The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment
Simple Summary
Abstract
1. Introduction
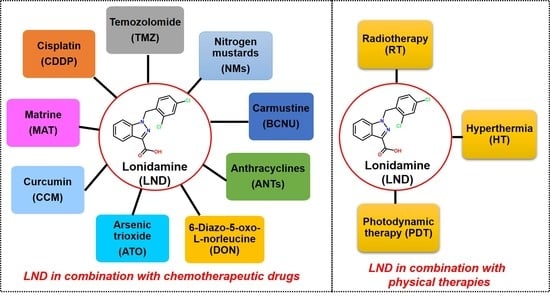
2. Combination of LND with Chemotherapeutic Drugs
2.1. LND in Combination with Cisplatin
2.2. LND in Combination with Temozolomide
2.3. LND in Combination with Nitrogen Mustards
2.4. LND in Combination with Carmustine
2.5. LND in Combination with Anthracyclines
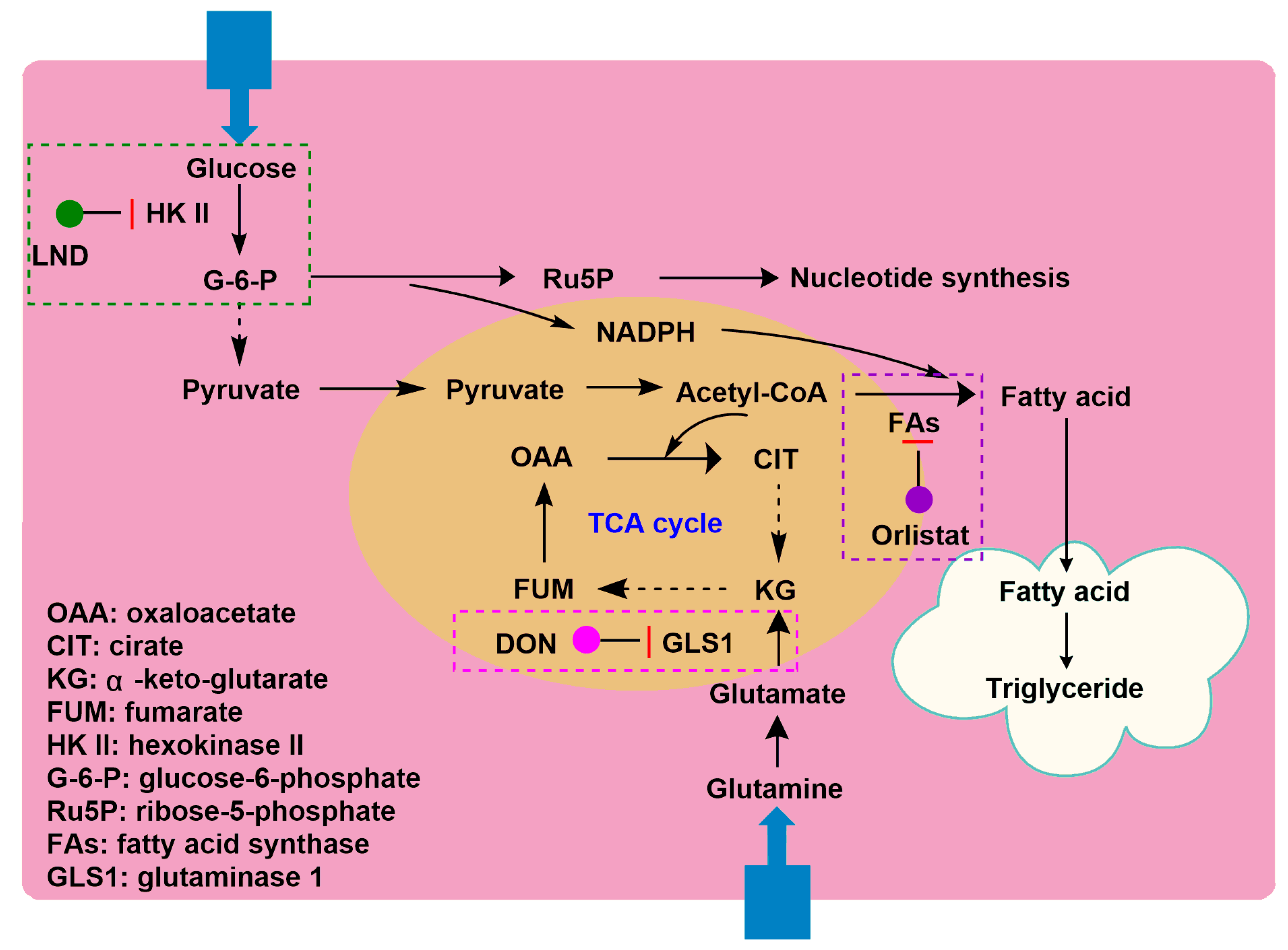
2.6. LND in Combination with 6-Diazo-5-oxo-l-norleucine
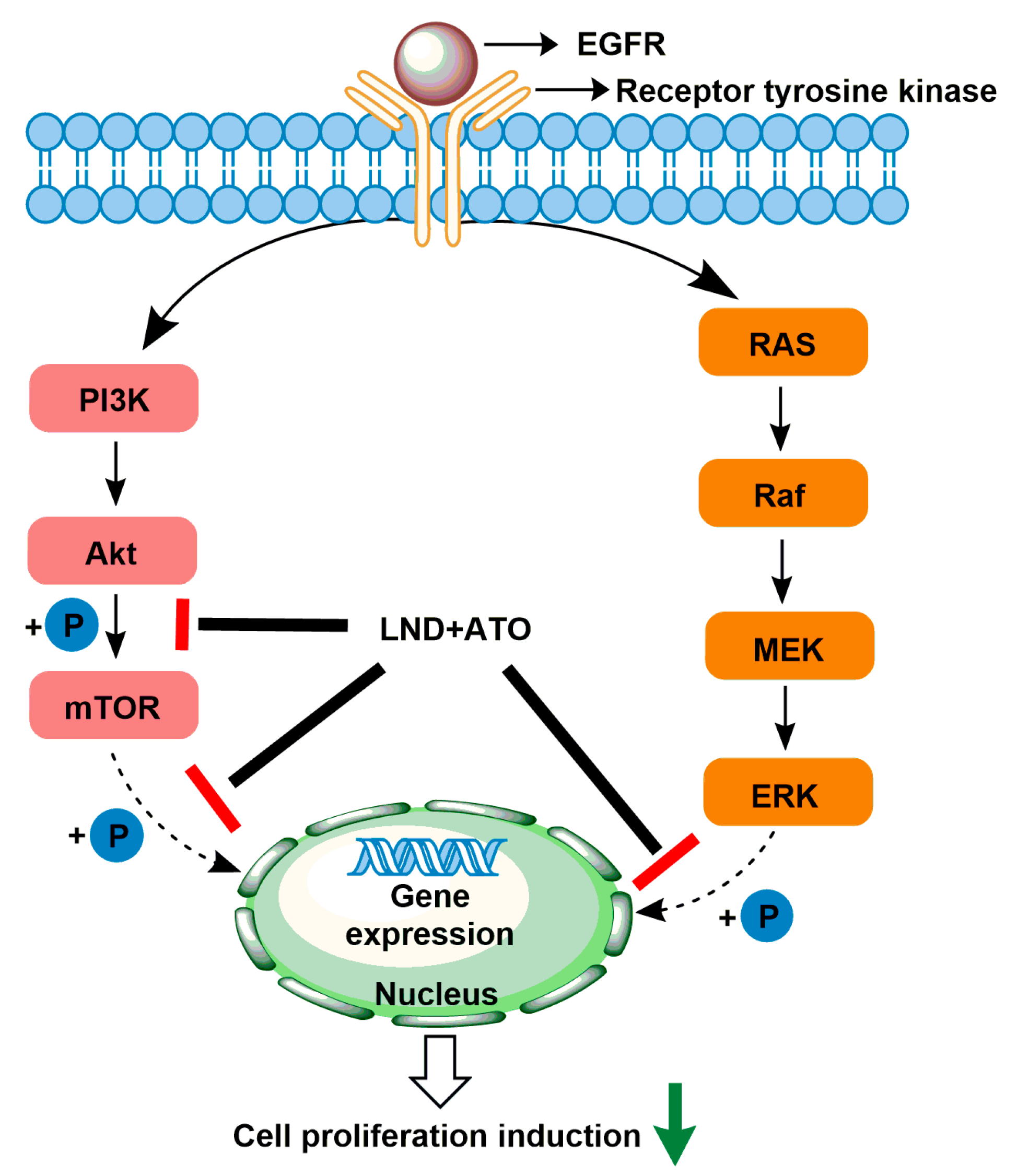
2.7. LND in Combination with Arsenic Trioxide
2.8. LND in Combination with Curcumin
2.9. LND in Combination with Matrine
3. LND Sensitizes Tumor Cells to Physical Therapy
3.1. LND Sensitizes Tumor Cells to Radiotherapy
3.2. LND Sensitizes Tumor Cells to Hyperthermia
3.3. LND Sensitizes Tumor Cells to Photodynamic Therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cioli, V.; Bellocci, B.; Putzolu, S.; Malorni, W.; Demartino, C. Anti-spermogenic activity of lonidamine (AF-1890) in rabbit. Ultramicroscopy 1980, 5, 418. [Google Scholar]
- Nath, K.; Guo, L.; Nancolas, B.; Nelson, D.S.; Shestov, A.A.; Lee, S.-C.; Roman, J.; Zhou, R.; Leeper, D.B.; Halestrap, A.P.; et al. Mechanism of antineoplastic activity of lonidamine. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1866, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shestov, A.A.; Worth, A.J.; Nath, K.; Nelson, D.S.; Leeper, D.B.; Glickson, J.D.; Blair, I.A. Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J. Biol. Chem. 2016, 291, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Ravagnan, L.; Marzo, I.; Costantini, P.; Susin, S.A.; Zamzami, N.; Petit, P.X.; Hirsch, F.; Goulbern, M.; Poupon, M.-F.; Miccoli, L.; et al. Lonidamine triggers apoptosis via a direct, Bcl-2-inhibited effect on the mitochondrial permeability transition pore. Oncogene 1999, 18, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Floridi, A.; Bellocci, M.; Paggi, M.G.; Marcante, M.L.; De Martino, C. Changes of energy metabolism in the germ cells and Ehrlich sscites tumor cells. Chemotherapy 1981, 27, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Davidescu, M.; Macchioni, L.; Scaramozzino, G.; Marchetti, M.C.; Migliorati, G.; Vitale, R.; Corcelli, A.; Roberti, R.; Castigli, E.; Corazzi, L. The energy blockers bromopyruvate and lonidamine lead GL15 glioblastoma cells to death by different p53-dependent routes. Sci. Rep. 2015, 5, 14343. [Google Scholar] [CrossRef]
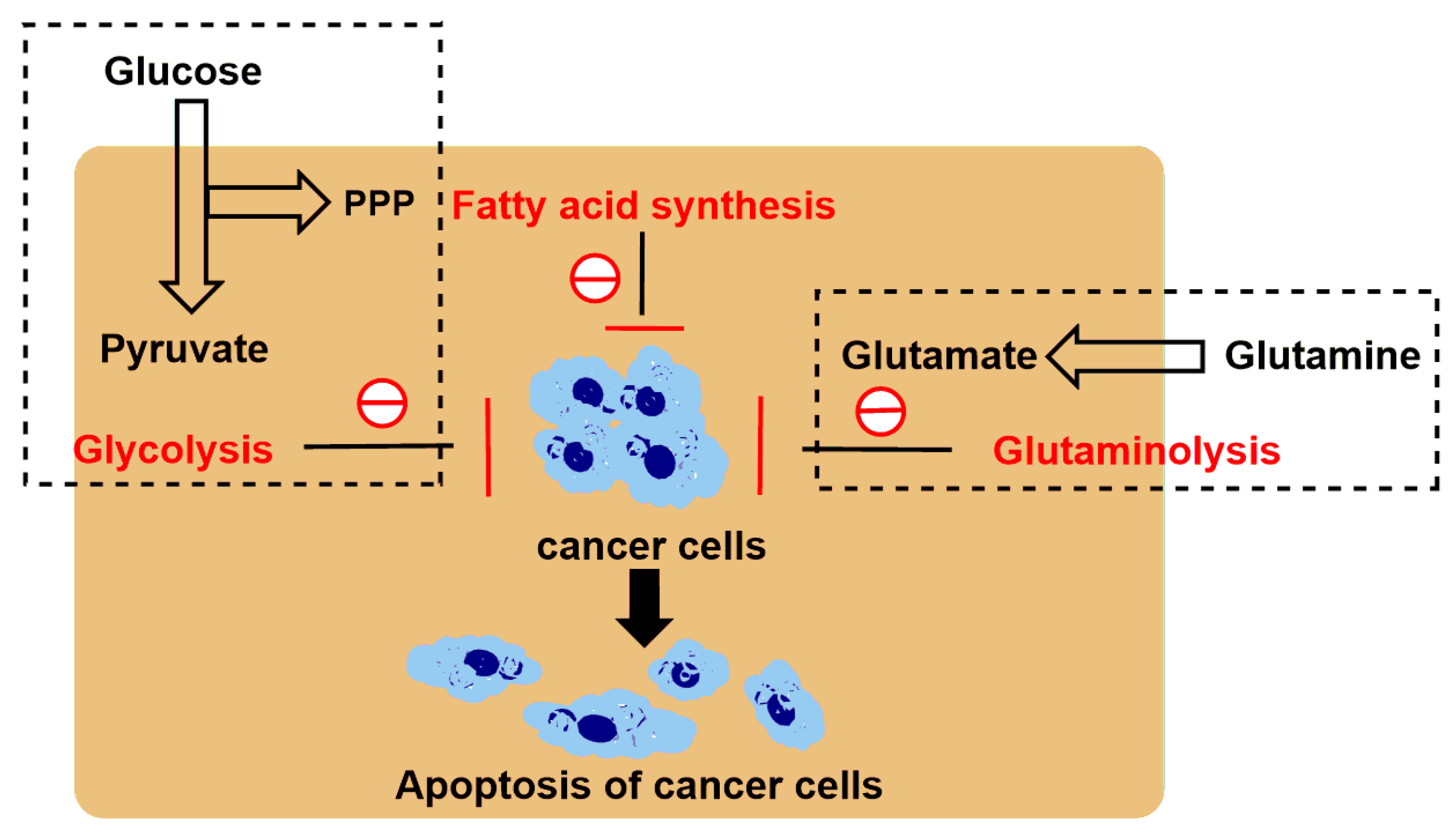
- Cervantes-Madrid, D.; Duenas-Gonzalez, A. Antitumor effects of a drug combination targeting glycolysis, glutaminolysis and de novo synthesis of fatty acids. Oncol. Rep. 2015, 34, 1533–1542. [Google Scholar] [CrossRef]
- Cervantes-Madrid, D.; Domínguez-Gómez, G.; Gonzalez-Fierro, A.; Pérez-Cárdenas, E.; Taja-Chayeb, L.; Trejo-Becerril, C.; Duenas-Gonzalez, A. Feasibility and antitumor efficacy in vivo, of simultaneously targeting glycolysis, glutaminolysis and fatty acid synthesis using lonidamine, 6-diazo-5-oxo-L-norleucine and orlistat in colon cancer. Oncol. Lett. 2017, 13, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yoseph, O.; Lyons, J.C.; Song, C.W.; Ross, B.D. Mechanism of action of lonidamine in the 9L brain tumor model involves inhibition of lactate efflux and intracellular acidification. J. Neurooncol. 1998, 36, 149–157. [Google Scholar] [CrossRef]
- Nath, K.; Nelson, D.S.; Heitjan, D.F.; Zhou, R.; Leeper, D.B.; Glickson, J.D. Effects of hyperglycemia on lonidamine-induced acidification and de-energization of human melanoma xenografts and sensitization to melphalan. NMR Biomed. 2015, 28, 395–403. [Google Scholar] [CrossRef]
- Scarantino, C.W.; Mccunniff, A.J.; Evans, G.; Young, C.W.; Paggiarino, D.A. A prospective randomized comparison of radiation therapy plus lonidamine versus radiation therapy plus placebo as initial treatment of clinically localized but nonresectable non-small cell lung cancer. Int. J. Radiat. Oncol. 1994, 29, 999–1004. [Google Scholar] [CrossRef]
- Pacini, P.; Rinaldini, M.; Algeri, R.; Guarneri, A.; Tucci, E.; Barsanti, G.; Neri, B.; Bastiani, P.; Marzano, S.; Fallai, C. FEC (5-fluorouracil, epidoxorubicin and cyclophosphamide) versus EM (epidoxorubicin and mitomycin-C) with or without lonidamine as first-line treatment for advanced breast cancer. A multicentric randomised study. Final results. Eur. J. Cancer 2000, 36, 966–975. [Google Scholar] [CrossRef]
