Poziotinib Inhibits the Efflux Activity of the ABCB1 and ABCG2 Transporters and the Expression of the ABCG2 Transporter Protein in Multidrug Resistant Colon Cancer Cells
Simple Summary
Abstract
1. Introduction
2. Results
2.1. The Cytotoxicity of Poziotinib in Parental and MDR Colon Cancer Cell Lines
2.2. Poziotinib Increases the Anticancer Efficacy of Substrate Chemotherapeutic Drugs in Colon Cancer Cells Overexpressing ABCG2 and ABCB1 Trasnporters
2.3. Poziotinib Significantly Downregulates the ABCG2 but Not the ABCB1 Transporter Protein Expression Level Without Affecting the Membrane Localization of the ABCG2 and ABCB1 Transporters
2.4. Poziotinib Increases [3H]- Mitoxantrone Accumulation in S1-M1-80 Colon Cancer Cells by Downregulating the Expression of the ABCG2 Transporter Protein
2.5. Poziotinib Significantly Stimulates ABCB1 and ABCG2 ATPase Activity
2.6. Poziotinib Significantly Increases the Intracellular Accumulation of ABCG2 and ABCB1 Transporter Substrates in Colon Cancer Cells Overexpressing the ABCG2 and ABCB1 Transporter
2.7. Poziotinib Decreases the Efflux of the ABCB1 and ABCG2 Substrates, [3H]-Mitoxantrone and [3H]-Paclitaxel, Respectively, from MDR Colon Cancer Cells
2.8. Poziotinib Reversiblely Inhibits the Efflux Function of the ABCG2 and ABCB1 Transporters
2.9. Poziotinib Binds to the Drug-Substrate Pocket in the Human ABCB1and ABCG2 Transporters
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Cell Culture
4.3. MTT Cell-Proliferation Assay
4.4. Immunoblotting
4.5. Immunofluorescence Microscopy
4.6. [3H]-Substrate Accumulation and Efflux Assay
4.7. Evaluation of ABCB1 and ABCG2 ATPase Activity
4.8. In Silico Molecular Docking Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davies, R.J.; Miller, R.; Coleman, N. Colorectal cancer screening: Prospects for molecular stool analysis. Nat. Rev. Cancer 2005, 5, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Eisterer, W.; Prager, G. Chemotherapy, still an option in the twenty-first century in metastatic colorectal cancer? Cardiovasc. Interv. Radiol. 2019, 42, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Begicevic, R.R.; Falasca, M. ABC Transporters in cancer stem cells: Beyond chemoresistance. Int. J. Mol. Sci. 2017, 18, 2362. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer Chemother. 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer Chemother. 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Mohammad, I.S.; He, W.; Yin, L. Understanding of human ATP binding cassette superfamily and novel multidrug resistance modulators to overcome MDR. Biomed. Pharmacother. 2018, 100, 335–348. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Linton, K.J. Structure and function of ABC transporters. Physiology 2007, 22, 122–130. [Google Scholar] [CrossRef]
- Hu, T.; Li, Z.; Gao, C.Y.; Cho, C.H. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J. Gastroenterol. 2016, 22, 6876–6889. [Google Scholar] [CrossRef]
- Wolking, S.; Schaeffeler, E.; Lerche, H.; Schwab, M.; Nies, A.T. Impact of genetic polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on drug disposition and potential clinical implications: Update of the literature. Clin. Pharmacokinet. 2015, 54, 709–735. [Google Scholar] [CrossRef]
