Simple Summary
Cancer is a major cause of human mortality worldwide. No life on earth can live without iron. Persistent oxidative stress resulting from continuous use of iron and oxygen may be a fundamental cause of carcinogenesis. Many animal models demonstrated that excess iron may lead to carcinogenesis. This is supported by a variety of human epidemiological data on cancer risk and prognosis. Cancer is basically a disease of the genome with persistently activated oncogenes and inactivated tumor suppressor genes through which iron addiction with ferroptosis-resistance is established. We predict that fine use of nanomaterials and non-thermal plasma may be able to reverse this situation.
Abstract
Evolution from the first life on earth to humans took ~3.8 billion years. During the time there have been countless struggles among the species. Mycobacterium tuberculosis was the last major uncontrollable species against the human public health worldwide. After the victory with antibiotics, cancer has become the leading cause of death since 1981 in Japan. Considering that life inevitably depends on ceaseless electron transfers through iron and oxygen, we believe that carcinogenesis is intrinsically unavoidable side effects of using iron and oxygen. Many animal models unequivocally revealed that excess iron is a risk for carcinogenesis. This is supported by a variety of human epidemiological data on cancer risk and prognosis. Cancer is basically a disease of the genome with persistently activated oncogenes and inactivated tumor suppressor genes through which iron addiction with ferroptosis-resistance is maintained. Engineering has made a great advance in the past 50 years. In particular, nanotechnology is distinct in that the size of the engineered molecules is similar to that of our biomolecules. While some nano-molecules are found carcinogenic, there are principles to avoid such carcinogenicity with a smart possibility to use nano-molecules to specifically kill cancer cells. Non-thermal plasma is another modality to fight against cancer.
1. Introduction
Space started to expand through the Big Bang 13.8 billion years ago (Gya) [1] and Earth came into existence 4.6 Gya [2]. Evolution from the first life on Earth to humans took ~3.8 Gy [3]. At present, there are 1.75 million species on Earth [4]. Symbiosis of all the species on Earth is generally established in equilibrium currently, except for a fraction of endangered or extinct species, such as dinosaurs [5]. Some species are parasites of the other higher species [6]. There have been countless intense struggles among the species, which humans may sometimes call infection. The last major fight in the history of human public health was the one against mycobacterium tuberculosis, which was finally stopped by the discovery of antibiotics, such as streptomycin and isoniazid in the 1940s and 1950s [7].
Thereafter, in Japan, cancer has been the leading cause of death since 1981 (https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai19/dl/gaikyouR1.pdf), and mortality is still increasing. In the United States, some cancer mortality, such as colorectal cancer, is decreasing [8] presumably due to the success for early detection (secondary prevention) through screening with endoscopy [9,10]. However, nearly one third of the population dies from cancer in high-income countries worldwide (https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death). The other major cause of death is atherosclerosis, leading to ischemic heart disease and stroke over time. However, we should not forget that the major causes of death in low-income countries are still various infections. Both cancer incidence and progression of atherosclerosis are proportionally age-dependent. In this review article, we consider the molecular cause of cancer from the highest global point of view, and then discuss the biological significance of nanomaterials and finally provide a perspective on the novel procedures to counteract cancer.
2. What Is the Major Cause of Carcinogenesis in Humans?
2.1. Epidemiology and Hypothesis
After the end of longstanding countless wars against the other species, cancer is now one of the leading causes of human mortality in high-income countries worldwide. Here, it would be important to consider major causes of carcinogenesis in humans. We hypothesize that persistent use of iron and oxygen is the overlooked major cause of carcinogenesis in humans. Cancers may be classified into the two types: one with unequivocal risk factor(s), including endogenous and exogenous, and the other with more ambiguous or no identified risk factor(s), which is not necessarily consistent with a tumor mutation burden [11] (Figure 1). Typical examples of the first category are malignant mesothelioma (MM) by respiratory exposure to asbestos fibers [12,13] and hereditary breast/ovarian cancer in those with BRCA1/2 mutations [14], namely occupational cancers and familial cancer syndromes. It is not surprising that these hereditary cancers share a relatively small fraction (5%~10%) even though there are hundreds of cancer-prone syndromes reported [15].
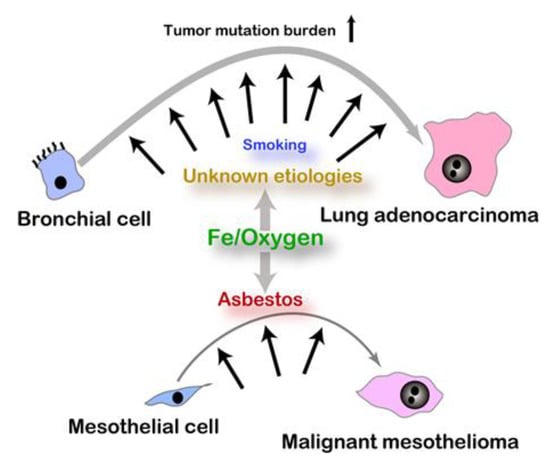
Figure 1.
Etiology and somatic mutation burden in carcinogenesis. Asbestos exposure is a well-established risk for malignant mesothelioma whereas risks for lung adenocarcinoma with high somatic mutation burden are various and still vague.
It is often difficult to identify the responsible risk(s) for most of the cancers, thus, falling into the second category. Current literatures discuss the importance of smoking [16] and a Western diet (high calorie, saturated oil, red meat, etc.) [17] as carcinogenic risks. We are not in a position to oppose these statements. Systematic reviews clearly reveal that smoking is a risk for various type of cancers including oral, laryngeal, lung, stomach, renal, and bladder cancers [18]. However, we believe that there are more fundamental factors to be considered as carcinogenic risks when looking at the steady global increase in the fraction of cancer as a major cause of human mortality where major infective diseases have been overcome. Furthermore, laboratory mice and rats suffer from a high incidence of cancer in old age [19,20,21] even if they are usually not exposed to apparent carcinogens or cancer-risk factors. Based on these facts, we started to consider the significance of the origin and evolution of life in carcinogenesis.
2.2. Iron, Sulfur, and Oxygen
As far as we are aware, no independent life on Earth can live without iron [22]. Geological studies revealed that the ancient sea contained a high concentration of catalytic ferrous iron [Fe(II)] when the first life on Earth was born [23,24]. It is true that iron is a fundamental element, existing in space because we can find many meteors which consist largely of iron [25]. Iron in the ancient ocean reacted with a subtle amount of oxygen to generate ores at the bottom of the sea [24]. Life might be defined as a continuous flow of electrons with reproductive activity of the next generation. Iron is a transition metal [26,27], efficient in electron flow, and, thus, preferentially used as a media for the first life on Earth [28].
Thereafter, a great oxidation event (GOE) occurred when an evolved life as cyanobacteria could transform light (solar) energy to electron flow to trigger rapid oxygenation [29]. Reportedly, sulfur was abundant in the environment at this period, where sulfur was firmly integrated in the life system not only as a coexistence of iron (Fe and S have a high affinity) but also as a competitor [24,30]. S is present in the sulfhydryl function of cysteine, which is in major use for reducing activity of polypeptides/proteins by counteracting as antioxidants. Representative ones are glutathione and thioredoxin, which are also used as a reducing unit for enzymes [31]. Furthermore, -SH works as an intracellular sensor for oxidative stress, as in the case of Keap1 and Nrf2 [32]. Recently, persulfides are regarded as a potent antioxidant mechanism [33].
After the GOE, the concentration of atmospheric oxygen started to rise gradually from ~0.6 Gya and reached a stable state of ~21% after several fluctuations [24]. Oxygen molecule, albeit a biradical, is relatively stable at the ground condition on the Earth and works flexibly as a media for electron flow. The most distinctive characteristic of O2 is its oxidizing ability, accepting a single electron to four electrons. At the same time, O2 is reduced ultimately to H2O. This process is quite versatile in that there can be electron flow of one to four, depending on the condition. O2 → O2− (superoxide) → H2O2 → •OH (hydroxyl radical) → H2O. In this case, the first two reactions are mediated by various enzymes whereas the third reaction is a chemical reaction designated as a Fenton reaction [27,34]. The hydroxyl radical is the most reactive species in the biological system on the Earth [35]. Therefore, higher animals hold and employ various enzymes, including catalase, peroxidases, and peroxiredoxins to directly decompose H2O2 to H2O [36,37,38]. In this way, the order of major elements used by the fundamental life is Fe → S → O during the evolution [39].
