Physiological Roles of ERM Proteins and Transcriptional Regulators in Supporting Membrane Expression of Efflux Transporters as Factors of Drug Resistance in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
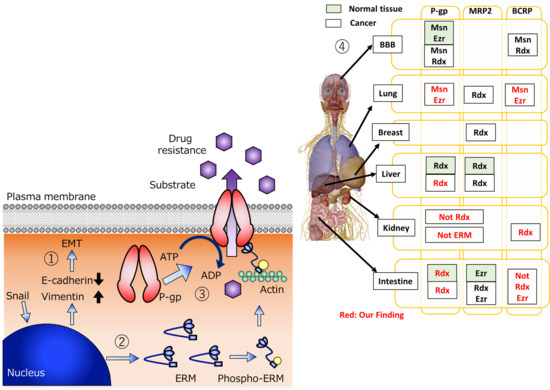
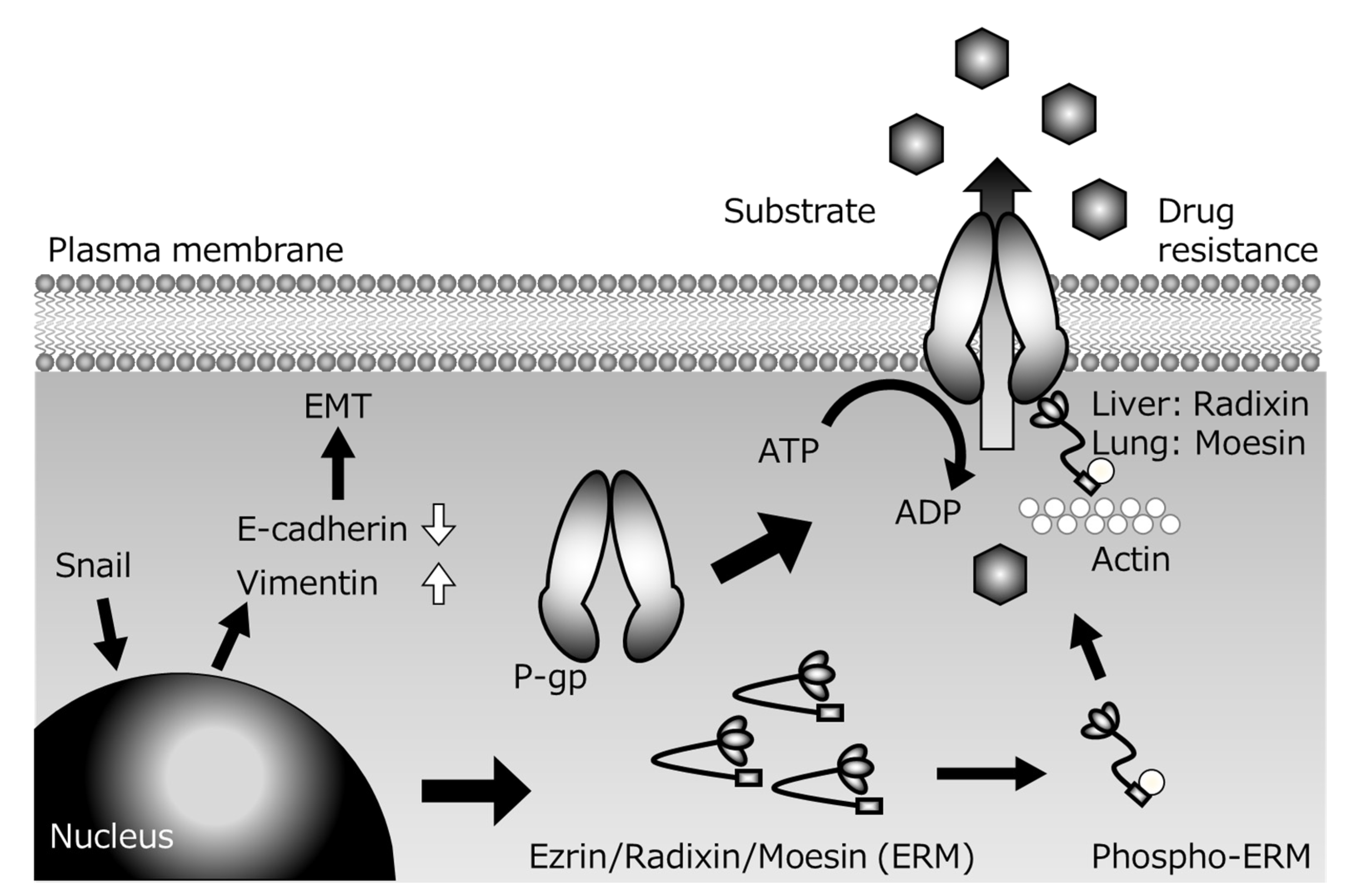
2. Relationship between ERM Scaffold Proteins and Efflux Transporter P-gp
3. Relationship between ERM Scaffold Proteins and Efflux Transporter MRP2
4. Relationship between ERM Scaffold Proteins and Efflux Transporter BCRP
5. Change of P-gp Activity Associated with EMT
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zilfou, J.T.; Lowe, S.W. Tumor suppressive functions of p53. Cold Spring Harb. Perspect. Biol. 2009, 1, a001883. [Google Scholar] [CrossRef]
- Pogrebniak, H.W.; Prewitt, T.W.; Matthews, W.A.; Pass, H.I.; Wallace, R.B. Tumor necrosis factor-alpha alters response of lung cancer cells to oxidative stress. J. Thorac. Cardiovasc. Surg. 1991, 102, 904–907. [Google Scholar] [CrossRef]
- Bierie, B.; Moses, H.L. Tumour microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 2006, 6, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Polgar, O.; Bates, S.E. ABC transporters in the balance: Is there a role in multidrug resistance? Biochem. Soc. Trans. 2005, 33, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, S.; Nagy, B.; Benedetti, E.; Pacini, S.; Brizzi, S.; Caracciolo, F.; Papineschi, F.; Ciabatti, E.; Guerrini, F.; Fazzi, R.; et al. Evaluation of the MDR1, ABCG2, Topoisomerases IIalpha and GSTpi gene expression in patients affected by aggressive mantle cell lymphoma treated by the R-Hyper-CVAD regimen. Leuk. Lymphoma 2007, 48, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel-Eibrink, M.M.; van der Holt, B.; Burnett, A.K.; Knauf, W.U.; Fey, M.F.; Verhoef, G.E.; Vellenga, E.; Ossenkoppele, G.J.; Löwenberg, B.; Sonneveld, P. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann. Hematol. 2007, 86, 329–337. [Google Scholar] [CrossRef]
- Turner, J.G.; Gump, J.L.; Zhang, C.; Cook, J.M.; Marchion, D.; Hazlehurst, L.; Munster, P.; Schell, M.J.; Dalton, W.S.; Sullivan, D.M. ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood 2006, 108, 3881–3889. [Google Scholar] [CrossRef]
- Lagas, J.S.; Fan, L.; Wagenaar, E.; Vlaming, M.L.; van Tellingen, O.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein (P-gp/Abcb1), Abcc2, and Abcc3 determine the pharmacokinetics of etoposide. Clin. Cancer Res. 2010, 16, 130–140. [Google Scholar] [CrossRef]
- Evers, R.; de Haas, M.; Sparidans, R.; Beijnen, J.; Wielinga, P.R.; Lankelma, J.; Borst, P. Vinblastine and sulfinpyrazone export by the multidrug resistance protein MRP2 is associated with glutathione export. Br. J. Cancer 2000, 83, 375–383. [Google Scholar] [CrossRef]
- Maliepaard, M.; van Gastelen, M.A.; Tohgo, A.; Hausheer, F.H.; van Waardenburg, R.C.; de Jong, L.A.; Pluim, D.; Beijnen, J.H.; Schellens, J.H. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin. Cancer Res. 2001, 7, 935–941. [Google Scholar]