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC transporter-mediated multidrug-resistant cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580. [Google Scholar] [CrossRef]
- Candeil, L.; Gourdier, I.; Peyron, D.; Vezzio, N.; Copois, V.; Bibeau, F.; Orsetti, B.; Scheffer, G.L.; Ychou, M.; Khan, Q.A.; et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int. J. Cancer 2004, 109, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Stenvang, J.; Budinská, E.; van Cutsem, E.; Bosman, F.; Popovici, V.; Brünner, N. An explorative analysis of ABCG2/TOP-1 mRNA expression as a biomarker test for FOLFIRI treatment in stage III colon cancer patients: Results from retrospective analyses of the PETACC-3 trial. Cancers 2020, 12, 977. [Google Scholar] [CrossRef]
- Jensen, N.F.; Stenvang, J.; Beck, M.K.; Hanáková, B.; Belling, K.C.; Do, K.N.; Viuff, B.; Nygård, S.B.; Gupta, R.; Rasmussen, M.H.; et al. Establishment and characterization of models of chemotherapy resistance in colorectal cancer: Towards a predictive signature of chemoresistance. Mol. Oncol. 2015, 9, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra, E.C.; Dekker, H.; Schuurhuis, G.J.; Broxterman, H.J.; Lankelma, J. P-glycoprotein drug efflux pump involved in the mechanisms of intrinsic drug resistance in various colon cancer cell lines. Evidence for a saturation of active daunorubicin transport. Biochem. Pharmacol. 1991, 41, 349–359. [Google Scholar] [CrossRef]
- Meschini, S.; Calcabrini, A.; Monti, E.; Del Bufalo, D.; Stringaro, A.; Dolfini, E.; Arancia, G. Intracellular P-glycoprotein expression is associated with the intrinsic multidrug resistance phenotype in human colon adenocarcinoma cells. Int. J. Cancer 2000, 87, 615–628. [Google Scholar] [CrossRef]
- Ji, N.; Yang, Y.; Cai, C.Y.; Lei, Z.N.; Wang, J.Q.; Gupta, P.; Shukla, S.; Ambudkar, S.V.; Kong, D.; Chen, Z.S. Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1 and ABCG2-overexpressing cancer cells. Cancer Lett. 2019, 440, 82–93. [Google Scholar] [CrossRef]
- Wu, Z.X.; Peng, Z.; Yang, Y.; Wang, J.Q.; Teng, Q.X.; Lei, Z.N.; Fu, Y.G.; Patel, K.; Liu, L.; Lin, L.; et al. M3814, a DNA-PK inhibitor, modulates ABCG2-mediated multidrug resistance in lung cancer cells. Front. Oncol. 2020, 10, 674. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, F.; Zheng, Z.; Chen, Z.; Wah To, K.K.; Zhang, H.; Han, Q.; Fu, L. Rociletinib (CO-1686) enhanced the efficacy of chemotherapeutic agents in ABCG2-overexpressing cancer cells in vitro and in vivo. Acta Pharm. Sin. B 2020, 10, 799–811. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.Q.; Cai, C.Y.; Cui, Q.; Yang, Y.; Wu, Z.X.; Dong, X.; Zeng, L.; Zhao, L.; Yang, D.H.; et al. Reversal effect of ALK inhibitor NVP-TAE684 on ABCG2-overexpressing cancer cells. Front. Oncol. 2020, 10, 228. [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, J.Y.; Teng, Q.X.; Lei, Z.N.; Ji, N.; Cui, Q.; Zeng, L.; Pan, Y.; Yang, D.H.; Chen, Z.S. Venetoclax, a BCL-2 inhibitor, enhances the efficacy of chemotherapeutic agents in wild-type ABCG2-overexpression-mediated MDR cancer cells. Cancers 2020, 12, 466. [Google Scholar] [CrossRef]