- Gourdier, I.; Del Rio, M.; Crabbé, L.; Candeil, L.; Copois, V.; Ychou, M.; Auffray, C.; Martineau, P.; Mechti, N.; Pommier, Y.; et al. Drug specific resistance to oxaliplatin is associated with apoptosis defect in a cellular model of colon carcinoma. FEBS Lett. 2002, 529, 232–236. [Google Scholar] [CrossRef]
- Paggi, M.G.; Zupi, G.; Fanciulli, M.; Del Carlo, C.; Giorno, S.; Laudonio, N.; Silvestrini, B.; Caputo, A.; Floridi, A. Effect of lonidamine on the utilization of 14C-labeled glucose by human astrocytoma cells. Exp. Mol. Pathol. 1987, 47, 154–165. [Google Scholar] [CrossRef]
- Raaphorst, G.P.; Feeley, M.M.; Martin, L.; Danjoux, C.E.; Maroun, J.; DeSanctis, A.J.; Ko, D. Enhancement of sensitivity to hyperthermia by Lonidamine in human cancer cells. Int. J. Hyperth. 1991, 7, 763–772. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Silvestrini, B.; Grima, J.; Mo, M.-Y.; Zhu, L.-J.; Johansson, E.; Saso, L.; Leone, M.-G.; Palmery, M.; Mruk, D. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis1. Biol. Reprod. 2001, 65, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.R.; Liu, Y.; Shao, J.; Yang, J.; Liu, T.; Zhang, T.; Wang, B.; Mruk, D.D.; Silvestrini, B.; Cheng, C.Y.; et al. Male contraceptive Adjudin is a potential anti-cancer drug. Biochem. Pharmacol. 2013, 85, 345–355. [Google Scholar] [CrossRef]
- Yang, C.L.; Tu, K.; Gao, H.L.; Zhang, L.; Sun, Y.; Yang, T.; Kong, L.; Ouyang, D.; Zhang, Z.P. The novel platinum (IV) prodrug with self-assembly property and structure-transformable character against triple-negative breast cancer. Biomaterials 2020, 232, 119751. [Google Scholar] [CrossRef]
- Li, X.; Gao, C.X.; Wu, Y.P.; Cheng, C.-Y.; Xia, W.L.; Zhang, Z.P. Combination delivery of Adjudin and Doxorubicin via integrating drug conjugation and nanocarrier approaches for the treatment of drug-resistant cancer cells. J. Mater. Chem. B 2015, 3, 1556–1564. [Google Scholar] [CrossRef]
- Forster, R.; Campana, A.; D’Onofrio, E.; Henderson, L.; Mosesso, P.; Barcellona, P.S. Lonidamine: A non-mutagenic antitumor agent. Carcinogenesis 1990, 11, 1509–1515. [Google Scholar] [CrossRef]
- Mansi, J.L.; De Graeff, A.; Newell, D.R.; Glaholm, J.; Button, D.; Leach, M.O.; Payne, G.; Smith, I.E. A phase II clinical and pharmacokinetic study of Lonidamine in patients with advanced breast cancer. Br. J. Cancer 1991, 64, 593–597. [Google Scholar] [CrossRef]
- Band, P.R.; Deschamps, M.; Besner, J.-G.; LeClaire, R.; Gervais, P.; De Sanctis, A. Phase I toxicologic study of lonidamine in cancer patients. Oncology 1984, 41, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Band, P.R.; Maroun, J.; Pritchard, K.; Stewart, D.; Coppin, C.M.; Wilson, K.; Eisenhauer, E. Phase II study of lonidamine in patients with metastatic breast cancer: A National Cancer Institute of Canada Clinical Trials Group Study. Cancer Treat. Rep. 1986, 70, 1305–1310. [Google Scholar] [PubMed]
- Price, G.S.; Page, R.L.; Riviere, J.E.; Cline, J.M.; Thrall, D.E. Pharmacokinetics and toxicity of oral and intravenous lonidamine in dogs. Cancer Chemother. Pharmacol. 1996, 38, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, C.-X.; Wang, X.-X.; Zhang, L.; Ma, X.; Zhou, J.; Ju, R.-J.; Li, X.-Y.; Zhao, W.-Y.; Lu, W.-L. Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signaling pathways. Biomaterials 2013, 34, 3366–3380. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, J.; Tang, X.; Zhai, Z.; Xu, K.; Zhong, W. An enzyme-assisted self-delivery system of lonidamine–peptide conjugates for selectively killing cancer cells. Chem. Commun. 2019, 55, 14852–14855. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xia, L.; Liang, J.; Han, Y.; Wang, H.; Oyang, L.; Tan, S.; Tian, Y.; Rao, S.; Chen, X.; et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, Q.; Pan, J.; Lee, Y.; Ouari, O.; Hardy, M.; Zielonka, M.; Myers, C.R.; Zielonka, J.; Weh, K.; et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Milane, L.; Duan, Z.; Amiji, M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol. Pharm. 2011, 8, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.H.; Fan, T.J.; Zhao, L.J.; Zhou, Y.; Zhong, R.G. The potential of combi-molecules with DNA-damaging function as anticancer agents. Future Med. Chem. 2017, 9, 403–435. [Google Scholar] [CrossRef]
