bosio, E.; Solito, S.; Mesa, C.; Fernandez, A.; Dolcetti, L.; Ugel, S.; Sonda, N.; Bicciato, S.; Falisi, E.; et al. Tumor-Induced Tolerance and Immune Suppression Depend on the C/EBPβ Transcription Factor. Immunity 2010, 32, 790–802. [Google Scholar] [CrossRef]
- Kenkel, J.A.; Tseng, W.W.; Davidson, M.G.; Tolentino, L.L.; Choi, O.; Bhattacharya, N.; Seeley, E.S.; Winer, D.A.; Reticker-Flynn, N.E.; Engleman, E.G. An immunosuppressive dendritic cell subset accumulates at secondary sites and promotes metastasis in pancreatic cancer. Cancer Res. 2017. [Google Scholar] [CrossRef]
- Bernt, K.M.; Ni, S.; Tieu, A.T.; Lieber, A. Assessment of a combined, adenovirus-mediated oncolytic and immunostimulatory tumor therapy. Cancer Res. 2005. [Google Scholar] [CrossRef]
- Ramakrishna, E.; Woller, N.; Mundt, B.; Knocke, S.; Gürlevik, E.; Saborowski, M.; Malek, N.; Manns, M.P.; Wirth, T.; Kühnel, F.; et al. Antitumoral Immune Response by Recruitment and Expansion of Dendritic Cells in Tumors Infected with Telomerase-Dependent Oncolytic Viruses. Cancer Res. 2009, 69, 1448–1458. [Google Scholar] [CrossRef]
- Dias, J.D.; Hemminki, O.; Diaconu, I.; Hirvinen, M.; Bonetti, A.; Guse, K.; Escutenaire, S.; Kanerva, A.; Pesonen, S.; Löskog, A.; et al. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012, 19, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, K.; Rosewell Shaw, A.; Watanabe, N.; Porter, C.; Rana, B.; Gottschalk, S.; Brenner, M.; Suzuki, M. Armed Oncolytic Adenovirus–Expressing PD-L1 Mini-Body Enhances Antitumor Effects of Chimeric Antigen Receptor T Cells in Solid Tumors. Cancer Res. 2017, 77, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Rosewell Shaw, A.; Porter, C.E.; Watanabe, N.; Tanoue, K.; Sikora, A.; Gottschalk, S.; Brenner, M.K.; Suzuki, M. Adenovirotherapy Delivering Cytokine and Checkpoint Inhibitor Augments CAR T Cells against Metastatic Head and Neck Cancer. Mol. Ther. 2017, 25, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.S.; Gomes, E.M.; Butcher, L.D.; Hernandez-Alcoceba, R.; Chang, D.; Kansopon, J.; Newman, J.; Stone, M.J.; Tong, A.W. Growth Inhibition of Human Multiple Myeloma Cells by an Oncolytic Adenovirus Carrying the CD40 Ligand Transgene. Clin. Cancer Res. 2009, 15, 4847–4856. [Google Scholar] [CrossRef]
- Diaconu, I.; Cerullo, V.; Hirvinen, M.L.M.; Escutenaire, S.; Ugolini, M.; Pesonen, S.K.; Bramante, S.; Parviainen, S.; Kanerva, A.; Loskog, A.S.I.; et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012, 72, 2327–2338. [Google Scholar] [CrossRef]
- Pesonen, S.; Diaconu, I.; Kangasniemi, L.; Ranki, T.; Kanerva, A.; Pesonen, S.K.; Gerdemann, U.; Leen, A.M.; Kairemo, K.; Oksanen, M.; et al. Oncolytic Immunotherapy of Advanced Solid Tumors with a CD40L-Expressing Replicating Adenovirus: Assessment of Safety and Immunologic Responses in Patients. Cancer Res. 2012, 72, 1621–1631. [Google Scholar] [CrossRef]