- Wu, Z.X.; Teng, Q.X.; Cai, C.Y.; Wang, J.Q.; Lei, Z.N.; Yang, Y.; Fan, Y.F.; Zhang, J.Y.; Li, J.; Chen, Z.S. Tepotinib reverses ABCB1-mediated multidrug resistance in cancer cells. Biochem. Pharmacol. 2019, 166, 120–127. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, M.; Wu, Z.X.; Wang, J.Q.; Dong, X.D.; Yang, Y.; Teng, Q.X.; Chen, X.Y.; Cui, Q.; Yang, D.H. Erdafitinib antagonizes ABCB1-mediated multidrug resistance in cancer cells. Front. Oncol. 2020, 10, 955. [Google Scholar] [CrossRef]
- Wei, L.Y.; Wu, Z.X.; Yang, Y.; Zhao, M.; Ma, X.Y.; Li, J.S.; Yang, D.H.; Chen, Z.S.; Fan, Y.F. Overexpression of ABCG2 confers resistance to pevonedistat, an NAE inhibitor. Exp. Cell Res. 2020, 388, 111858. [Google Scholar] [CrossRef]
- Wu, C.P.; Murakami, M.; Hsiao, S.H.; Chou, A.W.; Li, Y.Q.; Huang, Y.H.; Hung, T.H.; Ambudkar, S.V. Overexpression of ATP-Binding cassette subfamily G member 2 confers resistance to Phosphatidylinositol 3-Kinase inhibitor PF-4989216 in cancer cells. Mol. Pharm. 2017, 14, 2368–2377. [Google Scholar] [CrossRef]
- Wu, Z.X.; Yang, Y.; Teng, Q.X.; Wang, J.Q.; Lei, Z.N.; Wang, J.Q.; Lusvarghi, S.; Ambudkar, S.V.; Yang, D.H.; Chen, Z.S. Tivantinib, a c-Met inhibitor in clinical trials, is susceptible to ABCG2-mediated drug resistance. Cancers 2020, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Cai, C.Y.; Gao, H.L.; Ren, L.; Ji, N.; Gupta, P.; Yang, Y.; Shukla, S.; Ambudkar, S.V.; Yang, D.H.; et al. Glesatinib, a c-MET/SMO dual inhibitor, antagonizes P-glycoprotein mediated multidrug resistance in cancer cells. Front. Oncol. 2019, 9, 313. [Google Scholar] [CrossRef]
- Alam, A.; Kowal, J.; Broude, E.; Roninson, I.; Locher, K.P. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science 2019, 363, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Zeng, L.; Xu, M.; Wang, X.Q.; Yang, D.H.; Chen, Z.S. Osimertinib (AZD9291), a Mutant-Selective EGFR Inhibitor, Reverses ABCB1-Mediated Drug Resistance in Cancer Cells. Molecules 2016, 21, 1236. [Google Scholar] [CrossRef]
- Kim, T.Y.; Han, H.S.; Lee, K.W.; Zang, D.Y.; Rha, S.Y.; Park, Y.I.; Kim, J.S.; Lee, K.H.; Park, S.H.; Song, E.K.; et al. A phase I/II study of poziotinib combined with paclitaxel and trastuzumab in patients with HER2-positive advanced gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2019, 22, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Lee, K.H.; Kim, S.W.; Min, Y.J.; Cho, E.; Lee, Y.; Lee, S.H.; Kim, H.Y.; Lee, G.K.; Nam, B.H.; et al. A phase II study of Poziotinib in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma who have acquired resistance to EGFR-tyrosine kinase inhibitors. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2017, 49, 10–19. [Google Scholar] [CrossRef]
- Kim, T.M.; Lee, K.W.; Oh, D.Y.; Lee, J.S.; Im, S.A.; Kim, D.W.; Han, S.W.; Kim, Y.J.; Kim, T.Y.; Kim, J.H.; et al. Phase 1 studies of Poziotinib, an irreversible pan-HER tyrosine kinase inhibitor in patients with advanced solid tumors. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2018, 50, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Lee, K.H.; Sohn, J.H.; Lee, K.S.; Jung, K.H.; Kim, J.H.; Lee, K.H.; Ahn, J.S.; Kim, T.Y.; Kim, G.M.; et al. A phase II trial of the pan-HER inhibitor poziotinib, in patients with HER2-positive metastatic breast cancer who had received at least two prior HER2-directed regimens: Results of the NOV120101-203 trial. Int. J. Cancer 2018, 143, 3240–3247. [Google Scholar] [CrossRef]