- Lehnert, M.; Emerson, S.; Dalton, W.S.; de Giuli, R.; Salmon, S.E. In vitro evaluation of chemosensitizers for clinical reversal of P-glycoprotein-associated Taxol resistance. J. Natl. Cancer Inst. Monogr. 1993, 15, 63–67. [Google Scholar]
- Han, L.; Wang, Y.F.; Zhang, Y.; Wang, N.; Guo, X.J.; Yang, J.K.; Wang, K.P.; Liu, S.N.; Fan, Q.X.; Li, K.; et al. Increased expression and function of P-glycoprotein in peripheral blood CD56+ cells is associated with the chemoresistance of non-small-cell lung cancer. Cancer Chemother. Pharmacol. 2012, 70, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Gottesman, M.M.; Ueda, K.; Lovelace, E.; Rutherford, A.V.; Willingham, M.C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4486–4490. [Google Scholar] [CrossRef]
- Traxl, A.; Wanek, T.; Mairinger, S.; Stanek, J.; Filip, T.; Sauberer, M.; Muller, M.; Kuntner, C.; Langer, O. Breast cancer resistance protein and P-glycoprotein influence in vivo disposition of 11C-Erlotinib. J. Nucl. Med. 2015, 56, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Eadie, L.N.; Hughes, T.P.; White, D.L. Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin. Pharmacol. Ther. 2014, 95, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Kort, A.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Brain accumulation of the EML4-ALK inhibitor ceritinib is restricted by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance protein (BCRP/ABCG2). Pharmacol. Res. 2015, 102, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Nguyen, L.N.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int. J. Cancer 2014, 134, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Arceci, R.J. Clinical significance of P-glycoprotein in multidrug resistance malignancies. Blood 1993, 81, 2215–2222. [Google Scholar] [CrossRef]
- Holmes, J.A.; West, R.R. The effect of MDR-1 gene expression on outcome in acute myeloblastic leukaemia. Br. J. Cancer 1994, 69, 382–384. [Google Scholar] [CrossRef]
- Juranka, P.F.; Zastawny, R.L.; Ling, V. P-glycoprotein: Multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989, 3, 2583–2592. [Google Scholar] [CrossRef]
- He, X.J.; Wang, W.R.; Zhang, Y.; Yang, Q. The effect of radixin knockdown on the expression and efflux function of MRP2 in SGC-7901 cells. Eur. J. Pharm. Sci. 2012, 46, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bello-Reuss, E.; Ernest, S.; Holland, O.B.; Hellmich, M.R. Role of multidrug resistance P-glycoprotein in the secretion of aldosterone by human adrenal NCI-H295 cells. Am. J. Physiol. Cell Physiol. 2000, 278, C1256–C1265. [Google Scholar] [CrossRef] [PubMed]
- Cordon-Cardo, C.; O’Brien, J.P.; Casals, D.; Rittman-Grauer, L.; Biedler, J.L.; Melamed, M.R.; Bertino, J.R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood–brain barrier sites. Proc. Natl. Acad. Sci. USA 1989, 86, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Nooter, K.; Herweijer, H. Multidrug resistance (mdr) genes in human cancer. Br. J. Cancer 1991, 63, 663–669. [Google Scholar] [CrossRef]