- Zafar, S.; Sorsa, S.; Siurala, M.; Hemminki, O.; Havunen, R.; Cervera-Carrascon, V.; Santos, J.M.; Wang, H.; Lieber, A.; De Gruijl, T.; et al. CD40L coding oncolytic adenovirus allows long-term survival of humanized mice receiving dendritic cell therapy. Oncoimmunology 2018, 7, e1490856. [Google Scholar] [CrossRef]
- Huang, J.H.; Zhang, S.N.; Choi, K.J.; Choi, I.K.; Kim, J.H.; Lee, M.; Kim, H.; Yun, C.O. Therapeutic and Tumor-specific Immunity Induced by Combination of Dendritic Cells and Oncolytic Adenovirus Expressing IL-12 and 4-1BBL. Mol. Ther. 2010, 18, 264–274. [Google Scholar] [CrossRef]
- Jiang, H.; Rivera-Molina, Y.; Gomez-Manzano, C.; Clise-Dwyer, K.; Bover, L.; Vence, L.M.; Yuan, Y.; Lang, F.F.; Toniatti, C.; Hossain, M.B.; et al. Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer Vaccination. Cancer Res. 2017, 77, 3894–3907. [Google Scholar] [CrossRef]
- Rivera-Molina, Y.; Jiang, H.; Fueyo, J.; Nguyen, T.; Shin, D.H.; Youssef, G.; Fan, X.; Gumin, J.; Alonso, M.M.; Phadnis, S.; et al. GITRL-armed Delta-24-RGD oncolytic adenovirus prolongs survival and induces anti-glioma immune memory. Neuro Oncol. Adv. 2019, 1. [Google Scholar] [CrossRef]
- Runcie, K.; Budman, D.R.; John, V.; Seetharamu, N. Bi-specific and tri-specific antibodies- the next big thing in solid tumor therapeutics. Mol. Med. 2018, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.A.; Guedan, S.; Rojas, L.A.; Moreno, R.; Arias-Badia, M.; de Sostoa, J.; June, C.H.; Alemany, R. Oncolytic Adenoviral Delivery of an EGFR-Targeting T-cell Engager Improves Antitumor Efficacy. Cancer Res. 2017, 77, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.; Fajardo, C.A.; Posey, A.D.; Shaw, C.; Da, T.; Young, R.M.; Alemany, R.; June, C.H.; Guedan, S. Improving CART-Cell Therapy of Solid Tumors with Oncolytic Virus–Driven Production of a Bispecific T-cell Engager. Cancer Immunol. Res. 2018, 6, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.D.; Hagel, J.; Scott, E.M.; Psallidas, I.; Gupta, A.; Spiers, L.; Miller, P.; Kanellakis, N.; Ashfield, R.; Fisher, K.D.; et al. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol. Med. 2017, 9, 1067–1087. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.D.; Duffy, M.R.; Lei-Rossmann, J.; Muntzer, A.; Scott, E.M.; Hagel, J.; Campo, L.; Bryant, R.J.; Verrill, C.; Lambert, A.; et al. An Oncolytic Virus Expressing a T-cell Engager Simultaneously Targets Cancer and Immunosuppressive Stromal Cells. Cancer Res. 2018, 78, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- de Sostoa, J.; Fajardo, C.A.; Moreno, R.; Ramos, M.D.; Farrera-Sal, M.; Alemany, R. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J. Immunother. Cancer 2019, 7, 19. [Google Scholar] [CrossRef]
- Scott, E.M.; Jacobus, E.J.; Lyons, B.; Frost, S.; Freedman, J.D.; Dyer, A.; Khalique, H.; Taverner, W.K.; Carr, A.; Champion, B.R.; et al. Bi- And tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J. Immunother. Cancer 2019. [Google Scholar] [CrossRef]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Sci. Transl. Med. 2014, 6, 226ra32. [Google Scholar] [CrossRef]