- Lin, X.W.; Zhang, Y.H. The progress of the platinum antitumor drugs and clinical evaluation. Eval. Anal. Drug Use Hosp. China 2011, 11, 4–7. [Google Scholar]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Herman, T.S.; Holden, A.S.; Epelbaum, R.; Liu, S.D.; Frei, E. Lonidamine as a modulator of alkylating agent activity in vitro and in vivo. Cancer Res. 1991, 51, 780–784. [Google Scholar] [PubMed]
- De Cesare, M.; Pratesi, G.; Giusti, A.; Polizzi, D.; Zunino, F. Stimulation of the apoptotic response as a basis for the therapeutic synergism of lonidamine and cisplatin in combination in human tumour xenografts. Br. J. Cancer 1998, 77, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.C.; Hahn, G.M. Combination therapy: Lonidamine, hyperthermia, and chemotherapy against the RIF-1 tumor in vivo. Cancer Res. 1991, 51, 5910–5914. [Google Scholar] [PubMed]
- Yu, C.; Sun, X.H.; Zhang, Y.; Zhao, J.B.; Liu, F.; Li, X.H.; Wang, Y. LND and NP regimen treament for advanced non-small cell lung cancer. Chin. Clin. Oncol. 2010, 37, 763–766. [Google Scholar] [CrossRef]
- Kopecký, J.; Priester, P.; Slováček, L.; Petera, J.; Kopecký, O.; Macingova, Z. Aplastic anemia as a cause of death in a patient with glioblastoma multiforme treated with temozolomide. Strahlenther. Onkol. 2010, 186, 452–457. [Google Scholar] [CrossRef]
- Denny, B.J.; Wheelhouse, R.T.; Stevens, M.F.G.; Tsang, L.L.H.; Slack, J.A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry 1994, 33, 9045–9051. [Google Scholar] [CrossRef]
- Nath, K.; Nelson, D.S.; Roman, J.; Putt, M.E.; Lee, S.-C.; Leeper, D.B.; Glickson, J.D. Effect of lonidamine on systemic therapy of DB-1 human melanoma xenografts with temozolomide. Anticancer. Res. 2017, 37, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Rajski, S.R.; Williams, R.M. DNA cross-linking agents as antitumor drugs. Chem. Rev. 1998, 98, 2723–2796. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Yunes, J.A.; Gillies, R.J.; Gatenby, R.A. The potential pole of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res. 2009, 69, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Nelson, D.S.; Putt, M.E.; Leeper, D.B.; Garman, B.; Nathanson, K.L.; Glickson, J.D. Comparison of the lonidamine potentiated effect of nitrogen mustard alkylating agents on the systemic treatment of DB-1 human melanoma xenografts in mice. PLoS ONE 2016, 11, e0157125. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Nelson, D.S.; Ho, A.M.; Lee, S.-C.; Darpolor, M.M.; Pickup, S.; Zhou, R.; Heitjan, D.F.; Leeper, D.B.; Glickson, J.D. 31P and 1H MRS of DB-1 melanoma xenografts: Lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2012, 26, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mardor, Y.; Kaplan, O.; Sterin, M.; Ruiz-Cabello, J.; Ash, E.; Roth, Y.; Ringel, I.; Cohen, J.S. Noninvasive real-time monitoring of intracellular cancer cell metabolism and response to lonidamine treatment using diffusion weighted proton magnetic resonance spectroscopy. Cancer Res. 2000, 60, 5179–5186. [Google Scholar]
- Kuin, A.; Aalders, M.; Lamfers, M.; Van Zuidam, D.J.; Essers, M.; Beijnen, J.H.; Smets, A.L. Potentiation of anti-cancer drug activity at low intratumoral pH induced by the mitochondrial inhibitor m-iodobenzylguanidine (MIBG) and its analogue benzylguanidine (BG). Br. J. Cancer 1999, 79, 793–801. [Google Scholar] [CrossRef]
- Wong, P.; Lee, C.; Tannock, I.F. Reduction of Intracellular pH as a strategy to enhance the pH-dependent cytotoxic effects of melphalan for human breast cancer cells. Clin. Cancer Res. 2005, 11, 3553–3557. [Google Scholar] [CrossRef]
- Smith, C.V. Effect of BCNU pretreatment on diquat-induced oxidant stress and hepatotoxicity. Biochem. Biophys. Res. Commun. 1987, 144, 415–421. [Google Scholar] [CrossRef]
- Smith, A.C.; Boyd, M.R. Preferential effects of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) on pulmonary glutathione reductase and glutathione/glutathione disulfide ratios: Possible implications for lung toxicity. J. Pharmacol. Exp. Ther. 1984, 229, 658–663. [Google Scholar]
- Sun, G.H.; Zhao, L.J.; Zhong, R.G.; Peng, Y.Z. The specific role of O6-methylguanine-DNA methyltransferase inhibitors in cancer chemotherapy. Future Med. Chem. 2018, 10, 1971–1996. [Google Scholar] [CrossRef]