2.3. Excess Iron and Carcinogenesis
Excess iron is a soil for carcinogenesis [40,41,42]. Even though iron is essential for every kind of life on Earth, iron presents a double-edged sword. On the one hand, iron deficiency causes anemia (decrease in hemoglobin in the blood) and muscle weakness in higher animals [43]. On the other hand, iron excess causes oxidative damage to various different kinds of cells, which may lead to carcinogenesis [44,45,46]. Therefore, we have to consider both sides of the thresholds. Children and pregnant women definitely require a high amount of iron for the growth of organs. In the low-income countries, this issue is closely associated with malnutrition with food deficiency, but iron fortification to foods is recently recognized to alter gut microbiome [47]. Furthermore, we believe that supplementary iron intake to all the populations, irrespective of the iron status, whether deficient or sufficient, is not recommended [48,49]. This is partially because some form of iron, such as a nanoparticle form of iron, is absorbed from the duodenum without regulation via endocytosis but not via Fe(II) transporters [50].
Here, we briefly explain important principles on iron metabolism. More detailed descriptions are found in other recent publications [51,52]. Humans hold 2.5 to 4 g of iron in the body, which is the most abundant heavy metal, with zinc (2~3 g) [53] and copper (50~120 mg) [54] being the second and the third, respectively. In total, 60% of iron is in the heme of hemoglobin for oxygen transport (affinity of Fe[II] to O2) in red blood cells. Iron as a transition metal (Fe[II] ↔ Fe[III]) is important for DNA replication (ribonucleotide reductase), ATP synthesis (cytochrome oxidases), and antioxidant activity (catalase) in which either Fe(II) [55], heme [56], or the Fe-S cluster [57] is integrated as a cofactor of a catalytic subunit. Iron metabolism in humans as well as in other higher species is a semi-closed system, where only 1 mg of iron is absorbed from the villous surface membrane of duodenal epithelial cells and 1 mg is lost from the dead or peeled-off cells of skin or gastrointestinal system [41,58]. Therefore, iron from most of the dead cells inside our body is completely recovered by macrophages or their analogues or deposits in the interstitium.
Iron is essential not only for all the cells of the individual but also for the invading or coexisting lower species. Thus, every species competes for iron and fight with various smart molecular mechanisms, such as siderophores [59], for iron. As such, cells undertake to take up, reserve, and accumulate iron in themselves from dead cells or interstitium in higher species. Excess iron or iron overload often occurs in such pathologic conditions. Iron excess is classified into the following categories: (1) excess absorption via dysregulation (e.g., genetic hemochromatosis) or iron supplements, (2) chronic infection, (3) non-infectious inflammation (e.g., exposure to a large amount of foreign body difficult to be removed, such as asbestos), (4) increased cell death, including that of red blood cells (e.g., thalassemia, sickle cell disease), (5) relative decrease or dysfunction in an iron scavenging mechanism (e.g., aging), and (6) others, including repeated transfusion (Figure 2).
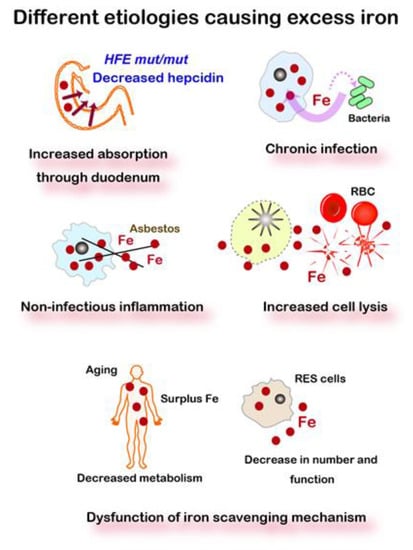
Figure 2.
Five different etiologies causing excess iron. Iron metabolism in mammals is a semi-closed system, starting from the absorption at the duodenal epithelia but with no active excreting pathway. Various pathologies, including hemolysis, inflammation, and aging, lead to excess iron. HFE, a responsible gene for genetic hemochromatosis. RBC, red blood cells. RES, reticuloendothelial system, including macrophages, histiocytes, dendritic cells, Kupffer cells, and microglia. Refer to text for details.
After briefly reviewing the molecular mechanisms associated with iron metabolisms, there are three independent lines of evidence available for the association of excess iron and carcinogenesis, (1) human observational data, either in specific diseases or in more broad population, (2) human interventional data, and (3) animal experiments. Representative human findings in the two categories are summarized in Table 1.

Table 1.
Representative human facts on the association of iron and carcinogenesis.
2.4. Iron-Induced Renal Carcinogenesis and Oxygenomics
Animal models are precious in that the comparisons among the experimental groups are the most precise due to the uniform genetic background (i.e., inbred strains) and living environment than the humans reported as epidemiological studies. There has been a key question whether Fe(II)-catalyzed the Fenton reaction of the repeated nature can induce carcinogenesis. The answer is positive. This came from a finding shed light by serendipity. Though iron is an important metal, the molecular understanding of iron metabolism required a long time and mostly started in the late 1990s. In the 1970s, only the transferrin system was recognized [51], but there was no in vivo method known to load iron to parenchymal cells of rodents. In those days, ferric nitrilotriacetate (Fe-NTA) was used to load iron to unsaturated transferrin in biochemical experiments [69]. NTA is a metal chelator with a structure of aminopolycarboxylic acid, solubilizing metals [70]. In the case of iron, both Fe(II)-NTA and Fe(III)-NTA are catalytic at neutral pH [26,71,72]. Intraperitoneal repeated injection of Fe-NTA to rats, for the first time, enabled iron loading to parenchymal cells (e.g., hepatocytes and β cells in Langerhans islets), showing similar signs of genetic hemochromatosis [73]. Unexpectedly, a long observation of this model revealed a high incidence (~90%) of renal cell carcinoma (RCC) with pulmonary metastasis in rats in 1982, and, later in mice, in the Department of Pathology, Kyoto University Faculty of Medicine [74,75,76,77].
At first, we could not imagine the responsible molecular mechanisms, but years later we found necrosis of renal proximal tubules with iron-catalyzed lipid peroxidation as early as 30 min after a single intraperitoneal injection of Fe-NTA [78,79,80]. Now, we sort out that this is ferroptosis vide infra [38,39,81], and this model unequivocally demonstrated that repeated oxidative stress catalyzed by iron leads to carcinogenesis in situ. This model contributed much to establishing oxidative stress markers, such as 4-hydroxy-2-nonenal (HNE) [82,83,84], 8-oxoguanine (8-oxoGua) [85,86], and thymine-tyrosine crosslinks [37,87].
We later revealed that genetic alterations in this rat renal carcinogenesis are similar to those in human cancers in that the homozygous deletion of p16Ink4a/p15Ink4b tumor suppressor gene and amplification of c-Met oncogene are frequently observed [88,89]. Hemiallelic loss of the p16Ink4 tumor suppressor gene is detected as early as three weeks after the start of Fe-NTA injections [90]. Furthermore, there are expressional and epigenetic alterations of substantial genes during carcinogenesis and tumor progression, such as annexin 2, thioredoxin-binding protein 2 (vitamin D3 up-regulated protein-1), and fibulin-5 [91,92,93,94,95]. Intriguingly, there is a marked difference between rats and mice regarding this renal carcinogenesis [96]. Most strains of rats (e.g., Wistar, Fischer-344, Brown-Norway, and Sprague-Dawley) provides a high incidence of renal cell carcinoma (RCC, 60–90%) whereas mice reveal a strain-specific susceptibility (e.g., C57BL/6, <10%, A/Jackson, ~60%). Grade of malignancy is also different. A half of RCCs metastasize to lung albeit wild-type animals in rats whereas a lower grade RCC is usually generated in mice with a low incidence of chromosomal aberrations [76,97] (Figure 3). Thus, our results on this RCC model confirm the fact that Rattus norvegicus are much closer than Mus musculus to Homo sapience in the evolutionary phylogeny.
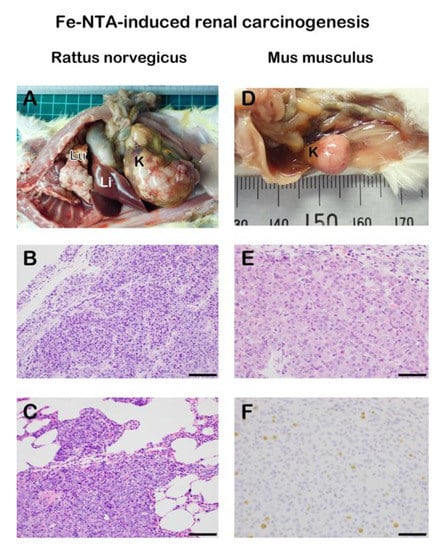
Figure 3.