- Kang, M.H.; Moon, S.U.; Sung, J.H.; Kim, J.W.; Lee, K.W.; Lee, H.S.; Lee, J.S.; Kim, J.H. Antitumor activity of HM781-36B, alone or in combination with chemotherapeutic agents, in colorectal cancer cells. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2016, 48, 355–364. [Google Scholar] [CrossRef]
- Nam, H.J.; Kim, H.P.; Yoon, Y.K.; Hur, H.S.; Song, S.H.; Kim, M.S.; Lee, G.S.; Han, S.W.; Im, S.A.; Kim, T.Y.; et al. Antitumor activity of HM781-36B, an irreversible pan-HER inhibitor, alone or in combination with cytotoxic chemotherapeutic agents in gastric cancer. Cancer Lett. 2011, 302, 155–165. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.P.; Yoon, Y.K.; Kim, M.S.; Lee, G.S.; Han, S.W.; Im, S.A.; Kim, T.Y.; Oh, D.Y.; Bang, Y.J. Antitumor activity of HM781-36B, a pan-HER tyrosine kinase inhibitor, in HER2-amplified breast cancer cells. Anti-Cancer Drugs 2012, 23, 288–297. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Suyama, K.; Baba, H. Recent advances in targeting the EGFR signaling pathway for the treatment of metastatic colorectal cancer. Int. J. Mol. Sci. 2017, 18, 752. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zhang, C.; Ma, Z. Epidermal growth factor receptor (EGFR): A rising star in the era of precision medicine of lung cancer. Oncotarget 2017, 8, 50209–50220. [Google Scholar] [CrossRef]
- Wang, L.; Lin, N.; Li, Y. The PI3K/AKT signaling pathway regulates ABCG2 expression and confers resistance to chemotherapy in human multiple myeloma. Oncol. Rep. 2019, 41, 1678–1690. [Google Scholar] [CrossRef]
- Ozvegy-Laczka, C.; Várady, G.; Köblös, G.; Ujhelly, O.; Cervenak, J.; Schuetz, J.D.; Sorrentino, B.P.; Koomen, G.J.; Váradi, A.; Német, K.; et al. Function-dependent conformational changes of the ABCG2 multidrug transporter modify its interaction with a monoclonal antibody on the cell surface. J. Biol. Chem. 2005, 280, 4219–4227. [Google Scholar] [CrossRef]
- Newman, M.J.; Rodarte, J.C.; Benbatoul, K.D.; Romano, S.J.; Zhang, C.; Krane, S.; Moran, E.J.; Uyeda, R.T.; Dixon, R.; Guns, E.S.; et al. Discovery and characterization of OC144-093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000, 60, 2964–2972. [Google Scholar]
- Miyake, K.; Mickley, L.; Litman, T.; Zhan, Z.; Robey, R.; Cristensen, B.; Brangi, M.; Greenberger, L.; Dean, M.; Fojo, T.; et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999, 59, 8–13. [Google Scholar] [PubMed]
- Lai, G.M.; Chen, Y.N.; Mickley, L.A.; Fojo, A.T.; Bates, S.E. P-glycoprotein expression and schedule dependence of adriamycin cytotoxicity in human colon carcinoma cell lines. Int. J. Cancer 1991, 49, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Yang, Y.; Cai, C.Y.; Lei, Z.N.; Wang, J.Q.; Gupta, P.; Teng, Q.X.; Chen, Z.S.; Kong, D.; Yang, D.H. VS-4718 antagonizes multidrug resistance in ABCB1 and ABCG2-overexpressing cancer cells by inhibiting the efflux function of ABC transporters. Front. Pharmacol. 2018, 9, 1236. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, G.N.; Wang, Y.J.; Patel, B.A.; Talele, T.T.; Yang, D.H.; Chen, Z.S. Bafetinib (INNO-406) reverses multidrug resistance by inhibiting the efflux function of ABCB1 and ABCG2 transporters. Sci. Rep. 2016, 6, 25694. [Google Scholar] [CrossRef]