- Demeule, M.; Jodoin, J.; Beaulieu, E.; Brossard, M.; Beliveau, R. Dexamethasone modulation of multidrug transporters in normal tissues. FEBS Lett. 1999, 442, 208–214. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, W.; Qin, L.; Tian, L.; Zhou, C. Enhancement of P-glycoprotein expression by hepatocyte transplantation in carbon tetrachloride-induced rat liver. Anat. Rec. 2010, 293, 1167–1174. [Google Scholar] [CrossRef]
- Ballinger, J.R.; Hua, H.A.; Berry, B.W.; Firby, P.; Boxen, I. 99Tcm-sestamibi as an agent for imaging P-glycoprotein-mediated multi-drug resistance: In vitro and in vivo studies in a rat breast tumour cell line and its doxorubicin-resistant variant. Nucl. Med. Commun. 1995, 16, 253–257. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Wu, J.; Li, J.; Qu, X.; Xu, W.; Tang, W. Strategies to overcome or circumvent P-glycoprotein mediated multidrug resistance. Curr. Med. Chem. 2008, 15, 470–476. [Google Scholar]
- Toyoda, Y.; Takada, T.; Suzuki, H. Inhibitors of Human ABCG2: From Technical Background to Recent Updates with Clinical Implications. Front. Pharmacol. 2019, 10, 208. [Google Scholar] [CrossRef]
- Leonard, G.D.; Fojo, T.; Bates, S.E. The role of ABC transporters in clinical practice. Oncologist 2003, 8, 411–424. [Google Scholar] [CrossRef]
- Schena, M.; Shalon, D.; Davis, R.W.; Brown, P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995, 270, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.H. Structure and function of P-glycoprotein. Cancer Treat. Res. 1989, 48, 37–53. [Google Scholar] [PubMed]
- Ogihara, T.; Kamiya, M.; Ozawa, M.; Fujita, T.; Yamamoto, A.; Yamashita, S.; Ohnishi, S.; Isomura, Y. What kinds of substrates show P-glycoprotein-dependent intestinal absorption? Comparison of verapamil with vinblastine. Drug Metab. Pharmacokinet. 2006, 21, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Berggren, S.; Gall, C.; Wollnitz, N.; Ekelund, M.; Karlbom, U.; Hoogstraate, J.; Schrenk, D.; Lennernäs, H. Gene and protein expression of P-glycoprotein, MRP1, MRP2, and CYP3A4 in the small and large human intestine. Mol. Pharm. 2007, 4, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Tomono, T.; Sakai, R.; Kano, T.; Morimoto, K.; Kato, Y.; Ogihara, T. Contribution of radixin to P-glycoprotein expression and transport activity in mouse small intestine in vivo. J. Pharm. Sci. 2013, 102, 2875–2881. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Okabe, C.; Fujii, K.; Kato, Y.; Ogihara, T. Regulation of breast cancer resistance protein and P-glycoprotein by ezrin, radixin and moesin in lung, intestinal and renal cancer cell lines. J. Pharm. Pharmacol. 2020, 72, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Tomono, T.; Ogihara, T. Advances in studies of P-glycoprotein and its expression regulators. Biol. Pharm. Bull. 2018, 41, 11–19. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Li, S.; Qin, X.; Chai, S.; Qu, C.; Wang, X.; Zhang, H. Modulation of E-cadherin expression promotes migration ability of esophageal cancer cells. Sci. Rep. 2016, 6, 21713. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Lu, D.; Shu, Y.; Shi, W.; Lu, S.; Wang, K. Expression of multidrug resistance-related proteins p-glycoprotein, glutathione-s-transferases, topoisomerase-II and lung resistance protein in primary gastric cardiac adenocarcinoma. Hepatogastroenterology 2008, 55, 1530–1536. [Google Scholar]