- Woller, N.; Gürlevik, E.; Fleischmann-Mundt, B.; Schumacher, A.; Knocke, S.; Kloos, A.M.; Saborowski, M.; Geffers, R.; Manns, M.P.; Wirth, T.C.; et al. Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Mol. Ther. 2015, 23, 1630–1640. [Google Scholar] [CrossRef]
- Leung, E.Y.L.; Ennis, D.P.; Kennedy, P.R.; Hansell, C.; Dowson, S.; Farquharson, M.; Spiliopoulou, P.; Nautiyal, J.; McNamara, S.; Carlin, L.M.; et al. NK Cells Augment Oncolytic Adenovirus Cytotoxicity in Ovarian Cancer. Mol. Ther. Oncolytics 2020, 16, 289–301. [Google Scholar] [CrossRef]
- Tähtinen, S.; Feola, S.; Capasso, C.; Laustio, N.; Groeneveldt, C.; Ylösmäki, E.O.; Ylösmäki, L.; Martins, B.; Fusciello, M.; Medeot, M.; et al. Exploiting Preexisting Immunity to Enhance Oncolytic Cancer Immunotherapy. Cancer Res. 2020, 80, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef]
- Schöne, D.; Hrycak, C.P.; Windmann, S.; Lapuente, D.; Dittmer, U.; Tenbusch, M.; Bayer, W. Immunodominance of Adenovirus-Derived CD8+ T Cell Epitopes Interferes with the Induction of Transgene-Specific Immunity in Adenovirus-Based Immunization. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Tysome, J.R.; Li, X.; Wang, S.; Wang, P.; Gao, D.; Du, P.; Chen, D.; Gangeswaran, R.; Chard, L.S.; Yuan, M.; et al. A Novel Therapeutic Regimen to Eradicate Established Solid Tumors with an Effective Induction of Tumor-Specific Immunity. Clin. Cancer Res. 2012, 18, 6679–6689. [Google Scholar] [CrossRef]
- Ilett, E.; Kottke, T.; Thompson, J.; Rajani, K.; Zaidi, S.; Evgin, L.; Coffey, M.; Ralph, C.; Diaz, R.; Pandha, H.; et al. Prime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumour therapy. Gene Ther. 2017, 24. [Google Scholar] [CrossRef]
- Bassett, J.D.; Yang, T.C.; Bernard, D.; Millar, J.B.; Swift, S.L.; McGray, A.J.R.; VanSeggelen, H.; Boudreau, J.E.; Finn, J.D.; Parsons, R.; et al. CD8+ T-cell expansion and maintenance after recombinant adenovirus immunization rely upon cooperation between hematopoietic and nonhematopoietic antigen-presenting cells. Blood 2011, 117, 1146–1155. [Google Scholar] [CrossRef]
- Lee, J.; Hashimoto, M.; Im, S.J.; Araki, K.; Jin, H.T.; Davis, C.W.; Konieczny, B.T.; Spies, G.A.; McElrath, M.J.; Ahmed, R. Adenovirus Serotype 5 Vaccination Results in Suboptimal CD4 T Helper 1 Responses in Mice. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Berkeley, R.A.; Steele, L.P.; Mulder, A.A.; van den Wollenberg, D.J.M.; Kottke, T.J.; Thompson, J.; Coffey, M.; Hoeben, R.C.; Vile, R.G.; Melcher, A.; et al. Antibody-Neutralized Reovirus Is Effective in Oncolytic Virotherapy. Cancer Immunol. Res. 2018, 6, 1161–1173. [Google Scholar] [CrossRef]
- Ricca, J.M.; Oseledchyk, A.; Walther, T.; Liu, C.; Mangarin, L.; Merghoub, T.; Wolchok, J.D.; Zamarin, D. Pre-existing Immunity to Oncolytic Virus Potentiates Its Immunotherapeutic Efficacy. Mol. Ther. 2018, 26, 1008–1019. [Google Scholar] [CrossRef]