- Stacy, A.E.; Jansson, P.J.; Richardson, D.R. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol. Pharmacol. 2013, 84, 655–669. [Google Scholar] [CrossRef]
- Ejendal, K.F.; Diop, N.K.; Schweiger, L.C.; Hrycyna, C.A. The nature of amino acid 482 of human ABCG2 affects substrate transport and ATP hydrolysis but not substrate binding. Protein Sci. Publ. Protein Soc. 2006, 15, 1597–1607. [Google Scholar] [CrossRef]
- Özvegy-Laczka, C.; Köblös, G.; Sarkadi, B.; Váradi, A. Single amino acid (482) variants of the ABCG2 multidrug transporter: Major differences in transport capacity and substrate recognition. Biochim. Biophys. Acta Biomembr. 2005, 1668, 53–63. [Google Scholar] [CrossRef]
- Li, J.; Kumar, P.; Anreddy, N.; Zhang, Y.K.; Wang, Y.J.; Chen, Y.; Talele, T.T.; Gupta, K.; Trombetta, L.D.; Chen, Z.S. Quizartinib (AC220) reverses ABCG2-mediated multidrug resistance: In vitro and in vivo studies. Oncotarget 2017, 8, 93785–93799. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Honjo, Y.; Morisaki, K.; Nadjem, T.A.; Runge, S.; Risbood, M.; Poruchynsky, M.S.; Bates, S.E. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br. J. Cancer 2003, 89, 1971–1978. [Google Scholar] [CrossRef]
- Li, X.Q.; Wang, L.; Lei, Y.; Hu, T.; Zhang, F.L.; Cho, C.H.; To, K.K. Reversal of P-gp and BCRP-mediated MDR by tariquidar derivatives. Eur. J. Med. Chem. 2015, 101, 560–572. [Google Scholar] [CrossRef]
- Dai, C.L.; Tiwari, A.K.; Wu, C.P.; Su, X.D.; Wang, S.R.; Liu, D.G.; Ashby, C.R., Jr.; Huang, Y.; Robey, R.W.; Liang, Y.J.; et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008, 68, 7905–7914. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Sodani, K.; Wang, S.R.; Kuang, Y.H.; Ashby, C.R., Jr.; Chen, X.; Chen, Z.S. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem. Pharmacol. 2009, 78, 153–161. [Google Scholar] [CrossRef]
- Vaidyanathan, A.; Sawers, L.; Gannon, A.L.; Chakravarty, P.; Scott, A.L.; Bray, S.E.; Ferguson, M.J.; Smith, G. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel and olaparib-resistant ovarian cancer cells. Br. J. Cancer 2016, 115, 431–441. [Google Scholar] [CrossRef]
- Fan, Y.F.; Zhang, W.; Zeng, L.; Lei, Z.N.; Cai, C.Y.; Gupta, P.; Yang, D.H.; Cui, Q.; Qin, Z.D.; Chen, Z.S.; et al. Dacomitinib antagonizes multidrug resistance (MDR) in cancer cells by inhibiting the efflux activity of ABCB1 and ABCG2 transporters. Cancer Lett. 2018, 421, 186–198. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Xu, M.; Chen, L.; Zhang, X.; To, K.K.W.; Zhao, H.; Wang, F.; Xia, Z.; Chen, X.; et al. Osimertinib (AZD9291) enhanced the efficacy of chemotherapeutic agents in ABCB1 and ABCG2-overexpressing cells in vitro, in vivo, and ex vivo. Mol. Cancer Ther. 2016, 15, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Honjo, Y.; van de Laar, A.; Miyake, K.; Regis, J.T.; Litman, T.; Bates, S.E. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochim. Biophys. Acta 2001, 1512, 171–182. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, H.; Zhang, G.N.; Wang, Y.J.; Kathawala, R.J.; Si, R.; Patel, B.A.; Xu, J.; Chen, Z.S. Semi-synthetic ocotillol analogues as selective ABCB1-mediated drug resistance reversal agents. Oncotarget 2015, 6, 24277–24290. [Google Scholar] [CrossRef]