- Chan, L.M.; Lowes, S.; Hirst, B.H. The ABCs of drug transport in intestine and liver: Efflux proteins limiting drug absorption and bioavailability. Eur. J. Pharm. Sci. 2004, 21, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Mottino, A.D.; Catania, V.A. Hepatic drug transporters and nuclear receptors: Regulation by therapeutic agents. World J. Gastroenterol. 2008, 14, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Tamai, I.; Sakata, A.; Tenda, Y.; Terasaki, T. Restricted transport of cyclosporin A across the blood–brain barrier by a multidrug transporter, P-glycoprotein. Biochem. Pharmacol. 1993, 46, 1096–1099. [Google Scholar] [CrossRef]
- Schinkel, A.H. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194. [Google Scholar] [CrossRef]
- Ichikawa-Haraguchi, M.; Sumizawa, T.; Yoshimura, A.; Furukawa, T.; Hiramoto, S.; Sugita, M.; Akiyama, S. Progesterone and its metabolites: The potent inhibitors of the transporting activity of P-glycoprotein in the adrenal gland. Biochim. Biophys. Acta. 1993, 1158, 201–208. [Google Scholar] [CrossRef]
- Sato, N.; Funayama, N.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J. Cell Sci. 1992, 103, 131–143. [Google Scholar]
- Tsukita, S.; Yonemura, S. Cortical actin organization: Lessons from ERM (ezrin/radixin/moesin) proteins. J. Biol. Chem. 1999, 274, 34507–34510. [Google Scholar] [CrossRef]
- Andréoli, C.; Martin, M.; Le Borgne, R.; Reggio, H.; Mangeat, P. Ezrin has properties to self-associate at the plasma membrane. J Cell Sci. 1994, 107, 2509–2521. [Google Scholar]
- Gary, R.; Bretscher, A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell 1995, 6, 1061–1075. [Google Scholar] [CrossRef]
- Magendantz, M.; Henry, M.D.; Lander, A.; Solomon, F. Interdomain interactions of radixin in vitro. J. Biol. Chem. 1995, 270, 25324–25327. [Google Scholar] [CrossRef]
- Tsukita, S.; Yonemura, S. ERM (ezrin/radixin/moesin) family: From cytoskeleton to signal transduction. Curr. Opin. Cell Biol. 1997, 9, 70–75. [Google Scholar] [CrossRef]
- Vaheri, A.; Carpén, O.; Heiska, L.; Helander, T.S.; Jääskeläinen, J.; Majander-Nordenswan, P.; Sainio, M.; Timonen, T.; Turunen, O. The ezrin protein family: Membrane-cytoskeleton interactions and disease associations. Curr. Opin. Cell Biol. 1997, 9, 659–666. [Google Scholar] [CrossRef]
- Del Pozo, M.A.; Nieto, M.; Serrador, J.M.; Sancho, D.; Vicente-Manzanares, M.; Martínez, C.; Sánchez-Madrid, F. The two poles of the lymphocyte: Specialized cell compartments for migration and recruitment. Cell Adhes. Commun. 1998, 6, 125–133. [Google Scholar] [CrossRef][Green Version]
- Bretscher, A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr. Opin. Cell Biol. 1999, 11, 109–116. [Google Scholar] [CrossRef]
- Mangeat, P.; Roy, C.; Martin, M. ERM proteins in cell adhesion and membrane dynamics. Trends. Cell Biol. 1999, 9, 187–192. [Google Scholar] [CrossRef]