- Niemann, J.; Woller, N.; Brooks, J.; Fleischmann-Mundt, B.; Martin, N.T.; Kloos, A.; Knocke, S.; Ernst, A.M.; Manns, M.P.; Kubicka, S.; et al. Molecular retargeting of antibodies converts immune defense against oncolytic viruses into cancer immunotherapy. Nat. Commun. 2019, 10, 3236. [Google Scholar] [CrossRef]
- Tahtinen, S.; Gronberg-Vaha-Koskela, S.; Lumen, D.; Merisalo-Soikkeli, M.; Siurala, M.; Airaksinen, A.J.; Vaha-Koskela, M.; Hemminki, A. Adenovirus Improves the Efficacy of Adoptive T-cell Therapy by Recruiting Immune Cells to and Promoting Their Activity at the Tumor. Cancer Immunol. Res. 2015, 3, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.E.; Rosewell Shaw, A.; Jung, Y.; Yip, T.; Castro, P.D.; Sandulache, V.C.; Sikora, A.; Gottschalk, S.; Ittman, M.M.; Brenner, M.K.; et al. Oncolytic Adenovirus Armed with BiTE, Cytokine, and Checkpoint Inhibitor Enables CAR T Cells to Control the Growth of Heterogeneous Tumors. Mol. Ther. 2020, 28, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
| Agent/Virus Name | Virus Type | Trial No. | Status/Start Date | Indication | Admin. | Phase | Co-Therapy | Arming |
|---|---|---|---|---|---|---|---|---|
| Phase I | ||||||||
| BM-hMSCs- DNX-2401 | Ad5-delta24-RGD (MSCs as carriers) | NCT03896568 | Recr. 02/2019 | Recurrent glioma | i.a. | I | Surgery | none |
| DNX-2401 | Ad5-delta24-RGD | NCT03178032 | Active, not recr. 05/2017 | Brainstem glioma DIPG | local | I | Radiotherapy, chemotherapy | none |
| DNX-2440 | Ad5-delta24-RGD-OX40L | NCT03714334 | Recr. 10/2018 | Glioblastoma | i.t. | I | - | OX40L |
| CAdVec | Binary oAd: Onc.Ad + helper-dependent (HD)-Ad | NCT03740256 | Active, not yet recr. 09/2020 | Diverse HER2 positive solid tumors | i.t. | I | HER2-specific autol. CAR T cells | not disclosed |
| Enadenotucirev/ Colo-Ad1 | Ad3/11 Chimera | NCT03916510 | Recr. 07/2019 | Locally adv. rectal cancer | I | Chemoradiation | none | |
| NG-641 | Ad3/11 Chimera | NCT04053283 | Recr. 01/2020 | Adv./Metastatic epithelial tumors | i.t., i.v. | I | Chemotherapy, checkpoint inhibitors | FAP/CD3, CXCL9, CXCL10, IFNα |
| NG-350A | Ad3/11 Chimera | NCT03852511 | Recr. 02/2019 | Adv./Metastatic epithelial tumors | i.t., i.v. | I | - | Anti-CD40 Ab |
| VCN-01 | Ad-DM-E2F-K-Δ24RGD- PH20 | NCT03284268 | Recr. 09/2017 | Refractory retinoblastoma | intravitreal | I | - | Hyaluronidase |
| VCN-01 | Ad-DM-E2F-K-Δ24RGD- PH20 | NCT03799744 | Recr. 05/2019 | Squamous cell carcinoma of head and neck | i.v. | I | Durvalumab | Hyaluronidase |
| VCN-01 | Ad-DM-E2F-K-Δ24RGD- PH20 | NCT02045602 | Active, not recr. 01/2014 | Adv. solid tumors PDAC | i.v. | I | Gemcitabine, Abraxane | Hyaluronidase |
| Ad5-yCD/mutTKSR39rep-hIL12 | Ad5-yCD/mutTKSR39rep- hIL12 | NCT02555397 | Unknown 08/2015 | Prostate cancer | intra prostatic | I | - | yCD/mutTk/IL-12 |
| Ad5-yCD/mutTKSR39rep-hIL12 | Ad5-yCD/mutTKSR39rep- hIL12 | NCT03281382 | Recr. 07/2017 | Metastatic PDAC | i.t. | I | 5-FC, chemotherapy | yCD/mutTk/IL-12 |
| OBP-301 | Ad5-hTert-E1A-IRES-E1B | NCT02293850 | Recr. 10/2014 | HCC | i.t. | I | - | none |