- Ning, S.C.; Hahn, G.M. Cytotoxicity of lonidamine alone and in combination with other drugs against murine RIF-1 and human HT1080 cells in vitro. Cancer Res. 1990, 50, 7867–7870. [Google Scholar]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Savini, S.; Zoli, W.; Nanni, O.; Volpi, A.; Frassineti, G.L.; Magni, E.; Flamigni, A.; Amadori, A.; Amadori, D. In vitro potentiation by lonidamine of the cytotoxic effect of adriamycin on primary and established breast cancer cell lines. Breast Cancer Res. Treat. 1992, 24, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Floridi, A.; Bruno, T.; Miccadei, S.; Fanciulli, M.; Federico, A.; Paggi, M.G. Enhancement of doxorubicin content by the antitumor drug lonidamine in resistant ehrlich ascites tumor cells through modulation of energy metabolism. Biochem. Pharmacol. 1998, 56, 841–849. [Google Scholar] [CrossRef]
- Nath, K.; Nelson, D.S.; Heitjan, D.F.; Leeper, D.B.; Zhou, R.; Glickson, J.D. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015, 28, 281–290. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Vijayappa, S.; Kozin, S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol. Cancer Ther. 2006, 5, 1275–1279. [Google Scholar] [CrossRef]
- Nath, K.; Roman, J.; Nelson, D.S.; Guo, L.; Lee, S.-C.; Orlovskiy, S.; Muriuki, K.; Heitjan, D.F.; Pickup, S.; Leeper, D.B.; et al. Effect of differences in metabolic activity of melanoma models on response to lonidamine plus doxorubicin. Sci. Rep. 2018, 8, 14654. [Google Scholar] [CrossRef]
- Nisticò, C.; Garufi, C.; Milella, M.; D’Ottavio, A.M.; Vaccaro, A.; Fabi, A.; Terzoli, E. Weekly epirubicin plus lonidamine in advanced breast carcinoma. Breast Cancer Res. Treat. 1999, 56, 231–235. [Google Scholar] [CrossRef]
- Cervantes-Madrid, D.; Romero, Y.; Dueñas-González, A. Reviving lonidamine and 6-diazo-5-oxo-L-norleucine to be used in combination for metabolic cancer therapy. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Kisner, D.L.; Catane, R.; Muggia, F.M. The rediscovery of DON (6-diazo-5-oxo-L-norleucine). Recent Results Cancer Res 1980, 74, 258–263. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Li, J.; Li, X.; Hou, J.; Zhao, Y.; Liu, X.; Han, X.; Hu, L.; Wang, S.; et al. Single-agent arsenic trioxide in the treatment of children with newly diagnosed acute promyelocytic leukemia. Blood 2010, 115, 1697–1702. [Google Scholar] [CrossRef]
- Miller, W.H.; Schipper, H.M.; Lee, J.S.; Singer, J.; Waxman, S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002, 62, 3893–3903. [Google Scholar] [PubMed]
- Miller, W.H. Molecular targets of arsenic trioxide in malignant cells. Oncologist 2002, 7, 14–19. [Google Scholar] [CrossRef]
- Sordet, O.; Rebe, C.; Leroy, I.; Bruey, J.-M.; Garrido, C.; Miguet, C.; Lizard, G.; Plenchette, S.; Corcos, L.; Solary, E. Mitochondria-targeting drugs arsenic trioxide and lonidamine bypass the resistance of TPA-differentiated leukemic cells to apoptosis. Blood 2001, 97, 3931–3940. [Google Scholar] [CrossRef]
- Calviño, E.; Estañ, M.C.; Simón, G.P.; Sancho, P.; Boyano-Adánez, M.D.C.; De Blas, E.; Breard, J.; Aller, P. Increased apoptotic efficacy of lonidamine plus arsenic trioxide combination in human leukemia cells. Reactive oxygen species generation and defensive protein kinase (MEK/ERK, Akt/mTOR) modulation. Biochem. Pharmacol. 2011, 82, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.; Scott, E.N.; Berry, D.P.; et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration—A clinical pilot study including assessment of patient acceptability. Cancer Prev. Res. 2012, 6, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Eifes, S.; Dicato, M.; Aggarwal, B.B.; Han, B.W. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem. Pharmacol. 2008, 76, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Y.; Simón, G.P.; Calviño, E.; De Blas, E.; Aller, P. Curcumin stimulates reactive oxygen species production and potentiates apoptosis induction by the antitumor drugs arsenic trioxide and lonidamine in human myeloid leukemia cell lines. J. Pharmacol. Exp. Ther. 2010, 335, 114–123. [Google Scholar] [CrossRef]
- Guo, L.; Xue, T.Y.; Xu, W.; Gao, J.Z. Matrine promotes G0/G1 arrest and down-regulates cyclin D1 expression in human rhabdomyosarcoma cells. Panminerva. Med. 2013, 55, 291–296. [Google Scholar]