- Matsui, T.; Yonemura, S.; Tsukita, S.; Tsukita, S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr. Biol. 1999, 9, 1259–1262. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Yoshida, S.; Hatano, R.; Asano, S. Pathophysiological Roles of Ezrin/Radixin/Moesin Proteins. Biol. Pharm. Bull. 2017, 40, 381–390. [Google Scholar] [CrossRef]
- Luciani, F.; Molinari, A.; Lozupone, F.; Calcabrini, A.; Lugini, L.; Stringaro, A.; Puddu, P.; Arancia, G.; Cianfriglia, M.; Fais, S. P-glycoprotein-actin association through ERM family proteins: A role in P-glycoprotein function in human cells of lymphoid origin. Blood 2002, 99, 641–648. [Google Scholar] [CrossRef]
- Brambilla, D.; Zamboni, S.; Federici, C.; Lugini, L.; Lozupone, F.; De Milito, A.; Cecchetti, S.; Cianfriglia, M.; Fais, S. P-glycoprotein binds to ezrin at amino acid residues 149-242 in the FERM domain and plays a key role in the multidrug resistance of human osteosarcoma. Int. J. Cancer 2012, 130, 2824–2834. [Google Scholar] [CrossRef]
- Wang, W.; Soroka, C.J.; Mennone, A.; Rahner, C.; Harry, K.; Pypaert, M.; Boyer, J.L. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology 2006, 131, 878–884. [Google Scholar] [CrossRef]
- Kano, T.; Wada, S.; Morimoto, K.; Kato, Y.; Ogihara, T. Effect of knockdown of ezrin, radixin, and moesin on P-glycoprotein function in HepG2 cells. J. Pharm. Sci. 2011, 100, 5308–5314. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Otsuka, K.; Kato, Y.; Kawabata, H.; Ohmori, S.; Arakawa, H.; Ogihara, T. Different regulation of P-glycoprotein function between Caco-2 and Caki-1 cells by ezrin, radixin and moesin proteins. J. Pharm. Pharmacol. 2016, 68, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Fujita-Hamabe, W.; Nishida, M.; Nawa, A.; Kobori, T.; Nakamoto, K.; Kishioka, S.; Tokuyama, S. Etoposide modulates the effects of oral morphine analgesia by targeting the intestinal P-glycoprotein. J. Pharm. Pharmacol. 2012, 64, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Kobori, T.; Harada, S.; Nakamoto, K.; Tokuyama, S. Activation of ERM-family proteins via RhoA-ROCK signaling increases intestinal P-gp expression and leads to attenuation of oral morphine analgesia. J. Pharm. Sci. 2013, 102, 1095–1105. [Google Scholar] [CrossRef]
- Kobori, T.; Harada, S.; Nakamoto, K.; Tokuyama, S. Time-dependent changes in the activation of RhoA/ROCK and ERM/p-ERM in the increased expression of intestinal P-glycoprotein by repeated oral treatment with etoposide. J. Pharm. Sci. 2013, 102, 1670–1682. [Google Scholar] [CrossRef]
- Kobori, T.; Fujiwara, S.; Miyagi, K.; Harada, S.; Nakamoto, K.; Nakagawa, T.; Takahashi, H.; Narita, M.; Tokuyama, S. Involvement of moesin in the development of morphine analgesic tolerance through P-glycoprotein at the blood–brain barrier. Drug Metab. Pharmacokinet. 2014, 9, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Uchida, Y.; Kuroda, T.; Tachikawa, M.; Couraud, P.O.; Suzuki, T.; Terasaki, T. Distinct roles of ezrin, radixin and moesin in maintaining the plasma membrane localizations and functions of human blood–brain barrier transporters. J. Cereb. Blood Flow Metab. 2020, 40, 1533–1545. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; Zhu, X.; Wang, W.; Yang, Q. The effect of sphingomyelin synthase 2 (SMS2) deficiency on the expression of drug transporters in mouse brain. Biochem. Pharmacol. 2011, 82, 287–294. [Google Scholar] [CrossRef]