- Wu, Z.X.; Yang, Y.; Wang, G.; Wang, J.Q.; Teng, Q.X.; Sun, L.; Lei, Z.N.; Lin, L.; Chen, Z.S.; Zou, C. Dual TTK/CLK2 inhibitor, CC-671, selectively antagonizes ABCG2-mediated multidrug resistance in lung cancer cells. Cancer Sci. 2020, 111, 2872–2882. [Google Scholar] [CrossRef]
- Ji, N.; Yang, Y.; Lei, Z.N.; Cai, C.Y.; Wang, J.Q.; Gupta, P.; Xian, X.; Yang, D.H.; Kong, D.; Chen, Z.S. Ulixertinib (BVD-523) antagonizes ABCB1 and ABCG2-mediated chemotherapeutic drug resistance. Biochem. Pharmacol. 2018, 158, 274–285. [Google Scholar] [CrossRef]
- Wu, C.P.; Hsiao, S.H.; Huang, Y.H.; Hung, L.C.; Yu, Y.J.; Chang, Y.T.; Hung, T.H.; Wu, Y.S. Sitravatinib sensitizes ABCB1 and ABCG2-overexpressing multidrug-resistant cancer cells to chemotherapeutic drugs. Cancers 2020, 12, 195. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Y.; To, K.K.; Wang, F.; Li, D.; Chen, L.; Fu, L. Alectinib (CH5424802) antagonizes ABCB1 and ABCG2-mediated multidrug resistance in vitro, in vivo and ex vivo. Exp. Mol. Med. 2017, 49, e303. [Google Scholar] [CrossRef]
- Wu, C.P.; Lusvarghi, S.; Wang, J.C.; Hsiao, S.H.; Huang, Y.H.; Hung, T.H.; Ambudkar, S.V. Avapritinib: A selective inhibitor of KIT and PDGFRα that reverses ABCB1 and ABCG2-mediated multidrug resistance in cancer lell Lines. Mol. Pharm. 2019, 16, 3040–3052. [Google Scholar] [CrossRef]
- Zhang, G.N.; Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Ashby, C.R., Jr.; Alqahtani, S.; Deng, T.; Bates, S.E.; Kaddoumi, A.; Wurpel, J.N.D.; et al. Epidermal growth factor receptor (EGFR) inhibitor PD153035 reverses ABCG2-mediated multidrug resistance in non-small cell lung cancer: In vitro and in vivo. Cancer Lett. 2018, 424, 19–29. [Google Scholar] [CrossRef]
- Pick, A.; Wiese, M. Tyrosine kinase inhibitors influence ABCG2 expression in EGFR-positive MDCK BCRP cells via the PI3K/Akt signaling pathway. ChemMedChem 2012, 7, 650–662. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Gao, S.; Hu, H.; Xie, P. HOXB4 knockdown enhances the cytotoxic effect of paclitaxel and cisplatin by downregulating ABC transporters in ovarian cancer cells. Gene 2018, 663, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y. Deoxyshikonin inhibits cisplatin resistance of non–small-cell lung cancer cells by repressing Akt-mediated ABCB1 expression and function. J. Biochem. Mol. Toxicol. 2020, 34, e22560. [Google Scholar] [CrossRef] [PubMed]
- Chau, W.K.; Ip, C.K.; Mak, A.S.C.; Lai, H.C.; Wong, A.S.T. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin–ATP-binding cassette G2 signaling. Oncogene 2013, 32, 2767–2781. [Google Scholar] [CrossRef]
- Yun, J.; Lee, S.H.; Kim, S.Y.; Jeong, S.Y.; Kim, J.H.; Pyo, K.H.; Park, C.W.; Heo, S.G.; Yun, M.R.; Lim, S.; et al. Antitumor activity of Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR Exon 20 insertion-driven NSCLC. Cancer Discov. 2020, 10, 1194–1209. [Google Scholar] [CrossRef]