- Lin, G.B.; Wu, Y.Z.; Cai, F.T.; Li, Z.; Su, S.X.; Wang, J.; Cao, J.L.; Ma, L.D. Matrine promotes human myeloid leukemia cells apoptosis through warburg effect mediated by hexokinase 2. Front. Pharmacol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt (II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Thiabaud, G.; McCall, R.; He, G.; Arambula, J.F.; Siddik, Z.H.; Sessler, J.L. Activation of platinum (IV) prodrugs by motexafin gadolinium as a redox mediator. Angew. Chem. Int. Ed. Engl. 2016, 55, 12626–12631. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, F.H.; Hu, W.W.; Gou, S.H. Effective platinum (IV) prodrugs conjugated with lonidamine as a functional group working on the mitochondria. J. Inorg. Biochem. 2018, 180, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Stavrovskaya, A.A.; Shushanov, S.S.; Rybalkina, E. Problems of glioblastoma multiforme drug resistance. Biochemistry 2016, 81, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.H.; Zhao, L.J.; Zhong, R.G. The induction and repair of DNA interstrand crosslinks and implications in cancer chemotherapy. Anticancer Agents Med. Chem. 2016, 16, 221–246. [Google Scholar]
- Gatenby, R.A.; Gillies, R.J. A microenvironmental model of carcinogenesis. Nat. Rev. Cancer 2008, 8, 56–61. [Google Scholar] [CrossRef]
- Sun, X.D.; Sun, G.H.; Huang, Y.X.; Hao, Y.X.; Tang, X.Y.; Zhang, N.; Zhao, L.J.; Zhong, R.G.; Peng, Y.Z. 3-Bromopyruvate regulates the status of glycolysis and BCNU sensitivity in human hepatocellular carcinoma cells. Biochem. Pharmacol. 2020, 177, 113988. [Google Scholar] [CrossRef]
- Sun, X.D.; Sun, G.H.; Huang, Y.X.; Zhang, S.F.; Tang, X.Y.; Zhang, N.; Zhao, L.J.; Zhong, R.G.; Peng, Y.Z. Glycolytic inhibition by 3-bromopyruvate increases the cytotoxic effects of chloroethylnitrosoureas to human glioma cells and the DNA interstrand cross-links formation. Toxicology 2020, 435, 152413. [Google Scholar] [CrossRef]
- Kaina, B.; Christmann, M. DNA repair in personalized brain cancer therapy with temozolomide and nitrosoureas. DNA Repair 2019, 78, 128–141. [Google Scholar] [CrossRef]
- Aghaabbasi, K.; Askari, N.; Kumleh, H.H.; Torkzadeh-Mahani, M.; Ramzani-Ghara, A. The Blepharis persica seed hydroalcoholic extract synergistically enhances the apoptotic effect of doxorubicin in human colon cancer and gastric cancer cells. Mol. Biol. Rep. 2019, 47, 843–853. [Google Scholar] [CrossRef]
- Nong, Q.; Zhang, C.; Liu, Q.; Xie, R.; Dong, M. Effect of daunorubicin on acute promyelocytic leukemia cells using nuclear magnetic resonance spectroscopy-based metabolomics. Environ. Toxicol. Pharmacol. 2020, 78, 103382. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, J.; Zhang, Z.; Lv, H. Doxorubicin combined with betulinic acid or lonidamine in RGD ligand-targeted pH-sensitive micellar system for ovarian cancer treatment. Int. J. Pharm. 2019, 571, 118751. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Qin, X.Y.; Yang, C.L.; Wu, T.T.; Qiao, Q.; Song, Q.L.; Zhang, Z.P. Micelle system based on molecular economy principle for overcoming multidrug resistance and inhibiting metastasis. Mol. Pharm. 2018, 15, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zou, C.M.; Wang, L.Y.; Gao, X.Q.; Wu, J.D.; Tan, S.W.; Wu, G. Doxorubicin and adjudin co-loaded pH-sensitive nanoparticles for the treatment of drug-resistant cancer. Acta Biomater. 2019, 94, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Heiden, M.G.V.; Kroemer, G. Metabolic targets for cancer therapy. Nat. Rev. Drug Discov. 2013, 12, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Vander, H.; Matthew, G. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug. Discov. 2011, 10, 671–684. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Gaglio, D.; Metallo, C.M.; Gameiro, A.P.; Hiller, K.; Danna, L.S.; Balestrieri, C.; Alberghina, L.; Stephanopoulos, G.; Chiaradonna, F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 2011, 7, 523. [Google Scholar] [CrossRef]
- Tarnowski, G.S.; Stock, C.C. Effects of combinations of azaserine and of 6-diazo-5-oxo-L-norleucine with purine analogs and other antimetabolites on the growth of two mouse mammary carcinomas. Cancer Res. 1957, 17, 1033–1039. [Google Scholar]
- Griffiths, M.; Keast, D.; Crawford, M.; Palmer, T.; Patrick, G. The role of glutamine and glucose analogues in metabolic inhibition of human myeloid leukaemia In Vitro. Int. J. Biochem. 1993, 25, 1749–1755. [Google Scholar] [CrossRef]