Species differences in ferric nitrilotriacetate (Fe-NTA)-induced renal carcinogenesis in wild-type rodents. Induction of an advanced renal cell carcinoma (RCC) with extensive pulmonary metastasis is frequently observed in rats whereas smaller RCC without metastasis is obtained with a much lower incidence in mice. (A) Macroscopic view of RCC with pulmonary metastasis and invasion in a male wild-type Sprague-Dawley rat 1 y after 11 weeks of repeated intraperitoneal 5–10 mg iron/kg Fe-NTA administration (3–5 times a week). Note primary RCC of 75 mm in diameter in the kidney and many metastatic nodules of 1–2 mm on the surface of lung. K, kidney. Li, liver. Lu, lung. (B) Histology of the primary RCC in the kidney. Proliferation of atypical glandular cells are observed in irregular glandular or solid structure (Hematoxylin and eosin staining). (C) Histology of the metastatic RCC in the lung. Similar adenocarcinoma to the primary site is invading the pulmonary alveolar structure. (D) Macroscopic view of RCC in a male wild-type A/J mouse 10 months after 12 weeks of repeated intraperitoneal 5–7 mg iron/kg Fe-NTA administration (6 times a week). Dose difference in the protocol between rats and mice comes from the difference in sensitivity to Fe-NTA. K, kidney. (E) Histology of the primary RCC in the kidney. Proliferation of atypical glandular cells are observed in irregular glandular or solid structure. (F) Ki-67 immunostaining of the RCC with an index of 5% (bar = 100 μm in B and C, 50 μm in E and F).
This RCC model also opened an avenue to understand the site specificity of oxidative genomic DNA damage in the nucleus [98,99,100,101]. We have developed a technique, called DNA immunoprecipitation for oxidative DNA base modification (e.g., 8-oxoGua), and showed that distribution of oxidative DNA damage is not random but influenced not only by chemical species involved (e.g., •OH and HNE) but also transcriptional activity, intranuclear localization (i.e., central or peripheral, near the nuclear membrane with Lamin B1 association) [102,103] and, thus, the structural fluctuation cycle [103,104]. We named such a research area as “Oxygenomics [98]”. Mutyh (an enzyme to repair 8-oxoGua in the genome)-deficient mice presented a higher incidence (26.7%) of RCC in comparison to wild-type mice (7.1%) [97].
2.5. Nanofiber-Induced Mesothelial Carcinogenesis and Excess Iron
Another important rodent carcinogenesis model associated with excess iron is asbestos-induced malignant mesothelioma (MM) [12]. This model also uses wild-type rats. Intraperitoneal injection of only 10 mg of asbestos (i.e., chrysotile, crocidolite or amosite, which correspond to white, blue, and brown asbestos, respectively) causes MM with an incidence of ~100% in two years [105]. Tremolite, which is a minor asbestos, also induces MM in rats [106]. This is extremely fast in comparison to human cases where 30~40 years of the latent period is usually after exposure to asbestos [66]. We believe that it is responsible that asbestos is directly exposed to mesothelial cells in our model whereas asbestos should go through lung parenchyma and pierce the visceral mesothelium to reach the parietal mesothelial cells in humans [13]. The essence of this carcinogenesis is local iron excess due to the affinity of asbestos to hemoglobin and phagocytic character of mesothelial cells [13,107,108]. Asbestos is a foreign material to our body, which is scavenged in situ through brave macrophages with resultant massive iron accumulation, which may be at least partially responsible for the deletion of the p16Ink4 tumor suppressor gene [109]. Iron deposition is responsible not only from the adsorbed iron on the asbestos surface but also from the basic defense mechanism as inflammation to remove as much iron as possible in the competing extracellular environments to suppress virtual microorganisms [110]. We have shown preclinically that iron removal, either by redox-inactive iron chelators (deferasirox [111] and desferal [112]) or phlebotomy [113], is beneficial for the prevention of MM even after exposure to asbestos.
Carcinogenicity of asbestos depends not only on its physical dimension but also bio-durability as a fibrous mineral to reach pulmonary alveoli and further pleural cavity. Long (>20 μm) and thin (<250 nm) asbestos fibers can disrupt macrophages, which exacerbates inflammation and iron deposition [12,114]. Mth1 (an enzyme to sanitize cytosolic nucleotide pool to remove 8-oxoGua) deficiency provided longer survival in asbestos-induced MM carcinogenesis, which meant that Mth1 is advantageous in crocidolite-induced mesothelial carcinogenesis in mice [115].
Of note, similar phenomena of the association between iron excess and carcinogenesis were reported on multiwalled carbon nanotubes (MWCNT) [116], which strictly depend on the diameter of the MWCNT [117,118]. MWCNT, which is a fibrous synthetic product purely from carbon, was discovered in 1991 [119], and is abundantly used to lengthen the lifetime of electric battery to strengthen rubber with thermal/electric conductivity and to compose biomedical sensors as hybrid composites with graphene [120]. MWCNT with a diameter of ~50 nm can cause MM when injected intraperitoneally [117,121]. Of note, homozygous deletion of p16Ink4a/p15Ink4b tumor suppressor gene is observed in almost all the cases of MM induced [117], which is the same for asbestos-induced MM. All of these results indicate that p16Ink4a/p15Ink4b tumor suppressor gene is a major target in excess iron-associated carcinogenesis [13,71,114,122]. Since the p16Ink4a/p15Ink4b tumor suppressor gene is the second major mutated gene in human cancers only after TP53 [123], we believe that persistent use of iron and oxygen is one of the major causes of human carcinogenesis.
2.6. Resistance to Ferroptosis
Light microscopy can differentiate apoptosis from necrosis morphologically. Apoptosis reveals nuclear and cytoplasmic fragmentation through caspase activation with little inflammatory responses [124] whereas necrosis generally shows cytoplasmic swelling with nuclear pyknosis and inflammatory responses. This is still a golden rule at present [125]. Formerly, necrosis was defined as an uncontrollable nature of passive cell death due to high levels of injury. Now, the concept of regulated necrosis is established, where some form of necrosis requires signal activation (i.e., not passive) and takes some time (i.e., mins to hours) for its execution [39].
Regulated cell death is currently divided into 12 different forms [125], among which ferroptosis was coined in 2012 [126]. Ferro- indicates Fe(II) whereas -ptosis means falling off. Ferroptosis is defined as a catalytic Fe(II)-dependent regulated necrosis accompanying lipid peroxidation [81]. Ferroptosis was first reported on the treatment of erastin (i.e., an inhibitor of cystine/glutamate antiporter, SLC7A11) on N-Ras mutant fibrosarcoma cells during the drug screening for Ras-activated cancers [126]. We immediately noticed that renal tubular necrosis induced by Fe-NTA [78,80,82] as described above is ferroptosis [38]. As an intriguing coincidence, ferroptosis of renal proximal tubules occurs after conditional knockout of glutathione peroxidase 4 (GPX4), which is the only membrane-specific isozyme of glutathione peroxidase [127]. Currently, we interpret that this is a fight between Fe and S and that a significantly higher Fe/S ratio than the control leads to ferroptosis [39]. Cancer cells require a high amount of iron to replicate DNA, proliferate, and invade. Therefore, they are rich in catalytic Fe(II) [128,129]. Carcinogenesis is a process to obtain this resistance to ferroptosis as shown in rodent RCC and MM models [30] (Figure 4).
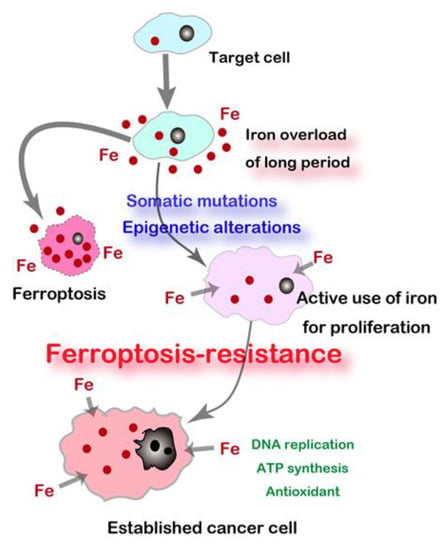
Figure 4.
Carcinogenesis as a process to establish “Iron addiction and ferroptosis-resistance”. Long-term iron overload is a soil for carcinogenesis.
2.7. Cancer Prognosis and Iron Metabolism
We have, thus, far discussed the iron-induced carcinogenic mechanisms. Here, we would mention the effects of iron deficiency or excess on the prognosis of cancer in humans. Several national surveys were performed in the 1980s and 1990s in the US and Finland. For example, 3287 men and 5269 women participated in the first national nutritional survey in which men and women were divided into five groups, based on baseline transferrin saturation (<30%, 30–40%, 40–50%, 50–60%, 60%<). For men and women combined, cancer risk for each group relative to the first was 1.0, 0.95, 1.16, 1.38, and 1.18 whereas mortality for each group was 1.0, 0.96, 1.22, 1.29, and 1.73 [130]. Other studies are summarized in a previous review article [40]. Here, we summarized the recent representative data in Table 2. Most of the data suggests that iron-rich status provides poorer prognosis in cancer patients.

Table 2.
Representative human facts on the association of iron and cancer prognosis.