- Liao, D.; Zhang, W.; Gupta, P.; Lei, Z.N.; Wang, J.Q.; Cai, C.Y.; Vera, A.A.; Zhang, L.; Chen, Z.S.; Yang, D.H. Tetrandrine interaction with ABCB1 reverses multidrug resistance in cancer cells through competition with anti-cancer drugs followed by downregulation of ABCB1 expression. Molecules 2019, 24, 4383. [Google Scholar] [CrossRef]
- Kontoyianni, M. Docking and virtual screening in drug discovery. Methods Mol. Biol. 2017, 1647, 255–266. [Google Scholar] [CrossRef]
- Ferreira, R.J.; Bonito, C.A.; Cordeiro, M.; Ferreira, M.U.; Dos Santos, D. Structure-function relationships in ABCG2: Insights from molecular dynamics simulations and molecular docking studies. Sci. Rep. 2017, 7, 15534. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, N.; Teng, Q.X.; Cai, C.Y.; Wang, J.Q.; Wu, Z.X.; Lei, Z.N.; Lusvarghi, S.; Ambudkar, S.V.; Chen, Z.S. Sitravatinib, a tyrosine kinase inhibitor, inhibits the transport function of ABCG2 and restores sensitivity to chemotherapy-resistant cancer cells in vitro. Front. Oncol. 2020, 10, 700. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, N.; Cai, C.Y.; Wang, J.Q.; Lei, Z.N.; Teng, Q.X.; Wu, Z.X.; Cui, Q.; Pan, Y.; Chen, Z.S. Modulating the function of ABCB1: In vitro and in vivo characterization of sitravatinib, a tyrosine kinase inhibitor. Cancer Commun. 2020, 40, 285–300. [Google Scholar] [CrossRef]
- Hu, C.M.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Sarisozen, C.; Vural, I.; Levchenko, T.; Hincal, A.A.; Torchilin, V.P. Long-circulating PEG-PE micelles co-loaded with paclitaxel and elacridar (GG918) overcome multidrug resistance. Drug Deliv. 2012, 19, 363–370. [Google Scholar] [CrossRef]
- Safdari, Y.; Ahmadzadeh, V.; Khalili, M.; Jaliani, H.Z.; Zarei, V.; Erfani-Moghadam, V. Use of single chain antibody derivatives for targeted drug delivery. Mol. Med. 2016, 22, 258–270. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, B.; Lei, Z.N.; Teng, Q.X.; Li, J.Y.; Zhang, W.; Ji, N.; Cai, C.Y.; Ma, L.Y.; Liu, H.M.; et al. Derivative of 5-cyano-6-phenylpyrimidin antagonizes ABCB1 and ABCG2-mediated multidrug resistance. Eur. J. Pharmacol. 2019, 863, 172611. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
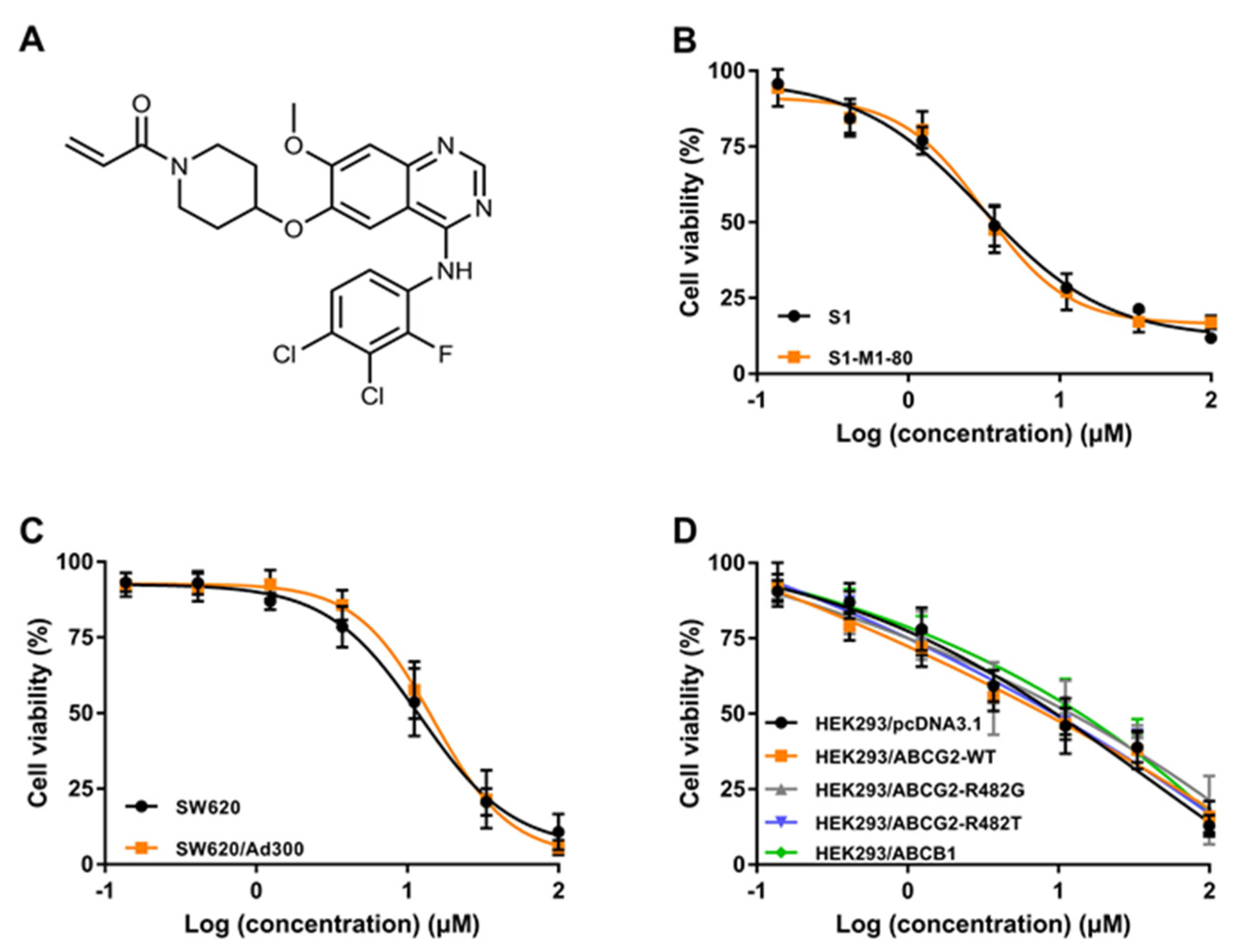

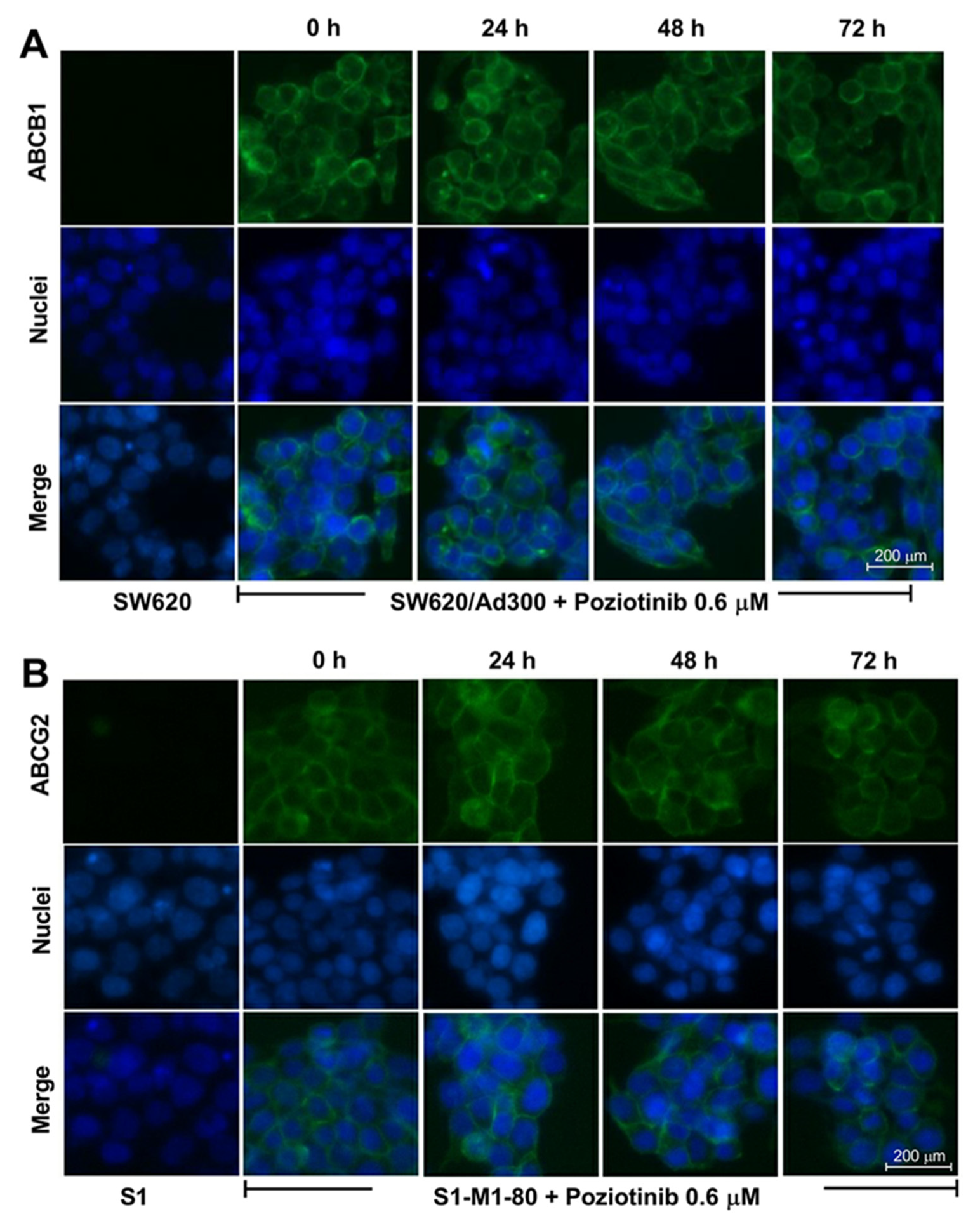

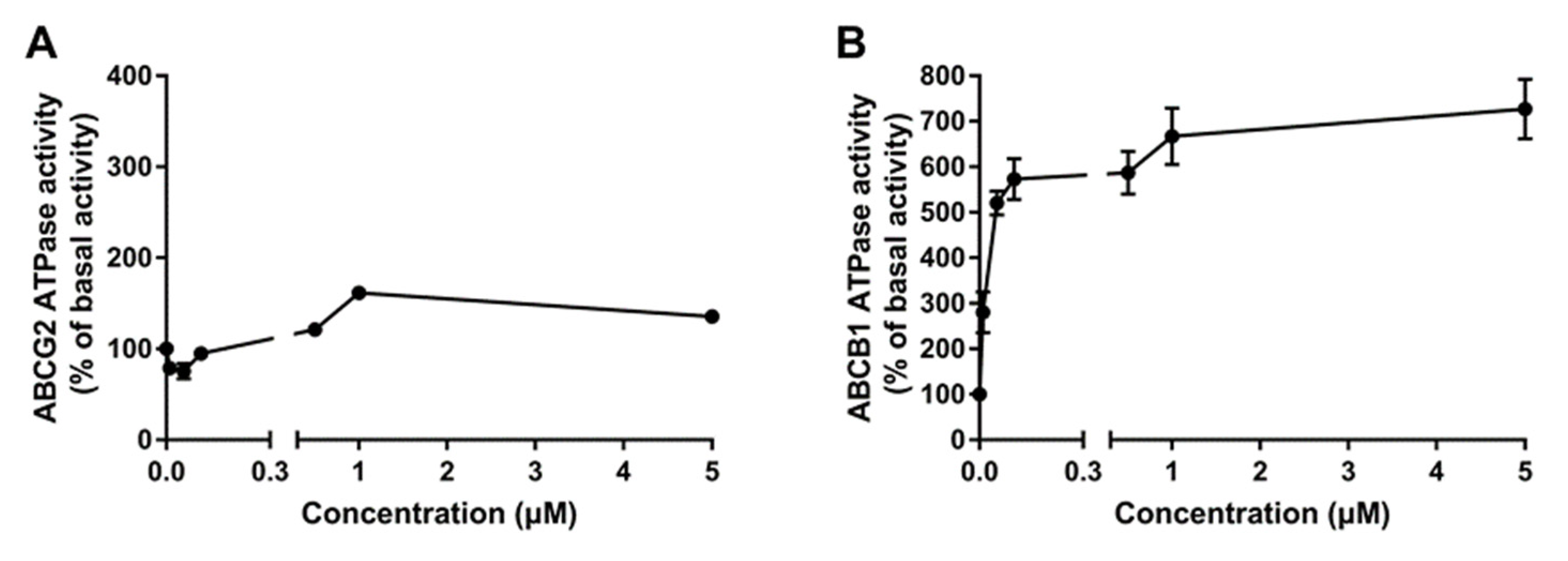





| Drugs | IC50 Value ± SD a (μM, RF b) | |
|---|---|---|
| S1 | S1-M1-80 | |
| Mitoxantrone | 0.046 ± 0.006 (1.00) | 5.768 ± 0.587 (125.75) |
| +Poziotinib 0.1 μM | 0.042 ± 0.012 (0.92) | 2.401 ± 0.486 (52.35) * |
| +Poziotinib 0.3 μM | 0.045 ± 0.020 (0.99) | 0.607 ± 0.225 (13.23) ** |
| +Poziotinib 0.6 μM | 0.045 ± 0.011 (0.98) | 0.342 ± 0.165 (7.45) * |
| +Ko143 0.6 μM | 0.050 ± 0.010 (1.09) | 0.291 ± 0.144 (6.34) * |
| SN-38 | 0.060 ± 0.015 (1.00) | 5.916 ± 0.783 (97.88) |
| +Poziotinib 0.1 μM | 0.052 ± 0.008 (0.85) | 2.847 ± 0.731 (47.11) |
| +Poziotinib 0.3 μM | 0.068 ± 0.027 (1.12) | 1.001 ± 0.143 (16.57) * |
| +Poziotinib 0.6 μM | 0.069 ± 0.019 (1.14) | 0.485 ± 0.012 (8.03) ** |
| +Ko143 0.6 μM | 0.086 ± 0.006 (1.42) | 0.328 ± 0.110 (5.42) ** |
| Oxaliplatin | 4.091 ± 0.525 (1.00) | 3.719 ± 0.622 (0.91) |
| +Poziotinib 0.1 μM | 3.763 ± 0.467 (0.92) | 4.061 ± 1.101 (0.99) |