- Kridel, S.J.; Houghton, P.J.; Germain, G.S.; Harwood, F.C.; Schuetz, J.D.; Stewart, C.F.; Buchdunger, E.; Traxler, P. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004, 64, 2070–2075. [Google Scholar] [CrossRef]
- Lin, J.-K. Molecular targets of curcumin. Biol. Mammary Gland 2007, 595, 227–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Yu, P.; Liu, Q.; Liu, K.; Duan, H.; Luan, G.; Yagasaki, K.; Zhang, G. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology 2009, 59, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhong, H.J.; Zhu, L.M.; Wu, X.G.; Ying, J.E.; Wang, X.H.; Lü, W.X.; Xu, Q.; Zhu, Y.L.; Huang, J. Inhibition of matrine against gastric cancer cell line MNK45 growth and its anti-tumor mechanism. Mol. Biol. Rep. 2011, 39, 5459–5464. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, Z.; Jiang, L.; Sun, X.; Jianyong, L. Matrine suppresses cell growth of human chronic myeloid leukemia cells via its inhibition of the IL-6/JAK/STAT3 signaling cohort. Leuk. Lymphoma 2015, 56, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, D.; Chen, X.; He, L.; Li, T.; Xu, X.; Li, M. Nuclear PKM2 contributes to gefitinib resistance via upregulation of STAT3 activation in colorectal cancer. Sci. Rep. 2015, 5, 16082. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, L.B.; Zhan, Q.; Gong, S.L.; Dong, L.H. Research progress on the relationship between tumor microenvironment and radiosensitivity of tumor cells. J. Jilin. Univ. Med. Edit. 2016, 42, 1038–1044. [Google Scholar]
- Hahn, G.M.; Van Kersen, I.; Silvestrini, B. Inhibition of the recovery from potentially lethal damage by lonidamine. Br. J. Cancer 1984, 50, 657–660. [Google Scholar] [CrossRef]
- Kim, J.H.; Alfieri, A.A.; Kim, S.H.; Young, C.W. The potentiation of radiation effects on two murine tumors by lonidamine. Cancer Res. 1986, 46, 1120–1123. [Google Scholar]
- Kim, J.H.; Kim, S.H.; He, S.; Alfieri, A.A.; Young, C.W. Potentiation of radiation effects on multicellular tumor spheroids (mts) of hela cells by lonidamine. Int. J. Radiat. Oncol. 1989, 16, 1277–1280. [Google Scholar] [CrossRef]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.H.; Alfieri, A.; Young, C.W.; Silvestrini, B. Lonidamine: A hyperthermic sensitizer of HeLa cells in culture and of the Meth-A tumor in vivo. Oncology 1984, 41, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, B.; Hahn, G.M.; Cioli, V.; De Martino, C. Effects of lonidamine alone or combined with hyperthermia in some experimental cell and tumour systems. Br. J. Cancer 1983, 47, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Coss, R.A.; Storck, C.W.; Wells, T.C.; Kulp, K.A.; Wahl, M.; Leeper, D.B. Thermal sensitisation by lonidamine of human melanoma cells grown at low extracellular pH. Int. J. Hyperth. 2013, 30, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Robinson, S.D. Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog. Brain Res. 2007, 162, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.E.; Lokeshwar, B.L.; Ferrell, S.M.; Block, N.L. Enhancement of hyperthermic toxicity by lonidarnine in the dunning R3327G rat prostatic adenocarcinorna. Prostate 1994, 24, 131–138. [Google Scholar] [CrossRef]
- Stanisaw, K.; Bartosz, K.; Dawid, P.; Jolanta, S.; Ewa, K.D.; Karolina, K.C.; Jolanta, K.; Olga, M.; Krzysztof, K.; Julita, K. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar]
- Ji, H.-T.; Chien, L.-T.; Lin, Y.-H.; Chien, H.-F.; Chen, C.-T. 5-ALA mediated photodynamic therapy induces autophagic cell death via AMP-activated protein kinase. Mol. Cancer 2010, 9, 91. [Google Scholar] [CrossRef]
- Shevchuk, I.; Chekulayev, V.; Moan, J.; Berg, K. Effects of the inhibitors of energy metabolism, lonidamine and levamisole, on 5-aminolevulinic-acidinduced photochemotherapy. Int. J. Cancer 1996, 67, 791–799. [Google Scholar] [CrossRef]
- Golding, J.; Wardhaugh, T.; Patrick, L.; Turner, M.; Phillips, J.B.; Bruce, I.J.; Kimani, S.G. Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. Br. J. Cancer 2013, 109, 976–982. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Little, J.B. The differential response of human tumors to fractionated radiation may be due to a post-radiation repair process. Br. J. Cancer 1982, 46, 532–537. [Google Scholar] [CrossRef]