3. Association of Cutting-Edge Engineering and Cancer
3.1. Nanomaterials and Carcinogenesis
Nanomaterials are defined as a material that contains at least 50% of the particles (by number) in the 1–100 nm range [135]. These materials are novel in that the dimension of the molecules generated through new developments are as small as the levels of our own biomolecules persistently used in our daily metabolism. It was socially meaningful to find that some of the fibrous nanomaterials (i.e., multiwalled carbon nanotube [MWCNT] of 50 nm diameter) are carcinogenic in rodents, causing MM after intraperitoneal administration (vide supra) [117].International Agency for Research on Cancer, thereafter, designated MWCNT of 50-nm diameter as Group 2B (possible human carcinogen) and other MWCNTs as Group 3 [136]. It was later reported that inhalation of MWCNT of a 50-nm diameter causes lung carcinoma in rats [137].
This kind of information is precious to differentiate management of MWCNT of different diameters toward safer work environments. MWCNT is already providing us with daily convenience as a high-power battery for smart phones and highly durable rubber for car tires and excavators [120]. The robotics automation process in the factory and avoidance of carcinogenic MWCNTs are helpful to decrease the carcinogenic risks for humans. We demonstrated in a 3-year rat study that MWCNT of 15 nm (tangled form) is not carcinogenic by intraperitoneal injection [121]. Based on these results, we believe that a subacute study by intraperitoneal injection with a four week observation predicts the carcinogenicity of the bio-persistent fibrous material, such as asbestos and MWCNT [117,118]. Commercial chlorine bleach can degrade MWCNT to CO2 ex vivo, which would facilitate the disposal of this nanomaterial [138].
Furthermore, it was recently reported that WS2 and MoS2 nanosheets (two-dimensional transition metal dicharcogenides [139]) induces ferroptosis through surface vacancies in bronchial epithelial and macrophage cells [140]. This is also an airborne risk and can be prevented by prior methanol treatment to passivate active particle surfaces [139].
3.2. Nanomaterials for Cancer Treatment by Designing the Death Code
Conversely, nanomaterials may be able to specifically kill cancer cells if designed optimally by exploring a structure-activity relationship (Figure 5). In this century, nanomaterials have been recognized as emerging media for drug delivery and a number of clinical trials are in progress. Currently, regulated cell death is classified into twelve and each has a fixed death code [125]. There is a huge possibility that nanomaterials can initiate and modify death codes in which ferroptosis acquired a high attention [141].
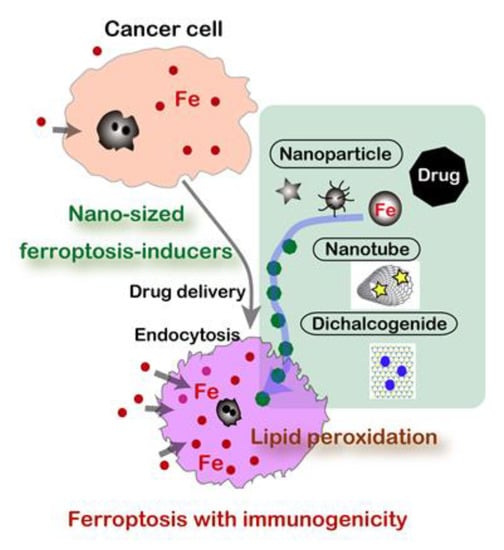
Figure 5.
Nanomaterials as novel ferroptosis inducers for cancer cells.
When we understand that carcinogenesis a process to establish “iron addiction with ferroptosis resistance” [13,30], cancer cells are expectedly rich in catalytic Fe(II) [128,129] in the cytosol to be easily utilized for enzymes toward unregulated endless proliferation, such as ribonucleotide reductase (DNA synthesis), cytochrome oxidase (ATP synthesis), and catalase (antioxidant). Fe(II) [55], Fe-S cluster [57], and heme [56] are important cofactors for these enzymes. Thus, this is the strategy to induce ferroptosis by using nanomaterials specifically in cancer cells, but not in non-tumorous cells. Most of the nanomaterials are actively taken up by cancer cells through endocytosis. This is an active research area in material science, and various forms of iron-based nanomaterials are preclinically proposed, including iron oxide nanoparticles (IONs) [142], lipid-hydroperoxide-tethered IONs [143], assembled IONs [144], amorphous iron nanoparticles [145], iron-organic frameworks [146], and FePt nanoparticles [147] in addition to small molecule chelators [148].
3.3. Non-Thermal Plasma
Plasma is the fourth condition of a physical state, which presents the highest energy over gas with ionization [149]. High-temperature plasma has been used from the 1960s for manufacturing semiconductors. Development of modern electronics produced plasma of a near body temperature (i.e., non-thermal plasma (NTP) or low-temperature plasma) [150,151,152]. Inert gas, such as Ar or He, is used as flow supply with high voltage/electron density to generate various reactive species from atmospheric O2 and N2, including •OH, H2O2, O2−, and NO. Fine adjustment of the concentration of each gas and humidity provides different fractions of each reactive species [150,153].
NTP was established as a novel method to load oxidative stress to the target coordinates [154]. In addition to direct exposure of NTP, plasma activated media and lactate (PAM [155,156,157,158] and PAL [159], respectively) are under intensive investigation even though the responsible chemical species have not been completely identified at present. NTP as preclinical experiments can be applied to multiple medical and biological purposes [152], including: (1) disinfection of viruses and bacteria, (2) promotion of wound healing, (3) specific killing of cancer cells [151,160,161], (4) removal of endometriotic lesions [162,163], and (5) increasing yield of plants [164] and fish [165]. The final biological effects depend on the relative strength of the oxidative stress loaded [37] (Figure 6).
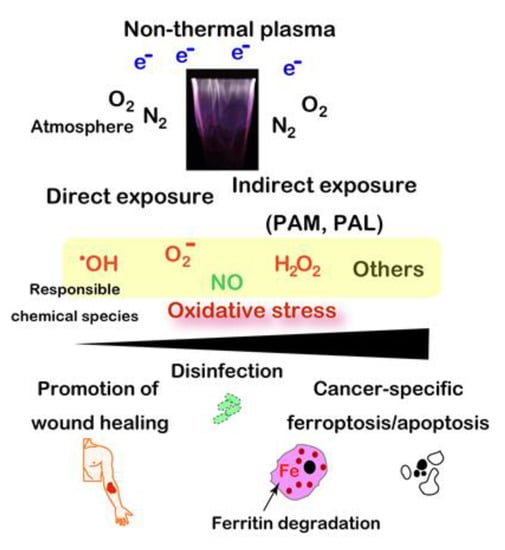
Figure 6.
Wide applicability of non-thermal plasma to biomedical field, including specific ferroptosis inducer for cancer cells. PAM, plasma-activated medium. PAL, plasma-activated Ringer’s lactate solution.
Regarding the specific killing of cancer cells, abundance of catalytic Fe(II) in cancer is important for the effects of NTP to cause the Fenton reaction eventually to ferroptosis [160,166]. Here, NTP-induced ferritin degradation with a simultaneous reduction to Fe(II) may be important [167]. This strategy is to attack the Achilles’ heel of cancer cells [39], which they obtained for their fundamental existence through the evolutionary process as discussed ibid. The drawback of NTP is that it reaches only a few mm in depth [154]. Thus, it would work for surface tumors in the situations of somatic cavity (e.g., peritonitis carcinomatosa) or operational margins, where other modalities are not presently easily applied.
4. Conclusions
Animal models suggest that carcinogenesis can be a side effect of using iron and oxygen for decades whereas there is a long list of carcinogenic agents. We may interpret that carcinogenic agents in the lists are intensifying the side effects of iron and oxygen. Animal models contributed to establish the concept of carcinogenesis as “iron addiction with ferroptosis-resistance”. Alternatively, there is a huge possibility to specifically kill cancer cells by attacking this Achilles’ heel of cancer cells with ultimate bioengineering.
