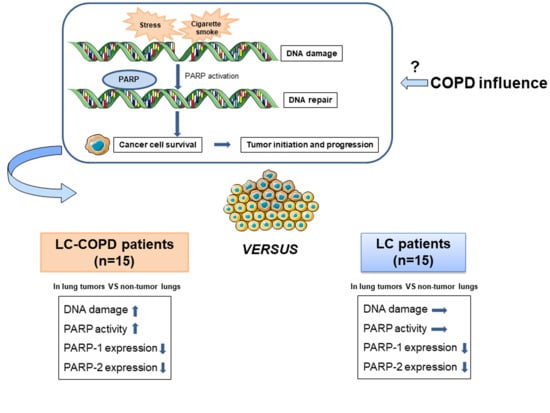
Increased PARP Activity and DNA Damage in NSCLC Patients: The Influence of COPD
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Clinical and Functional Characteristics of Study Patients
2.2. DNA Damage Increased in Lung Tumors of COPD Patients
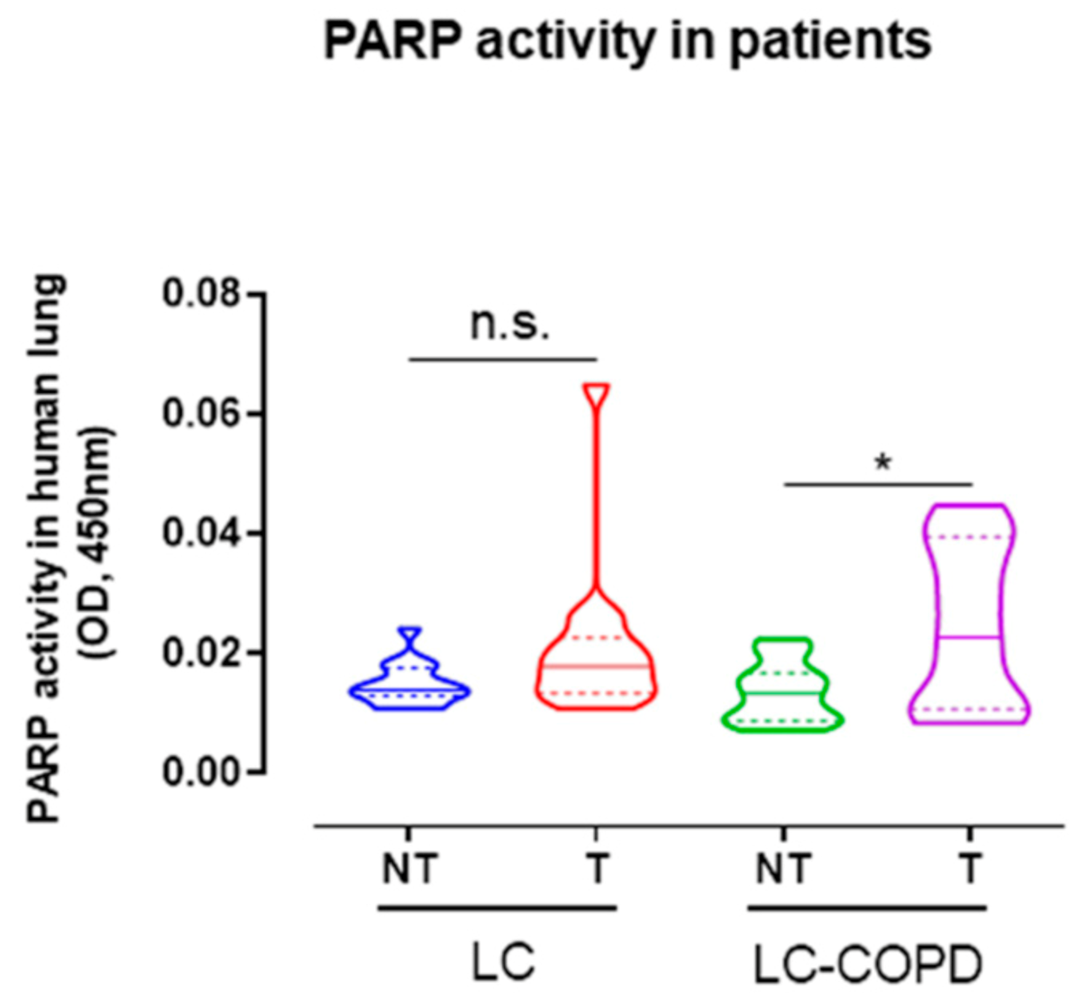
2.3. PARP Activity Increased in Lung Tumors of COPD Patients
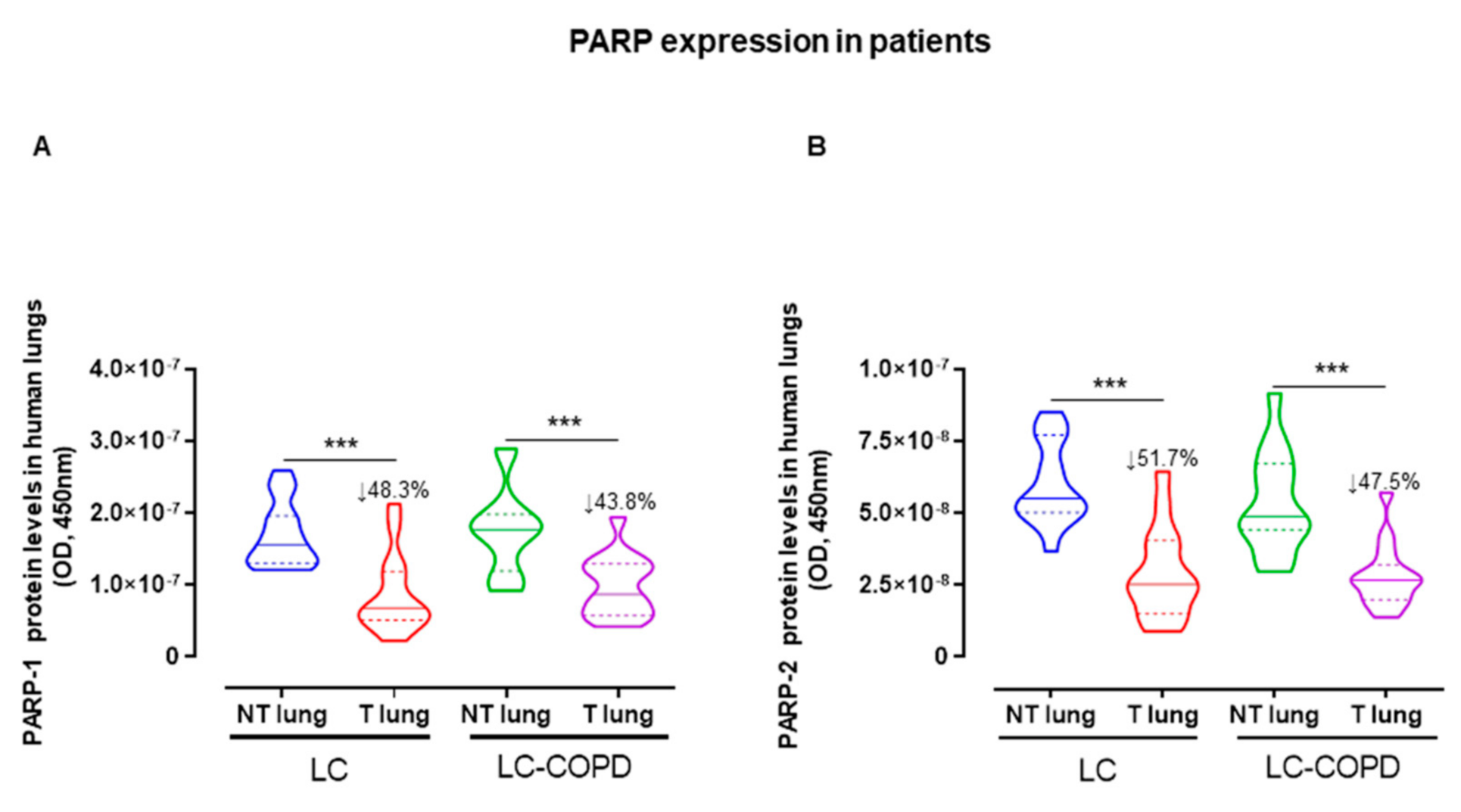
2.4. PARP Expression Decreased in Lung Tumors of All the Study Patients
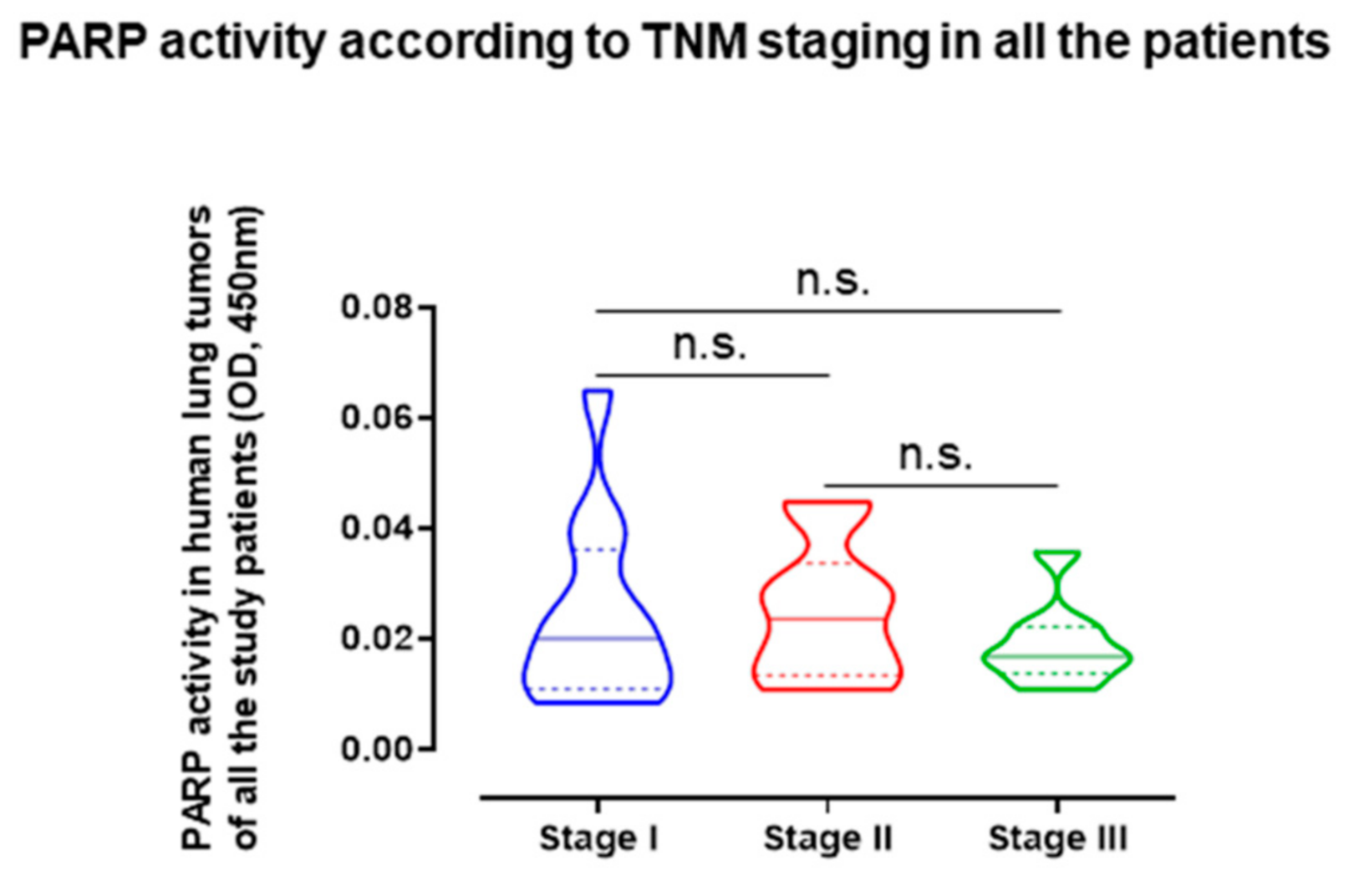
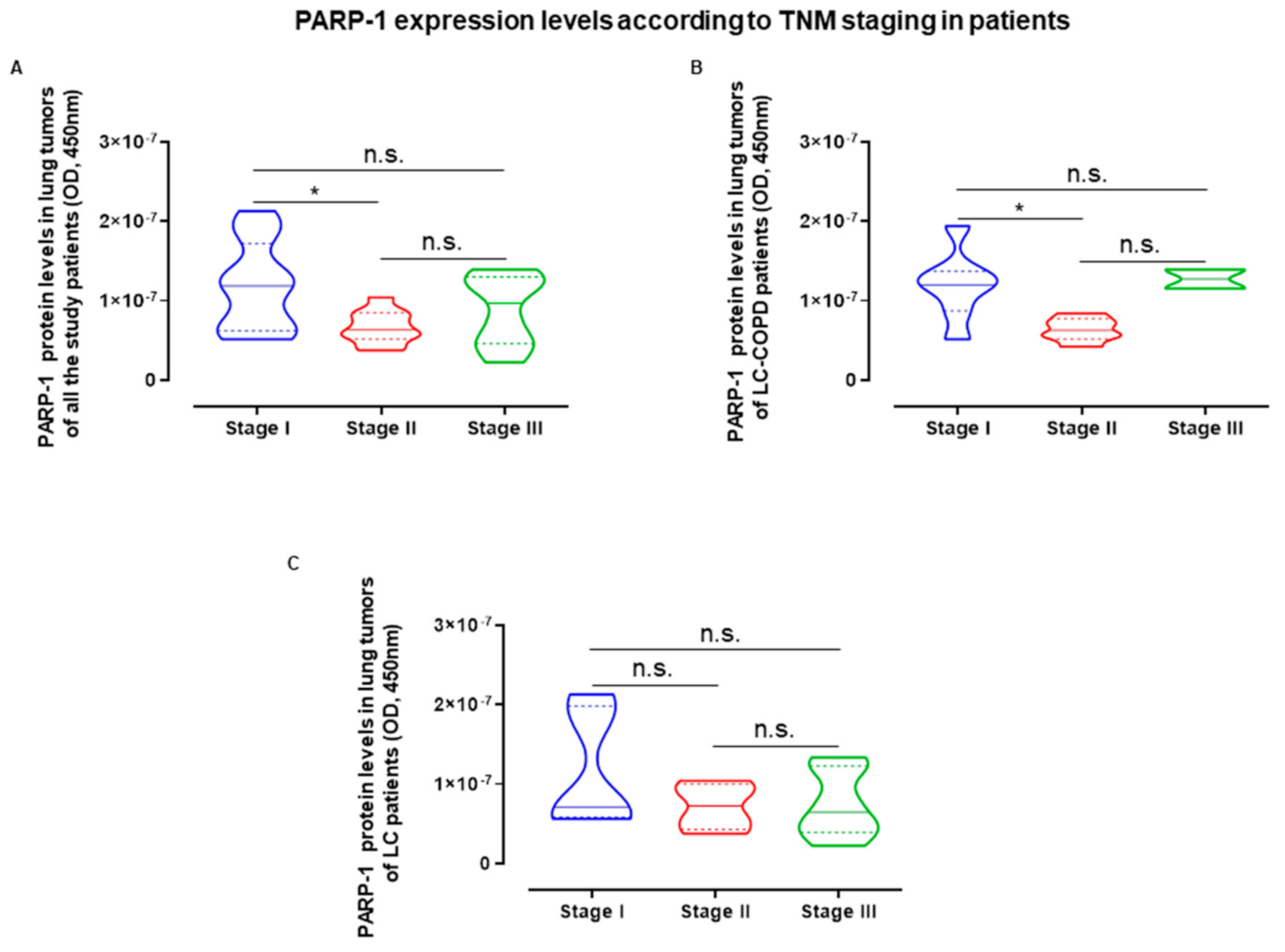
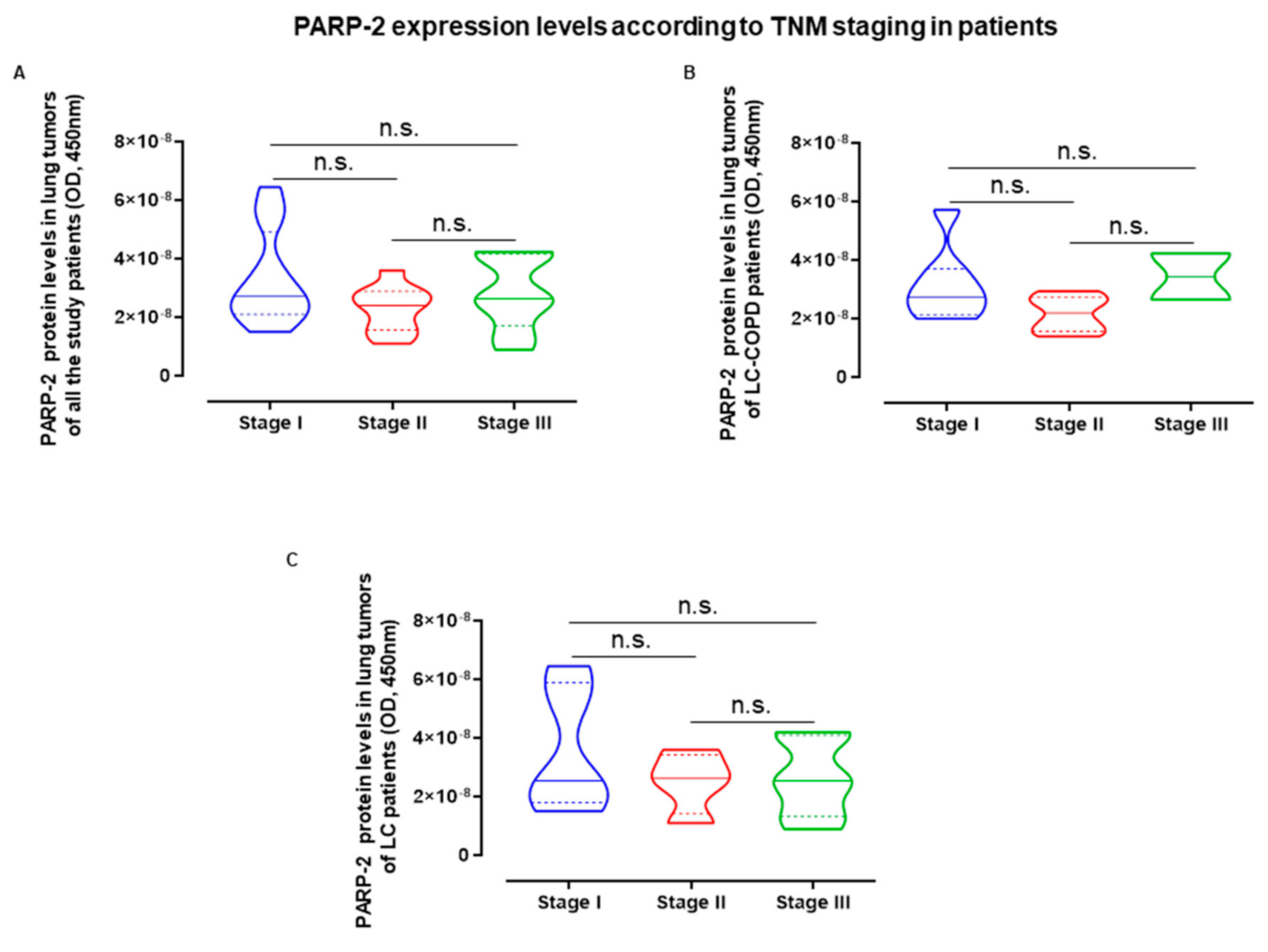
2.5. Influence of Staging in PARP Activity and Expression in LC and LC-COPD Patients
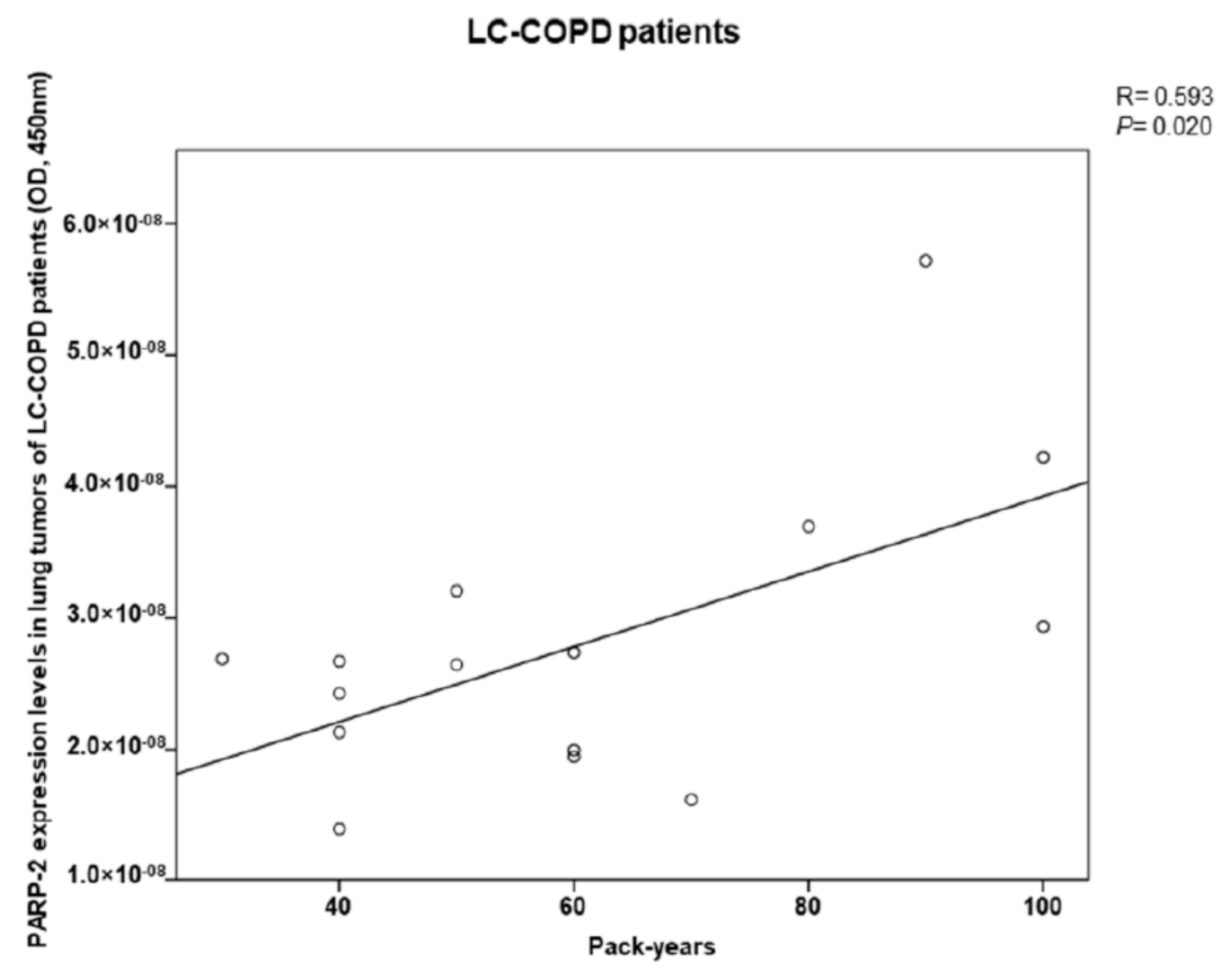
2.6. PARP-2 Expression in Lung Tumors of COPD Patients Correlates with Cigarette Smoking
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Study Design and Ethics
4.2. Clinical Assessment
4.3. Collection and Preservation of Samples
4.4. Molecular Biology Analysis
4.4.1. DNA Damage in Lungs of the Study Patients Using Immunohistochemistry
4.4.2. PARP Activity in Lungs of the Study Patients
4.4.3. PARP Expression in Human Lungs Using Enzyme Linked Immunosorbent Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de-Torres, J.P.; Wisnivesky, J.P.; Bastarrika, G.; Wilson, D.O.; Celli, B.R.; Zulueta, J.J. Exploring the Impact of Lung Cancer Screening on Lung Cancer Mortality of Smokers With Obstructive Lung Disease: Analysis of the NLST-ACRIN Cohort. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Cayuela, L.; López-Campos, J.L.; Otero, R.; Rodriguez Portal, J.A.; Rodríguez-Domínguez, S.; Cayuela, A. The Beginning of the Trend Change in Lung Cancer Mortality Trends in Spain, 1980–2018. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Clofent, D.; Culebras, M.; Loor, K.; Cruz, M.J. Environmental Pollution and Lung Cancer: The Carcinogenic Power of the Air We Breathe. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Fraile Olivero, C.A.; Pardina Solano, M.A.; Milla Collado, L. Intraoperatory Diagnosis of Partial Anomalous Pulmonary Venous Return During Pulmonary Resection Surgery in a Non-Small Cell Lung Cancer Patient. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef]
- Mesa-Guzmán, M.; González, J.; Alcaide, A.B.; Bertó, J.; de-Torres, J.P.; Campo, A.; Seijo, L.M.; Ocón, M.M.; Pueyo, J.C.; Bastarrika, G.; et al. Surgical Outcomes in a Lung Cancer-Screening Program Using Low Dose Computed Tomography. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Skillrud, D.M.; Offord, K.P.; Miller, D.W. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann. Intern. Med. 1986, 105, 503–507. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R.J.; Christmas, T.; Black, P.N.; Metcalf, P.; Gamble, G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2008, 34, 380–386. [Google Scholar] [CrossRef]