| ONCOS-102 | Ad5/3-D24-GMCSF | NCT03003676 | Active, not recr. 12/2016 | Advanced or unresectable melanoma | i.t. | I | Cyclophosphamide, pembrolizumab | GM-CSF |
| TILT 123 | Ad5/3-D24-TNFα-IRES-IL2 | NCT04217473 | Recr. 02/2020 | Metastatic melanoma | I | TIL | TNFα, IL2 | |
| Phase I/II | ||||||||
| ONCOS-102 | Ad5/3-D24-GMCSF | NCT02963831 | Recr. 09/2017 | Colorectal, chemoresistant ovarian, appendiceal cancer | i.p. | I/II | Durvalumab | GM-CSF |
| ONCOS-102 | Ad5/3-D24-GMCSF | NCT03514836 | Recr. 05/2018 | Castration-resistant advanced metastatic prostate cancer | i.t. | I/II | DCVAC/PCa | GM-CSF |
| ONCOS-102 | Ad5/3-D24-GMCSF | NCT02879669 | Active, not recr. 06/2016 | Unresectable malignant pleural mesothelioma | I/II | Carboplatin, cyclophosphamide | GM-CSF | |
| LOAd-703 | Ad5/35 | NCT04123470 | Recr. 01/2020 | Malignant melanoma | i.t. | I/II | Atezolizumab | CD40L, 4-1BBL |
| LOAd-703 | Ad5/35 | NCT03225989 | Recr. 03/2018 | PDAC/ovarian, biliary, colorectal cancer | i.t. | I/II | Standard chemotherapy or Gemcitabine | CD40L, 4-1BBL |
| LOAd-703 | Ad5/35 | NCT02705196 | Recr. 11/2016 | PDAC | i.t. | I/II | Gemcitabine, Nab-Paclitaxel, atezolizumab | CD40L, 4-1BBL |
| AdVince | Ad5(PTD)CgA-E1AmiR122 | NCT02749331 | Recr. 03/2016 | Neuroendocrine tumors | i.a. | I/II | - | none |
| ORCA-010 | Ad5-Δ24RGD; T1-mut. | NCT04097002 | Recr. 11/2019 | Prostate cancer | i.t. | I/II | - | none |
| Phase II | ||||||||
| ADV/HSV-tk | Ad5 | NCT03004183 | Recr. 07/2017 | Metastatic NSCLC TNBC | i.t. | II | Valacyclovir, SBRT radiation, pembrolizumab | HSV-tk |
| DNX-2401 | Ad5-delta24-RGD | NCT02798406 | Active, not recr. 06/2016 | Brain cancer | i.t. | II | Pembrolizumab | none |
| OBP-301 | Ad5-hTert-E1A-IRES-E1B | NCT03190824 | Active, not recr. 12/2016 | Melanoma stage III|stage IV | i.t. | II | - | none |
| OBP-301 | Ad5-hTert-E1A-IRES-E1B | NCT03921021 | Recr. 05/2019 | Esophagogastric adenocarcinoma | i.t. | II | Pembrolizumab | none |
| CG0070 | Ad-E2F-E1A-E3-GM- CSF | NCT04387461 | Not yet recr. 08/2015 | NMIBC | intra vesical | II | Pembrolizumab | GM-CSF |
| Phase III | ||||||||
| CG0070 | Ad-E2F-E1A-E3-GM- CSF | NCT04452591 | Not yet recr. 09/2020 | NMIBC | intra vesical | III | N-dodecyl-B-D-maltoside | GM-CSF |
| H101 | Ad5 | NCT03780049 | Recr. 10/2018 | Non-resectable HCC | i.a. | III | HAIC 5-FU, leucovorin | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, M.; Kühnel, F. Oncolytic Adenovirus in Cancer Immunotherapy. Cancers 2020, 12, 3354. https://doi.org/10.3390/cancers12113354
Peter M, Kühnel F. Oncolytic Adenovirus in Cancer Immunotherapy. Cancers. 2020; 12(11):3354. https://doi.org/10.3390/cancers12113354
Chicago/Turabian StylePeter, Malin, and Florian Kühnel. 2020. "Oncolytic Adenovirus in Cancer Immunotherapy" Cancers 12, no. 11: 3354. https://doi.org/10.3390/cancers12113354
APA StylePeter, M., & Kühnel, F. (2020). Oncolytic Adenovirus in Cancer Immunotherapy. Cancers, 12(11), 3354. https://doi.org/10.3390/cancers12113354