Funding
This work was supported, in part, by JST CREST (Grant Number JPMJCR19H4), JSPS Kakenhi (Grant Number JP17H04064, JP19H05462, and JP20H05502), and Research Grant of the Princess Takamatsu Cancer Research Fund to ST.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Fe-NTA | ferric nitrilotriacetate |
| GOE | great oxidation event |
| GPX4 | glutathione peroxidase 4 |
| Gya | billion years ago |
| HNE | 4-hydroxy-2-nonenal |
| ION | iron oxide nanoparticle |
| MM | malignant mesothelioma |
| MWCNT | multiwalled carbon nanotube |
| NTA | nitrilotriacetate |
| NTP | non-thermal plasma |
| 8-oxoGua | 8-oxoguanine |
| RCC | renal cell carcinoma |
| SWCNT | single walled carbon nanotube |
References
- Peplow, M. Planck snaps infant Universe. Nature 2013, 495, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, G.B. The age of the Earth in the twentieth century: A problem (mostly) solved. Geol. Soc. Spec. Publ. 2001, 190, 205–221. [Google Scholar] [CrossRef]
- Lanier, K.A.; Williams, L.D. The origin of life: Models and data. J. Mol. Evol. 2017, 84, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on earth and in the ocean? PLoS Biol. 2011, 9. [Google Scholar] [CrossRef]
- Chiarenza, A.A.; Farnsworth, A.; Mannion, P.D.; Lunt, D.J.; Valdes, P.J.; Morgan, J.V.; Allison, P.A. Asteroid impact, not volcanism, caused the end-Cretaceous dinosaur extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 17084–17093. [Google Scholar] [CrossRef]
- Poulin, R. Greater diversification of freshwater than marine parasites of fish. Int. J. Parasitol. 2016, 46, 275–279. [Google Scholar] [CrossRef]
- Kerantzas, C.A.; Jacobs, W.R., Jr. Origins of combination therapy for tuberculosis: Lessons for future antimicrobial development and application. mBio 2017, 8. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.J. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Shaukat, A.; Mongin, S.J.; Geisser, M.S.; Lederle, F.A.; Bond, J.H.; Mandel, J.S.; Church, T.R. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 2013, 369, 1106–1114. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Winawer, S.J.; Krauskopf, M.; Carlesimo, M.; Schnoll-Sussman, F.H.; Huang, K.; Weber, T.K.; Jandorf, L. New York Citywide colon cancer control coalition: A public health effort to increase colon cancer screening and address health disparities. Cancer 2016, 122, 269–277. [Google Scholar] [CrossRef]
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Mechanisms of asbestos-induced carcinogenesis. Nagoya J. Med. Sci. 2009, 71, 1–10. [Google Scholar] [PubMed]
- Toyokuni, S. Iron addiction with ferroptosis-resistance in asbestos-induced mesothelial carcinogenesis: Toward the era of mesothelioma prevention. Free Radic. Biol. Med. 2019, 133, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Manahan, E.R.; Kuerer, H.M.; Sebastian, M.; Hughes, K.S.; Boughey, J.C.; Euhus, D.M.; Boolbol, S.K.; Taylor, W.A. Consensus guidelines on genetic‘ testing for hereditary breast cancer from the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019, 26, 3025–3031. [Google Scholar] [CrossRef]
- Evans, D.G.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.M.; Lalloo, F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am. J. Med. Genet. A 2010, 152A, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Mena, J.M.; Schottker, B.; Mons, U.; Jenab, M.; Freisling, H.; Bueno-de-Mesquita, B.; O’Doherty, M.G.; Scott, A.; Kee, F.; Stricker, B.H.; et al. Quantification of the smoking-associated cancer risk with rate advancement periods: Meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 2016, 14, 62. [Google Scholar] [CrossRef]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Prejean, J.D.; Peckham, J.C.; Casey, A.E.; Griswold, D.P.; Weisburger, E.K.; Weisburger, J.H. Spontaneous tumors in Sprague-Dawley rats and Swiss mice. Cancer Res. 1973, 33, 2768–2773. [Google Scholar]
- Maekawa, A.; Onodera, H.; Tanigawa, H.; Furuta, K.; Matsuoka, C.; Kanno, J.; Ogiu, T.; Hayashi, Y. Spontaneous neoplastic and nonneoplastic lesions in aging donryu rats. Jpn. J. Cancer Res. 1986, 77, 882–890. [Google Scholar]
- Hojo, M.; Sakamoto, Y.; Maeno, A.; Tayama, K.; Tada, Y.; Yuzawa, K.; Ando, H.; Kubo, Y.; Nagasawa, A.; Tanaka, K.; et al. A histopathological analysis of spontaneous neoplastic and non-neoplastic lesions in aged male RccHan:WIST rats. J. Toxicol. Pathol. 2020, 33, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron and thiols as two major players in carcinogenesis: Friends or foes? Front. Pharmacol. 2014, 5, 200. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.A.; Sigman, D.M.; Morel, F.M.M. The bioinorganic chemistry of the ancient ocean: The co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean-Proterozoic boundary? Inorg. Chim. Acta 2003, 356, 308–318. [Google Scholar] [CrossRef]
- Olson, K.R.; Straub, K.D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef]
- Poole, G.M.; Rehkamper, M.; Coles, B.J.; Goldberg, T.; Smith, C.L. Nucleosynthetic molybdenum isotope anomalies in iron meteorites—New evidence for thermal processing of solar nebula material. Earth Planet Sci. Lett. 2017, 473, 215–226. [Google Scholar] [CrossRef]
- Toyokuni, S.; Sagripanti, J.L. Iron-mediated DNA damage: Sensitive detection of DNA strand breakage catalyzed by iron. J. Inorg. Biochem. 1992, 47, 241–248. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Hider, R.H. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef]
- Yoshiya, K.; Sato, T.; Omori, S.; Maruyama, S. The birthplace of proto-life: Role of secondary minerals in forming metallo-proteins through water-rock interaction of hadean rocks. Orig. Life Evol. Biosph. 2018, 48, 373–393. [Google Scholar] [CrossRef]
- Schirrmeister, B.E.; de Vos, J.M.; Antonelli, A.; Bagheri, H.C. Evolution of multicellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event. Proc. Natl. Acad. Sci. USA 2013, 110, 1791–1796. [Google Scholar] [CrossRef]
- Toyokuni, S.; Ito, F.; Yamashita, K.; Okazaki, Y.; Akatsuka, S. Iron and thiol redox signaling in cancer: An exquisite balance to escape ferroptosis. Free Radic. Biol. Med. 2017, 108, 610–626. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The redox code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Toyokuni, S. Reactive oxygen species-induced molecular damage and its applicaton in pathology. Pathol. Int. 1999, 49, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Akatsuka, S. Pathological investigation of oxidative stress in the post-genomic era. Pathol. Int. 2007, 57, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. The origin and future of oxidative stress pathology: From the recognition of carcinogenesis as an iron addiction with ferroptosis-resistance to non-thermal plasma therapy. Pathol. Int. 2016, 66, 245–259. [Google Scholar] [CrossRef]
- Toyokuni, S.; Yanatori, I.; Kong, Y.; Zheng, H.; Motooka, Y.; Jiang, L. Ferroptosis at the crossroads of infection, aging and cancer. Cancer Sci. 2020, 111, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron-induced carcinogenesis: The role of redox regulation. Free Radic. Biol. Med. 1996, 20, 553–566. [Google Scholar] [CrossRef]
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Harigae, H.; Hino, K.; Toyokuni, S. Iron as soul of life on earth revisited: From chemical reaction, ferroptosis to therapeutics. Free Radic. Biol. Med. 2019, 133, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron and carcinogenesis: From Fenton reaction to target genes. Redox Rep. 2002, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Novel aspects of oxidative stress-associated carcinogenesis. Antioxid. Redox Signal. 2006, 8, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Oxidative stress as an iceberg in carcinogenesis and cancer biology. Arch. Biochem. Biophys. 2016, 595, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C. Too much of a good thing? The problem of trace element fortification of foods. Trends Food Sci. Technol. 1996, 7, 139–142. [Google Scholar] [CrossRef]