- Tang, J.; Ramis-Cabrer, D.; Curull, V.; Wang, X.; Qin, L.; Mateu-Jiménez, M.; Duran, X.; Pijuan, L.; Rodríguez-Fuster, A.; Espases, R.A.; et al. Immune cell subtypes and cytokines in lung tumor microenvironment: Influence of COPD. Cancers 2020, 12, 1217. [Google Scholar] [CrossRef]
- Tang, J.; Ramis-Cabrer, D.; Curull, V.; Wang, X.; Mateu-Jiménez, M.; Pijuan, L.; Duran, X.; Qin, L.; Rodríguez-Fuster, A.; Aguiló, R.; et al. B cells and tertiary lymphoid structures influence survival in lung cancer patients with resectable tumors. Cancers 2020, 12, 2644. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ramis-Cabrer, D.; Curull, V.; Mateu-Jiménez, M.; Almagro, K.; Duran, X.; Pijuan, L.; Rodríguez-Fuster, A.; Aguiló Espases, R.; Barreiro, E. Markers of Stroma in Lung Cancer: Influence of COPD. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Curull, V.; Ramis-Cabrer, D.; Duran, X.; Rodríguez-Fuster, A.; Aguiló, R.; Barreiro, E. Preoperative Body Weight and Albumin Predict Survival in Patients With Resectable Lung Neoplasms: Role of COPD. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dharwal, V.; Naura, A.S. PARP-1 inhibition ameliorates elastase induced lung inflammation and emphysema in mice. Biochem. Pharmacol. 2018, 150, 24–34. [Google Scholar] [CrossRef]
- Yelamos, J.; Farres, J.; Llacuna, L.; Ampurdanes, C.; Martin-Caballero, J. PARP-1 and PARP-2: New players in tumour development. Am. J. Cancer Res. 2011, 1, 328–346. [Google Scholar]