- Evans, R.G.; Bagshaw, M.A.; Gordon, L.F.; Hahn, G.M.; Kurkjian, S.D. Modification of recovery from potentially lethal X-ray damage in plateau phase Chinese Hamster Cells. Radiat. Res. 1974, 59, 597. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Gurbuxania, S.; Ravagnanb, L.; Kroemer, G. Heat shock proteins: Endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 2001, 286, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Khdair, A.; Handa, H.; Mao, G.; Panyam, J. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro. Eur. J. Pharm. Biopharm. 2009, 71, 214–222. [Google Scholar] [CrossRef]
- Bown, S.G. How mainstream medicine sees photodynamic therapy in the United Kingdom. J. Natl. Compr. Cancer Netw. 2012, 10, S69–S74. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, L.M.; Rodríguez, M.E.; Cogno, I.S.; Vittar, N.B.R.; Pansa, M.F.; Lamberti, M.J.; Rivarola, V.A. Direct and indirect photodynamic therapy effects on the cellular and molecular components of the tumor microenvironment. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1835, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ruttala, H.B.; Ramasamy, T.; Poudel, B.K.; Ruttala, R.R.T.; Jin, S.G.; Choi, H.-G.; Ku, S.-K.; Yong, C.S.; Kim, J.O. Multi-responsive albumin-lonidamine conjugated hybridized gold nanoparticle as a combined photothermal-chemotherapy for synergistic tumor ablation. Acta Biomater. 2020, 101, 531–543. [Google Scholar] [CrossRef]

| Name | Structure | Mechanisms of Action | Tumor Type | Synergistic Mechanism | Adverse Effect | Ref. |
|---|---|---|---|---|---|---|
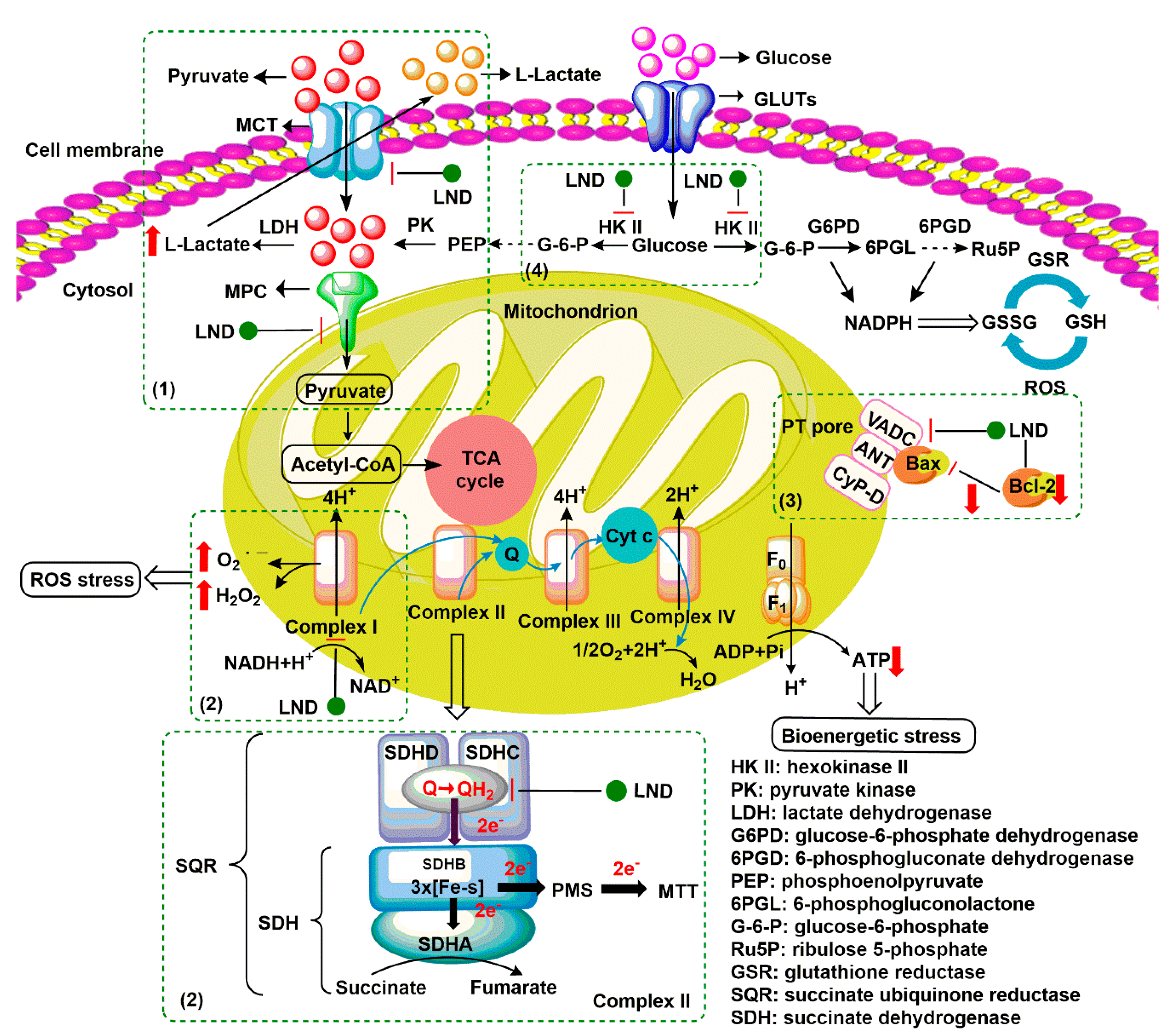
| Cisplatin (CDDP) | 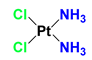 | CDDP induces DNA cross-linking to destroy the DNA structure. | MCF-7, MX-1 breast carcinoma cells (in vivo); A2780, IGROV-1 ovarian carcinoma cells (in vivo); NSCLC cells (in vivo); LNCaP cells (in vivo) | (1) Synergistically induces downregulation of Bcl-2, decrease antiapoptotic activity of cancer cells. (2) LND interferes with DNA repair process for the DNA damages induced by CDDP. | Myelosuppression; Nephrotoxicity; Neurotoxicity; Gastrointestinal reaction | [31,32,33,34,35,36] |
| Temozolomide (TMZ) | 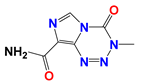 | TMZ degrades to generate active MTIC, which is converted to methyl diazonium ion, followed by alkylation on the O6 or N7 site of guanine. | DB-1 melanoma xenografts (in vivo) | LND inhibits the MDR efflux of TMZ through the reduction of the bioenergy state of the tumor. | Myelosuppression; Neurotoxicity; Thrombocytopenia; Granulocytosis | [37,38,39] |
| Nitrogen mustards (NMs) | 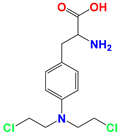 Melphalan | NMs can form electron-deficient ethyleneimine ions, and then covalently bind nucleophile groups (electron-rich) of biomacromolecules for alkylation, resulting in DNA intrastrand and interstrand cross-linking. | DB-1 melanoma xenografts (in vivo) | (1) LND induces intracellular acidification, leading to increased concentration of the active aziridinium ion intermediate that yields DNA damage. (2) LND induces de-energization prevents the energy-dependent MDR pump from pumping out the drugs. (3) LND-induced intracellular acidification inhibits GST activity, leading to decreased level of GSH which can quench the active aziridinium species. (4) DNA repair is reduced by acid inhibition of repair proteins. | Myelosuppression; Nephrotoxicity; Gastrointestinal reaction | [40,41,42,43,44,45,46] |
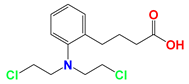 Chlorambucil | ||||||
 Cyclophosphamide | ||||||
 Bendamustine | ||||||
| Carmustine (BCNU) | 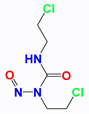 | BCNU induces DNA guanine O6-alkylation, followed by the formation of DNA interstrand cross-linking | HT1080 human fibrosarcoma cells (in vitro); Murine RIF-1 fibrosarcoma cells (in vivo) | LND induces acidic environment that may inhibit MGMT and potentiates the cytotoxicity of BCNU. | Myelosuppression; Vascular toxicity; Neurotoxicity; Liver impairment; Lung toxicity | [35,47,48,49,50] |
| Anthracyclines (ANTs) | 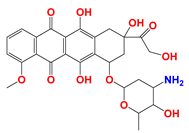 Doxorubicin | ANTs form special DNA adducts, thereby inhibiting DNA replication and RNA transcription. | Breast carcinoma cells (in vivo); WM983B, DB-1 melanoma xenografts (in vivo); Ehrlich ascites tumor cells (in vitro); H2030 cells (in vivo); H2030BrM3 cells (in vivo) | (1) LND inhibits the MDR efflux of ANTs through reducing the bioenergy state of tumors. (2) LND reverses pH acidity gradient, leading to high uptake of free basic DOX by tumors. | Myelosuppression; Cardiotoxicity | [51,52,53,54,55,56,57] |