- Hurrell, R.F. Efficacy and safety of iron fortification. Food Fortif. Glob. World 2018, 195–212. [Google Scholar] [CrossRef]
- Shirase, T.; Mori, K.; Okazaki, Y.; Itoh, K.; Yamamoto, M.; Tabuchi, M.; Kishi, F.; Jiang, L.; Akatsuka, S.; Nakao, K.; et al. Suppression of SLC11A2 expression is essential to maintain duodenal integrity during dietary iron overload. Am. J. Pathol. 2010, 177, 677–685. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Yanatori, I.; Kishi, F. DMT1 and iron transport. Free Radic. Biol. Med. 2019, 133, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Brunold, T.C.; Davis, M.I.; Kemsley, J.N.; Lee, S.K.; Lehnert, N.; Neese, F.; Skulan, A.J.; Yang, Y.S.; Zhou, J. Geometric and electronic structure/function correlations in non-heme iron enzymes. Chem. Rev. 2000, 100, 235–350. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L. Heme enzyme structure and function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef]
- Tsai, C.L.; Tainer, J.A. Robust production, crystallization, structure determination, and analysis of [Fe-S] proteins: Uncovering control of electron shuttling and gating in the respiratory metabolism of molybdopterin guanine dinucleotide enzymes. Methods Enzymol. 2018, 599, 157–196. [Google Scholar] [CrossRef]
- Harigae, H. Iron metabolism and related diseases: An overview. Int. J. Hematol. 2018, 107, 5–6. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef]
- Kowdley, K.V. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 2004, 127, S79–S86. [Google Scholar] [CrossRef]
- Finianos, A.; Matar, C.F.; Taher, A. Hepatocellular carcinoma in beta-thalassemia patients: Review of the literature with molecular insight into liver carcinogenesis. Int. J. Mol. Sci. 2018, 19, 4070. [Google Scholar] [CrossRef]
- Miyanishi, K.; Tanaka, S.; Sakamoto, H.; Kato, J. The role of iron in hepatic inflammation and hepatocellular carcinoma. Free Radic. Biol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Yanatori, I.; Tanaka, A.; Kishi, F.; Lemasters, J.J.; Nishina, S.; Sasaki, K.; Hino, K. Iron loss triggers mitophagy through induction of mitochondrial ferritin. EMBO Rep. 2020, e50202. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Kajiyama, H.; Suzuki, S.; Yoshihara, M.; Tamauchi, S.; Yoshikawa, N.; Niimi, K.; Shibata, K.; Kikkawa, F. Endometriosis and cancer. Free Radic. Biol. Med. 2018, 133, 186–192. [Google Scholar] [CrossRef]
- IARC; WHO. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens; Part C: Arsenic, Metals, Fibres, and Dusts; IARC Publications: Lyon, France, 2012; Volume 100C, pp. 219–309. [Google Scholar]
- Chua, A.C.; Knuiman, M.W.; Trinder, D.; Divitini, M.L.; Olynyk, J.K. Higher concentrations of serum iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am. J. Clin. Nutr. 2016, 104, 736–742. [Google Scholar] [CrossRef]
- Zacharski, L.; Chow, B.; Howes, P.; Shamayeva, G.; Baron, J.; Dalman, R.; Malenka, D.; Ozaki, C.; Levori, P. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: Results from a randomized trial. J. Natl. Cancer Inst. 2008, 100, 996–1002. [Google Scholar] [CrossRef]
- Bates, G.W.; Wernicke, J. The kinetics and mechanism of Iron (III) exchange between chelates and Transferrin IV. The reaction of transferrin with iron (III) nitrilotriacetate. J. Biol. Chem. 1971, 246, 3679–3685. [Google Scholar]
- Mottola, H.A. Nitrilotriacetic acid as a chelating agent: Applications, toxicology, and bio-environmental impact. Toxicol. Environ. Chem. Rev. 1974, 71, 99–161. [Google Scholar] [CrossRef]
- Toyokuni, S.; Sagripanti, J.L. DNA single- and double-strand breaks produced by ferric nitrilotriacetate in relation to renal tubular carcinogenesis. Carcinogenesis 1993, 14, 223–227. [Google Scholar] [CrossRef]
- Toyokuni, S.; Sagripanti, J.-L. Association between 8-hydroxy-2′-deoxyguanosine formation and DNA strand breaks mediated by copper and iron. Free Radic. Biol. Med. 1996, 20, 859–864. [Google Scholar] [CrossRef]
- Awai, M.; Narasaki, M.; Yamanoi, Y.; Seno, S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate: A model of experimental hemochromatosis. Am. J. Pathol. 1979, 95, 663–674. [Google Scholar] [PubMed]
- Okada, S.; Midorikawa, O. Induction of rat renal adenocarcinoma by Fe-nitrilotriacetate (Fe-NTA). Jpn. Arch. Intern Med. 1982, 29, 485–491. [Google Scholar]
- Ebina, Y.; Okada, S.; Hamazaki, S.; Ogino, F.; Li, J.L.; Midorikawa, O. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum- nitrilotriacetate complexes in rats. J. Natl. Cancer Inst. 1986, 76, 107–113. [Google Scholar]
- Li, J.L.; Okada, S.; Hamazaki, S.; Ebina, Y.; Midorikawa, O. Subacute nephrotoxicity and induction of renal cell carcinoma in mice treated with ferric nitrilotriacetate. Cancer Res. 1987, 47, 1867–1869. [Google Scholar] [PubMed]
- Nishiyama, Y.; Suwa, H.; Okamoto, K.; Fukumoto, M.; Hiai, H.; Toyokuni, S. Low incidence of point mutations in H-, K- and N-ras oncogenes and p53 tumor suppressor gene in renal cell carcinoma and peritoneal mesothelioma of Wistar rats induced by ferric nitrilotriacetate. Jpn. J. Cancer Res. 1995, 86, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, S.; Okada, S.; Ebina, Y.; Midorikawa, O. Acute renal failure and glucosuria induced by ferric nitrilotriacetate in rats. Toxicol. Appl. Pharmacol. 1985, 77, 267–274. [Google Scholar] [CrossRef]
- Hamazaki, S.; Okada, S.; Ebina, Y.; Fujioka, M.; Midorikawa, O. Nephrotoxicity of ferric nitrilotriacetate: An electron-microscopic and metabolic study. Am. J. Pathol. 1986, 123, 343–350. [Google Scholar] [PubMed]
- Toyokuni, S.; Okada, S.; Hamazaki, S.; Minamiyama, Y.; Yamada, Y.; Liang, P.; Fukunaga, Y.; Midorikawa, O. Combined histochemical and biochemical analysis of sex hormone dependence of ferric nitrilotriacetate-induced renal lipid peroxidation in ddY mice. Cancer Res. 1990, 50, 5574–5580. [Google Scholar] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Toyokuni, S.; Uchida, K.; Okamoto, K.; Hattori-Nakakuki, Y.; Hiai, H.; Stadtman, E.R. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc. Natl. Acad. Sci. USA 1994, 91, 2616–2620. [Google Scholar] [CrossRef]
- Toyokuni, S.; Miyake, N.; Hiai, H.; Hagiwara, M.; Kawakishi, S.; Osawa, T.; Uchida, K. The monoclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995, 359, 189–191. [Google Scholar] [CrossRef]
- Toyokuni, S.; Luo, X.P.; Tanaka, T.; Uchida, K.; Hiai, H.; Lehotay, D.C. Induction of a wide range of C2-12 aldehydes and C7-12 acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic. Biol. Med. 1997, 22, 1019–1027. [Google Scholar] [CrossRef]
- Toyokuni, S.; Mori, T.; Dizdaroglu, M. DNA base modifications in renal chromatin of Wistar rats treated with a renal carcinogen, ferric nitrilotriacetate. Int. J. Cancer 1994, 57, 123–128. [Google Scholar] [CrossRef]
- Toyokuni, S.; Tanaka, T.; Hattori, Y.; Nishiyama, Y.; Ochi, H.; Hiai, H.; Uchida, K.; Osawa, T. Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: Its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab. Investig. 1997, 76, 365–374. [Google Scholar]
- Toyokuni, S.; Mori, T.; Hiai, H.; Dizdaroglu, M. Treatment of Wistar rats with a renal carcinogen, ferric nitrilotriacetate, causes DNA-protein cross-linking between thymine and tyrosine in their renal chromatin. Int. J. Cancer 1995, 62, 309–313. [Google Scholar] [CrossRef]
- Tanaka, T.; Iwasa, Y.; Kondo, S.; Hiai, H.; Toyokuni, S. High incidence of allelic loss on chromosome 5 and inactivation of p15 INK4B and p16 INK4A tumor suppressor genes in oxystress-induced renal cell carcinoma of rats. Oncogene 1999, 18, 3793–3797. [Google Scholar] [CrossRef]
- Akatsuka, S.; Yamashita, Y.; Ohara, H.; Liu, Y.T.; Izumiya, M.; Abe, K.; Ochiai, M.; Jiang, L.; Nagai, H.; Okazaki, Y.; et al. Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLoS ONE 2012, 7, e43403. [Google Scholar] [CrossRef]
- Hiroyasu, M.; Ozeki, M.; Kohda, H.; Echizenya, M.; Tanaka, T.; Hiai, H.; Toyokuni, S. Specific allelic loss of p16 (INK4A) tumor suppressor gene after weeks of iron-mediated oxidative damage during rat renal carcinogenesis. Am. J. Pathol. 2002, 160, 419–424. [Google Scholar] [CrossRef]