- Daniels, C.M.; Ong, S.E.; Leung, A.K.L. The Promise of Proteomics for the Study of ADP-Ribosylation. Mol. Cell 2015, 58, 911–924. [Google Scholar] [CrossRef]
- Alemasova, E.E.; Lavrik, O.I. Poly(ADP-ribosyl)ation by PARP1: Reaction mechanism and regulatory proteins. Nucleic Acids Res. 2019, 47, 3811–3827. [Google Scholar] [CrossRef]
- Bai, P. Biology of Poly(ADP-Ribose) Polymerases: The Factotums of Cell Maintenance. Mol. Cell 2015, 58, 947–958. [Google Scholar] [CrossRef]
- Césaire, M.; Thariat, J.; Candéias, S.M.; Stefan, D.; Saintigny, Y.; Chevalier, F. Combining PARP inhibition, radiation, and immunotherapy: A possible strategy to improve the treatment of cancer? Int. J. Mol. Sci. 2018, 19, 3793. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef]
- Weil, M.K.; Chen, A.P. PARP Inhibitor Treatment in Ovarian and Breast Cancer. Curr. Probl. Cancer 2011, 35, 7–50. [Google Scholar] [CrossRef]
- Lallo, A.; Frese, K.K.; Morrow, C.J.; Sloane, R.; Gulati, S.; Schenk, M.W.; Trapani, F.; Simms, N.; Galvin, M.; Brown, S.; et al. The combination of the PARP inhibitor olaparib and the WEE1 Inhibitor AZD1775 as a new therapeutic option for small cell lung cancer. Clin. Cancer Res. 2018, 24, 5153–5164. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Cantó, C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012, 16, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Amé, J.C.; Spenlehauer, C.; De Murcia, G. The PARP superfamily. BioEssays 2004, 26, 882–893. [Google Scholar] [CrossRef]