- Amieva, M.R.; Wilgenbus, K.K.; Furthmayr, H. Radixin is a component of hepatocyte microvilli in situ. Exp. Cell Res. 1994, 210, 140–144. [Google Scholar] [CrossRef]
- Schwartz-Albiez, R.; Merling, A.; Spring, H.; Möller, P.; Koretz, K. Differential expression of the microspike-associated protein moesin in human tissues. Eur. J. Cell Biol. 1995, 67, 189–198. [Google Scholar]
- Berryman, M.; Franck, Z.; Bretscher, A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J. Cell Sci. 1993, 105, 1025–1043. [Google Scholar] [PubMed]
- Saeki, J.; Sekine, S.; Horie, T. LPS-induced dissociation of multidrug resistance-associated protein 2 (Mrp2) and radixin is associated with Mrp2 selective internalization in rats. Biochem. Pharmacol. 2011, 81, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Nies, A.T.; König, J.; Hagmann, W.; Spring, H.; Uemura, M.; Fukui, H.; Keppler, D. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J. Hepatol. 2003, 39, 693–702. [Google Scholar] [CrossRef]
- Kawase, A.; Inoue, Y.; Nakazaki, S.; Koizumi, E.; Iwaki, M. Radixin knockdown improves the accumulation and efficiency of methotrexate in tumor cells. Oncol. Rep. 2019, 42, 283–290. [Google Scholar] [CrossRef]
- Suda, J.; Zhu, L.; Karvar, S. Phosphorylation of radixin regulates cell polarity and Mrp-2 distribution in hepatocytes. Am. J. Physiol. Cell Physiol. 2011, 300, C416–C424. [Google Scholar] [CrossRef]
- Nakano, T.; Sekine, S.; Ito, K.; Horie, T. Correlation between apical localization of Abcc2/Mrp2 and phosphorylation status of ezrin in rat intestine. Drug Metab. Dispos. 2009, 37, 1521–1527. [Google Scholar] [CrossRef]
- Nakano, T.; Sekine, S.; Ito, K.; Horie, T. Ezrin regulates the expression of Mrp2/Abcc2 and Mdr1/Abcb1 along the rat small intestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G807–G817. [Google Scholar] [CrossRef]
- Yang, Q.; Onuki, R.; Nakai, C.; Sugiyama, Y. Ezrin and radixin both regulate the apical membrane localization of ABCC2 (MRP2) in human intestinal epithelial Caco-2 cells. Exp. Cell Res. 2007, 313, 3517–3525. [Google Scholar] [CrossRef]
- Zhou, S.F.; Wang, L.L.; Di, Y.M.; Xue, C.C.; Duan, W.; Li, C.G.; Li, Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Med. Chem. 2008, 15, 1981–2039. [Google Scholar] [CrossRef]
- Sasaki, M.; Suzuki, H.; Ito, K.; Abe, T.; Sugiyama, Y. Transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and multidrug resistance-associated protein 2 (MRP2/ABCC2). J. Biol. Chem. 2002, 277, 6497–6503. [Google Scholar] [CrossRef]
- Kouzuki, H.; Suzuki, H.; Sugiyama, Y. Pharmacokinetic study of the hepatobiliary transport of indomethacin. Pharm. Res. 2000, 17, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.Y.; Kato, Y.; Niinuma, K.; Sudo, K.I.; Hakusui, H.; Sugiyama, Y. Multispecific organic anion transporter is responsible for the biliary excretion of the camptothecin derivative irinotecan and its metabolites in rats. J. Pharmacol. Exp. Ther. 1997, 281, 304–314. [Google Scholar] [PubMed]