- Toyokuni, S. Oxidative stress and cancer: The role of redox regulation. Biotherapy 1998, 11, 147–154. [Google Scholar] [CrossRef]
- Tanaka, T.; Kondo, S.; Iwasa, Y.; Hiai, H.; Toyokuni, S. Expression of stress-response and cell proliferation genes in renal cell carcinoma induced by oxidative stress. Am. J. Pathol. 2000, 156, 2149–2157. [Google Scholar] [CrossRef]
- Tanaka, T.; Akatsuka, S.; Ozeki, M.; Shirase, T.; Hiai, H.; Toyokuni, S. Redox regulation of annexin 2 and its implications for oxidative stess-induced renal carcinogenesis and metastasis. Oncogene 2004, 23, 3980–3989. [Google Scholar]
- Dutta, K.K.; Nishinaka, Y.; Masutani, H.; Akatsuka, S.; Aung, T.T.; Shirase, T.; Lee, W.H.; Yamada, Y.; Hiai, H.; Yodoi, J.; et al. Two distinct mechanisms for loss of thioredoxin-binding protein-2 in oxidative stress-induced renal carcinogenesis. Lab. Investig. 2005, 85, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Akatsuka, S.; Nagai, H.; Liu, Y.T.; Jiang, L.; Okazaki, Y.; Yamashita, Y.; Nakamura, T.; Toyokuni, S. Stage-specific roles of fibulin-5 during oxidative stress-induced renal carcinogenesis in rats. Free Radic. Res. 2011, 45, 211–220. [Google Scholar] [PubMed]
- Akatsuka, S.; Li, G.H.; Toyokuni, S. Superiority of rat over murine model for studies on the evolution of cancer genome. Free Radac. Res. 2018, 52, 1323–1327. [Google Scholar] [CrossRef]
- Li, G.H.; Akatsuka, S.; Chew, S.H.; Jiang, L.; Nishiyama, T.; Sakamoto, A.; Takahashi, T.; Futakuchi, M.; Suzuki, H.; Sakumi, K.; et al. Fenton reaction-induced renal carcinogenesis in Mutyh-deficient mice exhibits less chromosomal aberrations than the rat model. Pathol. Int. 2017, 67, 564–574. [Google Scholar] [CrossRef]
- Toyokuni, S.; Akatsuka, S. What has been learned from the studies of oxidative stress-induced carcinogenesis: Proposal of the concept of oxygenomics. J. Clin. Biochem. Nutr. 2006, 39, 3–10. [Google Scholar] [CrossRef]
- Toyokuni, S. Molecular mechanisms of oxidative stress-induced carcinogenesis: From epidemiology to oxygenomics. IUBMB Life 2008, 60, 441–447. [Google Scholar] [CrossRef]
- Akatsuka, S.; Toyokuni, S. Genome-scale approaches to investigate oxidative DNA damage. J. Clin. Biochem. Nutr. 2010, 47, 91–97. [Google Scholar] [CrossRef]
- Akatsuka, S.; Toyokuni, S. Genome-wide assessment of oxidatively generated DNA damage. Free Radic. Res. 2012, 46, 523–530. [Google Scholar] [CrossRef]
- Akatsuka, S.; Aung, T.T.; Dutta, K.K.; Jiang, L.; Lee, W.H.; Liu, Y.T.; Onuki, J.; Shirase, T.; Yamasaki, K.; Ochi, H.; et al. Contrasting genome-wide distribution of 8-hydroxyguanine and acrolein-modified adenine during oxidative stress-induced renal carcinogenesis. Am. J. Pathol. 2006, 169, 1328–1342. [Google Scholar] [CrossRef]
- Yoshihara, M.; Jiang, L.; Akatsuka, S.; Suyama, M.; Toyokuni, S. Genome-wide profiling of 8-Oxoguanine reveals its association with spatial positioning in nucleus. DNA Res. 2014, 21, 603–612. [Google Scholar] [CrossRef]
- Akatsuka, S.; Li, G.H.; Kawaguchi, S.; Takahashi, T.; Yoshihara, M.; Suyama, M.; Toyokuni, S. Augmented oxidative stress increases 8-oxoguanine preferentially in the transcriptionally active genomic regions. Free Radic. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.; Akatsuka, S.; Nagai, H.; Chew, S.H.; Ohara, H.; Okazaki, Y.; Yamashita, Y.; Yoshikawa, Y.; Yasui, H.; Ikuta, K.; et al. Iron overload signature in chrysotile-induced malignant mesothelioma. J. Pathol. 2012, 228, 366–377. [Google Scholar]
- Aierken, D.; Okazaki, Y.; Chew, S.H.; Sakai, A.; Wang, Y.; Nagai, H.; Misawa, N.; Kohyama, N.; Toyokuni, S. Rat model demonstrates a high risk of tremolite but a low risk of anthophyllite for mesothelial carcinogenesis. Nagoya J. Med. Sci. 2014, 76, 149–160. [Google Scholar]
- Nagai, H.; Toyokuni, S. Biopersistent fiber-induced inflammation and carcinogenesis: Lessons learned from asbestos toward safety of fibrous nanomaterials. Arch. Biochem. Biophys. 2010, 502, 1–7. [Google Scholar] [CrossRef]
- Kubo, Y.; Takenaka, H.; Nagai, H.; Toyokuni, S. Distinct affinity of nuclear proteins to the surface of chrysotile and crocidolite. J. Clin. Biochem. Nutr. 2012, 51, 221–226. [Google Scholar] [CrossRef]
- Ito, F.; Yanatori, I.; Maeda, Y.; Nimura, K.; Ito, S.; Hirayama, T.; Nagasawa, H.; Kohyama, N.; Okazaki, Y.; Akatsuka, S.; et al. Asbestos conceives Fe(II)-dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and beta-catenin induction in mesothelium. Redox Biol. 2020, 36, 101616. [Google Scholar] [CrossRef]
- Nagai, H.; Ishihara, T.; Lee, W.H.; Ohara, H.; Okazaki, Y.; Okawa, K.; Toyokuni, S. Asbestos surface provides a niche for oxidative modification. Cancer Sci. 2011, 102, 2118–2125. [Google Scholar]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yasui, H.; Toyokuni, S. Deferasirox induces mesenchymal-epithelial transition in crocidolite-induced mesothelial carcinogenesis in rats. Cancer Prev. Res. 2013, 6, 1222–1230. [Google Scholar] [CrossRef]
- Jiang, L.; Chew, S.H.; Nakamura, K.; Ohara, Y.; Akatsuka, S.; Toyokuni, S. Dual preventive benefits of iron elimination by desferal in asbestos-induced mesothelial carcinogenesis. Cancer Sci. 2016, 107, 908–915. [Google Scholar] [CrossRef]
- Ohara, Y.; Chew, S.H.; Shibata, T.; Okazaki, Y.; Yamashita, K.; Toyokuni, S. Phlebotomy as a preventive measure for crocidolite-induced mesothelioma in male rats. Cancer Sci. 2018, 109, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron overload as a major targetable pathogenesis of asbestos-induced mesothelial carcinogenesis. Redox Rep. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, S.; Okazaki, Y.; Akatsuka, S.; Takahashi, T.; Sakumi, K.; Nakabeppu, Y.; Toyokuni, S. Mth1 deficiency provides longer survival upon intraperitoneal crocidolite injection in female mice. Free Radic. Res. 2020, 54, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Okazaki, Y.; Shi, L.; Kohda, H.; Tanaka, M.; Taki, K.; Nishioka, T.; Hirayama, T.; Nagasawa, H.; Yamashita, Y.; et al. Role of hemoglobin and transferrin in multi-wall carbon nanotube-induced mesothelial injury and carcinogenesis. Cancer Sci. 2016, 107, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Okazaki, Y.; Chew, S.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Yamashita, K.; Ishihara, T.; Yoshikawa, Y.; Jiang, L.; et al. Diameter of multi-walled carbon nanotubes is a critical factor in mesothelial injury and subsequent carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1330–E1338. [Google Scholar] [CrossRef]
- Toyokuni, S. Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv. Drug Deliv. Rev. 2013, 65, 2098–2110. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Qian, W.Z.; Zhang, Y.Y.; Wei, F. The road for nanomaterials industry: A review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Miyata, Y.; Shinohara, H.; Toyokuni, S. Intraperitoneal administration of tangled multiwalled carbon nanotubes of 15 nm in diameter does not induce mesothelial carcinogenesis in rats. Pathol. Int. 2013, 63, 457–462. [Google Scholar] [CrossRef]
- Toyokuni, S. Mysterious link between iron overload and CDKN2A/2B. J. Clin. Biochem. Nutr. 2011, 48, 46–49. [Google Scholar] [CrossRef]
- Liggett, W.H., Jr.; Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis—Basic biological phenomenon with wide-ranging implications in tissue kinetics. Brit. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Ito, F.; Nishiyama, T.; Shi, L.; Mori, M.; Hirayama, T.; Nagasawa, H.; Yasui, H.; Toyokuni, S. Contrasting intra- and extracellular distribution of catalytic ferrous iron in ovalbumin-induced peritonitis. Biochem. Biophys. Res. Commun. 2016, 476, 600–606. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Bradley, M.D.; Wagner, B.A.; Buettner, G.R.; Monga, V.; Milhem, M.; Spitz, D.R.; Allen, B.G. Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol. 2018, 14, 417–422. [Google Scholar] [CrossRef]