- Ha, H.C.; Snyder, S.H. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl. Acad. Sci. USA 1999, 96, 13978–13982. [Google Scholar] [CrossRef] [PubMed]
- Künzi, L.; Holt, G.E. Cigarette smoke activates the parthanatos pathway of cell death in human bronchial epithelial cells. Cell Death Discov. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Hageman, G.J.; Larik, I.; Pennings, H.J.; Haenen, G.R.M.M.; Wouters, E.F.M.; Bast, A. Systemic poly(ADP-ribose) polymerase-1 activation, chronic inflammation, and oxidative stress in COPD patients. Free Radic. Biol. Med. 2003, 35, 140–148. [Google Scholar] [CrossRef]
- Zaremba, T.; Ketzer, P.; Cole, M.; Coulthard, S.; Plummer, E.R.; Curtin, N.J. Poly(ADP-ribose) polymerase-1 polymorphisms, expression and activity in selected human tumour cell lines. Br. J. Cancer 2009, 101, 256–262. [Google Scholar] [CrossRef]
- Campelo, R.G.; Arrieta Rodriguez, O.G.; Massuti, B.; Rodriguez-Abreu, D.; Ortega Granados, A.L.; Majem, M.; Vicente, D.; Lianes, P.; Bosch-Barrera, J.; Insa, A.; et al. Combination of gefitinib and olaparib versus gefitinib alone in EGFR mutant non-small-cell lung cancer (NSCLC): A randomized phase 2 study (GOAL, Spanish Lung Cancer Group). J. Clin. Oncol. 2018, 36, 9012. [Google Scholar] [CrossRef]
- Slatore, C.G.; Horeweg, N.; Jett, J.R.; Midthun, D.E.; Powell, C.A.; Wiener, R.S.; Wisnivesky, J.P.; Gould, M.K. An Official American Thoracic Society research statement: A research framework for pulmonary nodule evaluation and management. Am. J. Respir. Crit. Care Med. 2015, 192, 500–514. [Google Scholar] [CrossRef]