- Kikuchi, S.; Hata, M.; Fukumoto, K.; Yamane, Y.; Matsui, T.; Tamura, A.; Yonemura, S.; Yamagishi, H.; Keppler, D.; Tsukita, S.; et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat. Genet. 2002, 31, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Sugiura, T.; Wakayama, T.; Kijima, A.; Nakamichi, N.; Iseki, S.; Silver, D.L.; Kato, Y. PDZK1 regulates breast cancer resistance protein in small intestine. Drug Metab. Dispos. 2011, 39, 2148–2154. [Google Scholar] [CrossRef]
- Zhao, R.; Raub, T.J.; Sawada, G.A.; Kasper, S.C.; Bacon, J.A.; Bridges, A.S.; Pollack, G.M. Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood–brain barrier. Drug Metab. Dispos. 2009, 37, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.; Biswas, R.; Steplock, D.; Shenolikar, S.; Weinman, E. Role of NHERF and scaffolding proteins in proximal tubule transport. Urol. Res. 2010, 38, 257–262. [Google Scholar] [CrossRef]
- Terawaki, S.; Maesaki, R.; Hakoshima, T. Structural basis for NHERF recognition by ERM proteins. Structure 2006, 14, 777–789. [Google Scholar] [CrossRef]
- Perl, A.K.; Wilgenbus, P.; Dahl, U.; Semb, H.; Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392, 190–193. [Google Scholar] [CrossRef]
- Kevans, D.; Wang, L.M.; Sheahan, K.; Hyland, J.; O’Donoghue, D.; Mulcahy, H.; O’Sullivan, J. Epithelial–mesenchymal transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int. J. Surg. Pathol. 2011, 19, 751–760. [Google Scholar] [CrossRef]
- Chen, J.; Imanaka, N.; Chen, J.; Griffin, J.D. Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br. J. Cancer 2010, 102, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Shirako, H.; Takeuchi, T.; Kawakami, Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 2009, 15, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Elloul, S.; Elstrand, M.B.; Nesland, J.M.; Tropé, C.G.; Kvalheim, G.; Goldberg, I.; Reich, R.; Davidson, B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 2005, 103, 1631–1643. [Google Scholar] [CrossRef]
- Roy, H.K.; Smyrk, T.C.; Koetsier, J.; Victor, T.A.; Wali, R.K. The transcriptional repressor SNAIL is overexpressed in human colon cancer. Dig. Dis. Sci. 2005, 50, 42–46. [Google Scholar] [CrossRef]
- Woo, H.Y.; Min, A.L.; Choi, J.Y.; Bae, S.H.; Yoon, S.K.; Jung, C.K. Clinicopathologic significance of the expression of Snail in hepatocellular carcinoma. Korean J. Hepatol. 2011, 17, 12–18. [Google Scholar] [CrossRef]
- Hung, J.J.; Yang, M.H.; Hsu, H.S.; Hsu, W.H.; Liu, J.S.; Wu, K.J. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax 2009, 64, 1082–1089. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Hunag, Y.Y.; Liu, X.G.; He, J.Y.; Chen, D.D.; Zeng, F.; Zhou, J.H.; Zhang, Y.K. Prognostic evaluation of CapG, gelsolin, P-gp, GSTP1, and Topo-II proteins in non-small cell lung cancer. Anat. Rec. 2012, 295, 208–214. [Google Scholar] [CrossRef]
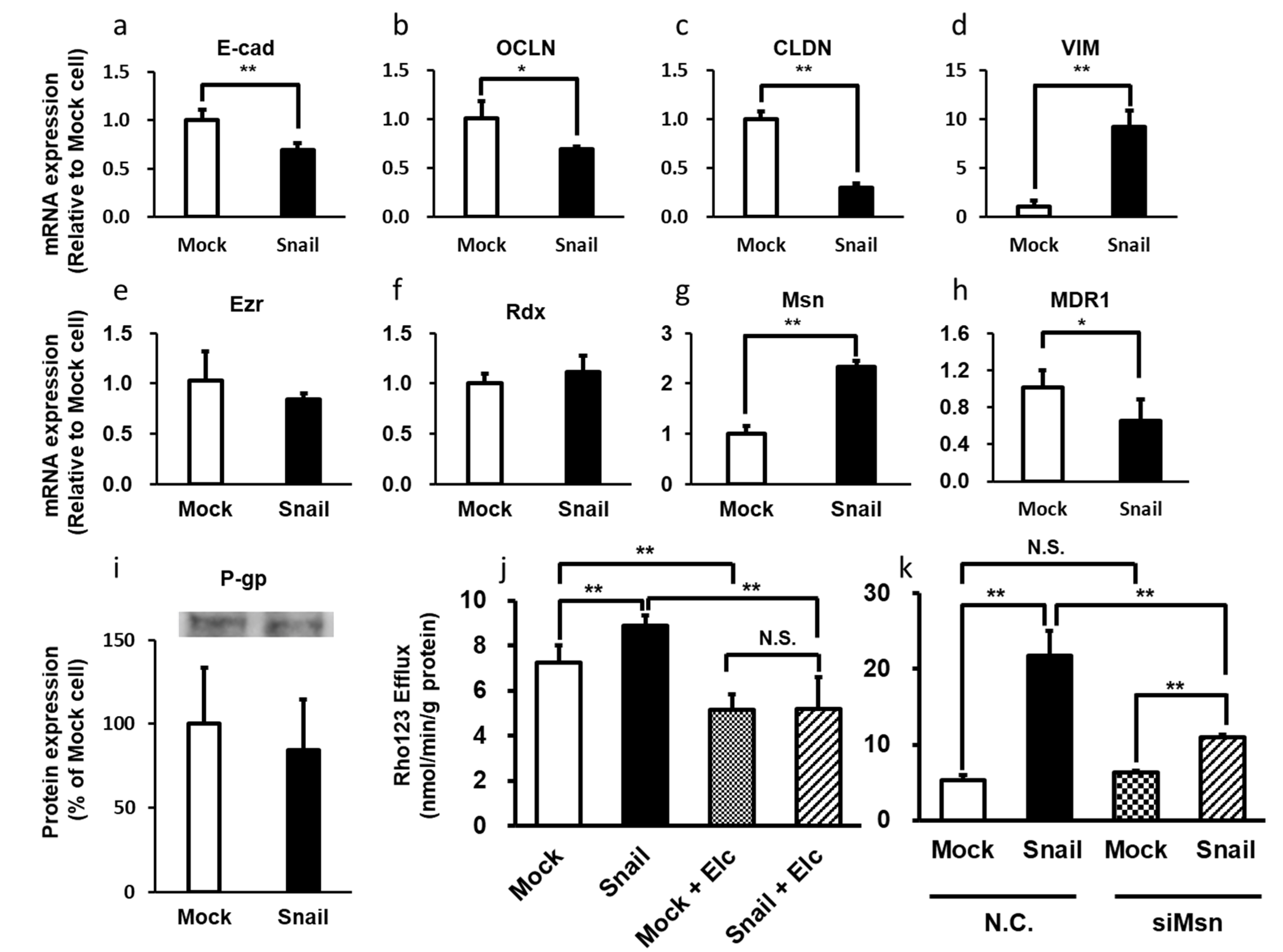
- Tomono, T.; Yano, K.; Ogihara, T. Snail-induced epithelial-to-mesenchymal transition enhances P-gp-mediated multidrug resistance in HCC827 cells. J. Pharm. Sci. 2017, 106, 2642–2649. [Google Scholar] [CrossRef]