- Stevens, R.G.; Graubard, B.I.; Micozzi, M.S.; Neriishi, K.; Blumberg, B.S. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Int. J. Cancer 1994, 56, 364–369. [Google Scholar] [CrossRef]
- Lee, S.; Song, A.; Eo, W. Serum ferritin as a prognostic biomarker for survival in relapsed or refractory metastatic colorectal cancer. J. Cancer 2016, 7, 957–964. [Google Scholar] [CrossRef]
- Lee, S.; Eo, W.; Jeon, H.; Park, S.; Chae, J. Prognostic significance of host-related biomarkers for survival in patients with advanced non-small cell lung cancer. J. Cancer 2017, 8, 2974–2983. [Google Scholar] [CrossRef]
- Wu, S.J.; Zhang, Z.Z.; Cheng, N.S.; Xiong, X.Z.; Yang, L. Preoperative serum ferritin is an independent prognostic factor for liver cancer after hepatectomy. Surg. Oncol. 2019, 29, 159–167. [Google Scholar] [CrossRef]
- Habashy, H.O.; Powe, D.G.; Staka, C.M.; Rakha, E.A.; Ball, G.; Green, A.R.; Aleskandarany, M.; Paish, E.C.; Douglas Macmillan, R.; Nicholson, R.I.; et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res. Treat. 2010, 119, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Nanomaterials definition matters. Nat. Nanotechnol. 2019, 14, 193. [CrossRef] [PubMed]
- Grosse, Y.; Loomis, D.; Guyton, K.Z.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014, 15, 1427–1428. [Google Scholar] [CrossRef]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Mine, T.; Kondo, H.; Takeuchi, T.; Matsumoto, M.; Fukushima, S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part Fibre Toxicol. 2016, 13, 53. [Google Scholar] [CrossRef]
- Zhang, M.F.; Yang, M.; Nakajima, H.; Yudasaka, M.; Iijima, S.; Okazaki, T. Diameter-dependent degradation of 11 types of carbon nanotubes: Safety implications. ACS Appl. Nano Mater. 2019, 2, 4293–4301. [Google Scholar] [CrossRef]
- Hu, Z.H.; Wu, Z.T.; Han, C.; He, J.; Ni, Z.H.; Chen, W. Two-dimensional transition metal dichalcogenides: Interface and defect engineering. Chem. Soc. Rev. 2018, 47, 3100–3128. [Google Scholar] [CrossRef]
- Xu, S.J.; Zheng, H.Z.; Ma, R.L.; Wu, D.; Pan, Y.X.; Yin, C.Y.; Gao, M.; Wang, W.L.; Li, W.; Liu, S.J.; et al. Vacancies on 2D transition metal dichalcogenides elicit ferroptotic cell death. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Shen, Z.; Song, J.; Yung, B.C.; Zhou, Z.; Wu, A.; Chen, X. Emerging strategies of cancer therapy based on ferroptosis. Adv. Mater. 2018, 30, e1704007. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Tian, R.; Yang, Z.; Yu, G.; Lin, L.; Zhang, G.; Fan, W.; Zhang, F.; Niu, G.; et al. Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 6492–6496. [Google Scholar] [CrossRef] [PubMed]
- Li, W.P.; Su, C.H.; Chang, Y.C.; Lin, Y.J.; Yeh, C.S. Ultrasound-induced reactive oxygen species mediated therapy and imaging using a fenton reaction activable polymersome. ACS Nano 2016, 10, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Lei, Q.; Zhu, J.Y.; Fan, J.X.; Li, C.X.; Li, C.; Xu, Z.; Cheng, S.X.; Zhang, X.Z. Switching apoptosis to ferroptosis: Metal-organic network for high-efficiency anticancer therapy. Nano Lett. 2017, 17, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, J.; Dai, Z.; Hu, Z.; Chen, X.; Qi, Y.; Zheng, X.; Yu, D. pH-Responsive, self-sacrificial nanotheranostic agent for potential in vivo and in vitro dual modal MRI/CT imaging, real-time, and in situ monitoring of cancer therapy. Bioconjug. Chem. 2017, 28, 400–409. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Stefani, C.; Toyokuni, S.; Ganz, T.; Anderson, G.J.; Subramaniam, N.V.; Trinder, D.; Olynyk, J.K.; Chua, A.; Jansson, P.J.; et al. Redox cycling metals: Pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochim. Biophys. Acta 2016, 1863, 727–748. [Google Scholar] [CrossRef]
- Rehman, M.U.; Jawaid, P.; Uchiyama, H.; Kondo, T. Comparison of free radicals formation induced by cold atmospheric plasma, ultrasound, and ionizing radiation. Arch. Biochem. Biophys. 2016, 605, 19–25. [Google Scholar] [CrossRef]
- Tanaka, H.; Hori, M. Medical applications of non-thermal atmospheric pressure plasma. J. Clin. Biochem. Nutr. 2017, 60, 29–32. [Google Scholar] [CrossRef]
- Kajiyama, H.; Utsumi, F.; Nakamura, K.; Tanaka, H.; Toyokuni, S.; Hori, M.; Kikkawa, F. Future perspective of strategic non-thermal plasma therapy for cancer treatment. J. Clin. Biochem. Nutr. 2017, 60, 33–38. [Google Scholar] [CrossRef]
- Toyokuni, S.; Ikehara, Y.; Kikkawa, F.; Hori, M. Plasma Medical Science; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Tanaka, H.; Mizuno, M.; Toyokuni, S.; Maruyama, S.; Kodera, Y.; Terasaki, H.; Adachi, T.; Kato, M.; Kikkawa, F.; Hori, M. Cancer therapy using non-thermal atmospheric pressure plasma with ultra-high electron density. Phys. Plasmas 2015, 22, 122004. [Google Scholar] [CrossRef]
- Okazaki, Y.; Wang, Y.; Tanaka, H.; Mizuno, M.; Nakamura, K.; Kajiyama, H.; Kano, H.; Uchida, K.; Kikkawa, F.; Hori, M.; et al. Direct exposure of non-equilibrium atmospheric pressure plasma confers simultaneous oxidative and ultraviolet modifications in biomolecules. J. Clin. Biochem. Nutr. 2014, 55, 207–215. [Google Scholar] [CrossRef]
- Adachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.; Hori, M. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F. Variable susceptibility of ovarian cancer cells to non-thermal plasma-activated medium. Oncol. Rep. 2016, 35, 3169–3177. [Google Scholar] [CrossRef]
- Nakamura, K.; Peng, Y.; Utsumi, F.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; Kajiyama, H. Novel intraperitoneal treatment with non-thermal plasma-activated medium inhibits metastatic potential of ovarian cancer cells. Sci. Rep. 2017, 7, 6085. [Google Scholar] [CrossRef]
- Ikeda, J.I.; Tanaka, H.; Ishikawa, K.; Sakakita, H.; Ikehara, Y.; Hori, M. Plasma-activated medium (PAM) kills human cancer-initiating cells. Pathol. Int. 2018, 68, 23–30. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef]
- Shi, L.; Ito, F.; Wang, Y.; Okazaki, Y.; Tanaka, H.; Mizuno, M.; Hori, M.; Hirayama, T.; Nagasawa, H.; Richardson, D.R.; et al. Non-thermal plasma induces a stress response in mesothelioma cells resulting in increased endocytosis, lysosome biogenesis and autophagy. Free Radic. Biol. Med. 2017, 108, 904–917. [Google Scholar] [CrossRef]
- Sato, K.; Shi, L.; Ito, F.; Ohara, Y.; Motooka, Y.; Tanaka, H.; Mizuno, M.; Hori, M.; Hirayama, T.; Hibi, H.; et al. Non-thermal plasma specifically kills oral squamous cell carcinoma cells in a catalytic Fe(II)-dependent manner. J. Clin. Biochem. Nutr. 2019, 65, 8–15. [Google Scholar] [CrossRef]
- Ishida, C.; Mori, M.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Hori, M.; Iwase, A.; Kikkawa, F.; Toyokuni, S. Non-thermal plasma prevents progression of endometriosis in mice. Free Radic. Res. 2016, 50, 1131–1139. [Google Scholar] [CrossRef]
- Hayashi, S.; Nakamura, T.; Motooka, Y.; Ito, F.; Jiang, L.; Akatsuka, S.; Iwase, A.; Kajiyama, H.; Kikkawa, F.; Toyokuni, S. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020, 37, 101726. [Google Scholar] [CrossRef]
- Randeniya, L.K.; de Groot, G.J.J.B. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review. Plasma Process Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Yun, S.; Yoon, S.Y.; Hong, E.J.; Giri, S.S.; Kim, S.G.; Kim, S.W.; Han, S.J.; Kwon, J.; Oh, W.T.; Lee, S.B.; et al. Effect of plasma-activated water, used as a disinfectant, on the hatch rate of dormant cysts of the Artemia salina. Aquaculture 2020, 523. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Ito, F.; Okazaki, Y.; Tanaka, H.; Mizuno, M.; Hori, M.; Richardson, D.R.; Toyokuni, S. Biphasic effects of l-ascorbate on the tumoricidal activity of non-thermal plasma against malignant mesothelioma cells. Arch. Biochem. Biophys. 2016, 605, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Shi, L.; Toyokuni, S. Non-thermal plasma as a simple ferroptosis inducer in cancer cells: A possible role of ferritin. Pathol. Int. 2018, 68, 442–443. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).