- Kozower, B.D.; Larner, J.M.; Detterbeck, F.C.; Jones, D.R. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e369S–e399S. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Calle, M.; Soler-Cataluña, J.J. GesEPOC 2021: One More Step Towards Personalized Treatment of COPD. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Soler-Cataluña, J.J.; Novella, L.; Soler, C.; Nieto, M.L.; Esteban, V.; Sánchez-Toril, F.; Miravitlles, M. Clinical Characteristics and Risk of Exacerbations Associated With Different Diagnostic Criteria of Asthma-COPD Overlap. Arch. Bronconeumol. 2019. [Google Scholar] [CrossRef]
- Miravitlles, M.; Soler-Cataluña, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Trigueros, J.A.; Cosío, B.G.; Casanova, C.; Antonio Riesco, J.; et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological Treatment of Stable Phase. Arch. Bronconeumol. 2017, 53, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martínez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch. Bronconeumol. 2017, 53, 128–149. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. γh2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Barreiro, E.; Tang, J.; Ramis-Cabrer, D.; Wang, X. Immunotherapy with monoclonal antibodies in lung cancer of mice: Oxidative stress and other biological events. Cancers 2019, 11, 1301. [Google Scholar] [CrossRef]
- Navarro, J.; Gozalbo-López, B.; Méndez, A.C.; Dantzer, F.; Schreiber, V.; Martínez, C.; Arana, D.M.; Farrés, J.; Revilla-Nuin, B.; Bueno, M.F.; et al. PARP-1/PARP-2 double deficiency in mouse T cells results in faulty immune responses and T lymphomas. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]

| Anthropometric Variables | LC (N = 15) | LC-COPD (N = 15) |
|---|---|---|
| Age, years | 64 (11) | 67 (9) |
| Male, N/Female, N | 6/9 | 10/5 |
| BMI, kg/m2 | 27 (4) | 28 (7) |
| Smoking history | ||
| Current: N, % | 5, 33.3 | 6, 40 |
| Ex-smoker: N, % | 5, 33.3 | 9, 60 |
| Never smoker: N, % | 5, 33.3 | 0 * |
| Pack-years | 23 (23) | 61 (23) *** |
| Lung function parameters | ||
| FEV1 | 88 (23) | 70 (20) * |
| FEV1/FVC, % | 76 (5) | 58 (11) *** |
| DLCO, % | 85 (17) | 57 (13) *** |
| KCO, % | 83 (13) | 55 (10) *** |
| GOLD stage | ||
| GOLD stage I: N, % | NA | 5, 33.3 |
| GOLD stage II: N, % | NA | 8, 53.3 |
| GOLD stage III: N, % | NA | 2, 13.3 |
| GOLD stage IV: N, % | NA | 0, 0 |
| TNM staging | ||
| Stage I: N, % | 5, 33.3 | 7, 46.7 |
| Stage II: N, % | 4, 26.7 | 6, 40 |
| Stage III: N, % | 6, 40 | 2, 13.3 |
| Histological diagnosis | ||
| Squamous cell carcinoma: N, % | 0, 0 | 0, 0 |
| Adenocarcinoma: N, % | 15, 100 | 15, 100 |
| Others: N, % | 0, 0 | 0, 0 |
| Blood parameters | ||
| Total leucocytes/μL | 9.3 (3.5) × 103 | 10.1 (4.4) × 103 |
| Total neutrophils/μL | 6.8 (3.9) × 103 | 7.5 (4.7) × 103 |