- Kamioka, H.; Tomono, T.; Fujita, A.; Onozato, R.; Iijima, M.; Tsuchida, S.; Arai, T.; Fujita, Y.; Zhang, X.; Yano, K.; et al. Moesin-mediated P-glycoprotein activation during Snail-induced epithelial–mesenchymal transition in lung cancer cells. J. Pharm. Sci. 2020, 109, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Leng, J.; Hu, M.; Zhang, L.; Wang, Z.; Liu, D.; Tong, X.; Yu, B.; Hu, Y.; Deng, C.; et al. Ezrin is a key molecule in the metastasis of MOLT4 cells induced by CCL25/CCR9. Leuk. Res. 2010, 34, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Todokoro, I.; Kamioka, H.; Tomono, T.; Ogihara, T. Functional alterations of multidrug resistance-associated proteins 2 and 5, and breast cancer resistance protein upon Snail-induced epithelial–mesenchymal transition in HCC827 cells. Bio. Pharm. Bull 2020, in press. [Google Scholar]
| Membrane proteins |
| CD44 |
| CD43 |
| CD95 (APO-1/Fas) |
| Intercellular adhesion molecule-1 (ICAM-1) |
| Intercellular adhesion molecule-1 (ICAM-2) |
| L-selectin |
| MRP2 |
| Na+,H+-exchanger (NHE1) |
| Na+ K+ 2Cl-cotransporter (NKCC2) |
| P-gp |
| Scaffoerd proteins |
| NHERF1 (EBP-50) |
| NHERF2 |
| NHERF3 |
| Rho-relate3 proteins |
| Dbl |
| Rho-GDP-dissociation inhibitor (Rho-GDI) |
| Tissues | P-gp | MRP2 | BCRP | |
|---|---|---|---|---|
| Liver | Normal | Rdx [60] | Rdx [72,73] | |
| Cancer cell lines | Rdx [61] | Rdx [74,75] | ||
| Intestine | Normal | Rdx [35] | Ezr [76,77] | |
| Cancer cell lines | Rdx [62] | Rdx [78], Ezr [78] | Not Ezr, Rdx [36] | |
| Kidney | Normal | Not Rdx [35] | ||
| Cancer cell lines | Not ERM [62] | Rdx [36] | ||
| Lung | Cancer cell lines | Ezr, Msn [36] | Rdx [74] | Ezr, Msn [36] |
| BBB | Normal | Msn [66], Ezr [68] | ||
| Cancer cell lines | Msn, Rdx [67] | Msn, Rdx [67] | ||
| Breast | Cancer cell lines | Rdx [74] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogihara, T.; Mizoi, K.; Kamioka, H.; Yano, K. Physiological Roles of ERM Proteins and Transcriptional Regulators in Supporting Membrane Expression of Efflux Transporters as Factors of Drug Resistance in Cancer. Cancers 2020, 12, 3352. https://doi.org/10.3390/cancers12113352
Ogihara T, Mizoi K, Kamioka H, Yano K. Physiological Roles of ERM Proteins and Transcriptional Regulators in Supporting Membrane Expression of Efflux Transporters as Factors of Drug Resistance in Cancer. Cancers. 2020; 12(11):3352. https://doi.org/10.3390/cancers12113352
Chicago/Turabian StyleOgihara, Takuo, Kenta Mizoi, Hiroki Kamioka, and Kentaro Yano. 2020. "Physiological Roles of ERM Proteins and Transcriptional Regulators in Supporting Membrane Expression of Efflux Transporters as Factors of Drug Resistance in Cancer" Cancers 12, no. 11: 3352. https://doi.org/10.3390/cancers12113352
APA StyleOgihara, T., Mizoi, K., Kamioka, H., & Yano, K. (2020). Physiological Roles of ERM Proteins and Transcriptional Regulators in Supporting Membrane Expression of Efflux Transporters as Factors of Drug Resistance in Cancer. Cancers, 12(11), 3352. https://doi.org/10.3390/cancers12113352