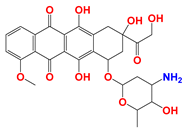 Epirubicin | ||||||
| 6-Diazo-5-oxo-L-norleucine (DON) | 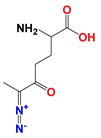 | DON inhibits glutaminolysis | HCC1806 breast carcinoma cells (in vitro); HeLa cells (in vitro); A375 melanin cells (in vitro); SW480 colon cancer cells (in vitro) | Combined with LND and orlistat, blocking three key pathways: glycolysis, glutaminolysis and fatty acid synthesis, achieve synergistic killing of cancer cells. | Myelotoxicity; Oral mucositis; Uremia; Gastrointestinal reaction | [7,8,58,59] |
| Arsenic trioxide (ATO) | 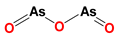 | (1) ATO induces PT pore opening and activates intrinsic apoptotic pathways. (2) ATO induce ROS production. | HL60 acute myelocytic leukemia cells (in vitro) | (1) ATO attenuates LND-mediated activation of MEK/ERK and AKT/mTOR defensive pathways; (2) LND-induced ROS production enhances the apoptosis-inducing capacity of ATO in cancer cells. | Hepatotoxicity; Cardiotoxicity | [60,61,62,63,64] |
| Curcumin (CCM) | 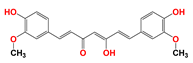 | CCM induces PT pore opening and the inhibition of defensive signaling pathway such as PI3K/AKT and NF-κB. | U937 acute myelocytic leukemia cells (in vitro) | CCM and LND synergistically destroy mitochondrial membrane structure and induce ROS production. | Gastrointestinal reaction | [65,66,67] |
| Matrine (MAT) | 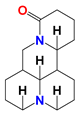 | (1) MAT reduces the expression of cell cycle protein mRNA in G0/G1 phase to block the cell cycle. (2) MAT reduces c-Myc binding to HK-II gene introns. | HL60 acute myelocytic leukemia cells (in vitro); K56 chronic myelocytic leukemia cells (in vivo) | MAT and LND synergistically downregulate or inhibit HK-II expression. | Degeneration of nerve cells in brain tissue | [68,69] |
| Therapy Method | Mechanism of Action | Tumor Type | Synergistic Mechanism | Ref. |
|---|---|---|---|---|
| Radiotherapy (RT) | Cancer cells exhibit sensitivity to radiation, poor tolerance, while the normal cell population is opposite | BALB/c, C3H/He mice fibrosarcoma cells (in vivo); HeLa cells (in vivo) | LND interferes with the energy-dependent PLD repair process. | [96,97,98,99] |
| Hyperthermia (HT) | Cancer cells can easily store more heat than normal cells. When heated to 40–43 °C, cancer cells undergo membrane structure destruction, cytoskeleton deformation, DNA synthesis inhibition, and blood vessel damage, thus resulting in death. | BALB/c mice fibrosarcoma cells (in vivo); HeLa cells (in vivo); human glioma cells (in vivo); Head-neck squamous cells (in vivo); DB-1 melanoma xenografts (in vivo); R3327G rat prostatic adenocarcinoma (in vivo) | (1) HT can lead t78o the formation of condensed mitochondria, which LND targets more easily. (2) HT increases blow flow and drug delivery. (3) HT increases cell uptake of drugs. (4) LND inhibits the repair of HT-induced (sub)lethal damage as well as the proteins involved in cell survival. | [100,101,102,103,104,105] |
| Photodynamic therapy (PDT) | PDT can cause mitochondrial damage, causing ATP consumption and ROS production. | MCF-7 human breast carcinoma cells (in vitro) | LND and PDT synergistically destruct mitochondrial structure, decrease intracellular ATP level, and induce the generation of ROS. | [106,107,108,109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Sun, G.; Sun, X.; Li, F.; Zhao, L.; Zhong, R.; Peng, Y. The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment. Cancers 2020, 12, 3332. https://doi.org/10.3390/cancers12113332
Huang Y, Sun G, Sun X, Li F, Zhao L, Zhong R, Peng Y. The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment. Cancers. 2020; 12(11):3332. https://doi.org/10.3390/cancers12113332
Chicago/Turabian StyleHuang, Yaxin, Guohui Sun, Xiaodong Sun, Feifan Li, Lijiao Zhao, Rugang Zhong, and Yongzhen Peng. 2020. "The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment" Cancers 12, no. 11: 3332. https://doi.org/10.3390/cancers12113332
APA StyleHuang, Y., Sun, G., Sun, X., Li, F., Zhao, L., Zhong, R., & Peng, Y. (2020). The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment. Cancers, 12(11), 3332. https://doi.org/10.3390/cancers12113332