| Total lymphocytes/μL | 1.8 (0.8) × 103 | 1.8 (0.9) × 103 |
| Albumin (g/dL) | 4.3 (0.4) | 4.4 (0.5) |
| Total proteins (g/dL) | 6.9 (0.4) | 7.1 (0.6) |
| Body weight loss, kg | ||
| 0, N, % | 13, 86.6 | 12, 80 |
| 1–5, N, % | 1,6.7 | 1, 6.7 |
| 6–10, N, % | 1,6,7 | 2, 13.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Curull, V.; Wang, X.; Ampurdanés, C.; Duran, X.; Pijuan, L.; Rodríguez-Fuster, A.; Aguiló, R.; Yélamos, J.; Barreiro, E. Increased PARP Activity and DNA Damage in NSCLC Patients: The Influence of COPD. Cancers 2020, 12, 3333. https://doi.org/10.3390/cancers12113333
Tang J, Curull V, Wang X, Ampurdanés C, Duran X, Pijuan L, Rodríguez-Fuster A, Aguiló R, Yélamos J, Barreiro E. Increased PARP Activity and DNA Damage in NSCLC Patients: The Influence of COPD. Cancers. 2020; 12(11):3333. https://doi.org/10.3390/cancers12113333
Chicago/Turabian StyleTang, Jun, Víctor Curull, Xuejie Wang, Coral Ampurdanés, Xavier Duran, Lara Pijuan, Alberto Rodríguez-Fuster, Rafael Aguiló, José Yélamos, and Esther Barreiro. 2020. "Increased PARP Activity and DNA Damage in NSCLC Patients: The Influence of COPD" Cancers 12, no. 11: 3333. https://doi.org/10.3390/cancers12113333
APA StyleTang, J., Curull, V., Wang, X., Ampurdanés, C., Duran, X., Pijuan, L., Rodríguez-Fuster, A., Aguiló, R., Yélamos, J., & Barreiro, E. (2020). Increased PARP Activity and DNA Damage in NSCLC Patients: The Influence of COPD. Cancers, 12(11), 3333. https://doi.org/10.3390/cancers12113333