| +Poziotinib 0.6 μM | 5.325 ± 0.492 (1.30) | 4.417 ± 1.083 (1.08) |
| +Ko143 0.6 μM | 3.719 ± 0.294 (0.91) | 4.780 ± 0.232 (1.17) |
| Drugs | IC50 Value ± SD a (μM, RF b) | |
|---|---|---|
| SW620 | SW620/Ad300 | |
| Paclitaxel | 0.190 ± 0.033 (1.00) | 14.680 ± 3.722 (77.30) |
| +Poziotinib 0.1 μM | 0.179 ± 0.062 (0.94) | 5.671 ± 0.469 (29.86) * |
| +Poziotinib 0.3 μM | 0.190 ± 0.005 (1.00) | 0.760 ± 0.181 (9.96) * |
| +Poziotinib 0.6 μM | 0.168 ± 0.006 (0.88) | 0.225 ± 0.012 (1.19) ** |
| +Verapamil 0.6 μM | 0.184 ± 0.012 (0.97) | 0.719 ± 0.070 (3.79) ** |
| Doxorubicin | 0.187 ± 0.028 (1.00) | 13.985 ± 2.170 (74.76) |
| +Poziotinib 0.1 μM | 0.193 ± 0.040 (1.03) | 6.596 ± 0.174 (35.26) * |
| +Poziotinib 0.3 μM | 0.180 ± 0.021 (0.96) | 1.065 ± 0.351 (5.69) ** |
| +Poziotinib 0.6 μM | 0.155 ± 0.007 (0.83) | 0.291 ± 0.085 (1.56) *** |
| +Verapamil 0.6 μM | 0.183 ± 0.021 (0.98) | 0.561 ± 0.104 (3.00) *** |
| Oxaliplatin | 12.484 ± 1.254 (1.00) | 13.614 ± 1.336 (1.09) |
| +Poziotinib 0.1 μM | 13.768 ± 0.924 (1.10) | 12.908 ± 1.364 (1.03) |
| +Poziotinib 0.6 μM | 11.708 ± 1.101 (0.94) | 14.048 ± 1.968 (1.13) |
| +Verapamil 0.6 μM | 11.378 ± 0.940 (0.91) | 13.458 ± 2.634 (1.08) |
| Treatment | IC50 Value ± SD a (μM, RF b) | |||
|---|---|---|---|---|
| pcDNA3.1 | ABCG2-WT | ABCG2-R482G | ABCG2-R482T | |
| Mitoxantrone | 0.105 ± 0.021 (1.00) | 2.551 ± 0.508 (24.01) | 2.827 ± 0.672 (27.03) | 2.197 ± 0.557 (21.01) |
| +Poziotinib 0.1 μM | 0.122 ± 0.019 (1.17) | 0.332 ± 0.018 (3.18) ** | 0.402 ± 0.020 (3.84) * | 0.907 ± 0.137 (8.67) * |
| +Poziotinib 0.3 μM | 0.098 ± 0.022 (0.94) | 0.266 ± 0.079 (2.55) * | 0.287 ± 0.074 (2.74) *** | 0.423 ± 0.191 (4.04) * |
| +Poziotinib 0.6 μM | 0.127 ± 0.027 (1.22) | 0.150 ± 0.003 (1.44) * | 0.273 ± 0.039 (2.61) * | 0.200 ± 0.086 (1.91) ** |
| +Ko143 0.6 μM | 0.104 ± 0.026 (0.99) | 0.232 ± 0.017 (2.21) * | 0.146 ± 0.043 (1.39) ** | 0.177 ± 0.088 (1.69) ** |
| SN-38 | 0.156 ± 0.050 (1.00) | 3.905 ± 0.245 (25.07) | 6.149 ± 0.582 (39.47) | 3.543 ± 0.122 (22.75) |
| +Poziotinib 0.1 μM | 0.203 ± 0.066 (1.31) | 1.363 ± 0.190 (8.75) * | 1.239 ± 0.208 (7.96) ** | 1.483 ± 0.200 (9.52) * |
| +Poziotinib 0.3 μM | 0.206 ± 0.039 (1.32) | 0.349 ± 0.120 (2.24) *** | 0.556 ± 0.195 (3.57) ** | 0.646 ± 0.190 (4.15) *** |
| +Poziotinib 0.6 μM | 0.198 ± 0.019 (1.27) | 0.182 ± 0.044 (1.17) *** | 0.226 ± 0.079 (1.45) *** | 0.363 ± 0.132 (2.33) *** |
| +Ko143 0.6 μM | 0.171 ± 0.038 (1.10) | 0.338 ± 0.141 (2.17) ** | 0.194 ± 0.064 (1.25) ** | 0.605 ± 0.141 (3.88) *** |
| Oxaliplatin | 7.004 ± 0.865 (1.00) | 9.412 ± 2.000 (1.34) | 6.321 ± 1.153 (0.90) | 5.836 ± 0.221 (0.83) |
| +Poziotinib 0.1 μM | 7.063 ± 0.984 (1.01) | 7.057 ± 1.939 (1.01) | 7.926 ± 1.839 (1.13) | 6.219 ± 1.127 (0.89) |
| +Poziotinib 0.6 μM | 5.672 ± 0.368 (0.81) | 7.318 ± 0.890 (0.04) | 6.638 ± 2.370 (0.95) | 6.785 ± 0.382 (0.97) |
| +Ko143 0.6 μM | 5.785 ± 0.374 (0.83) | 8.259 ± 0.832 (1.18) | 6.590 ± 1.544 (0.94) | 6.358 ± 1.138 (0.91) |
| Drugs | IC50 Value ± SD a (μM, RF b) | |
|---|---|---|
| HEK293/pcDNA3.1 | HEK293/ABCB1 | |
| Paclitaxel | 0.111 ± 0.006 (1.0) | 2.569 ± 0.603 (23.20) |
| +Poziotinib 0.1 μM | 0.126 ± 0.015 (1.14) | 1.301 ± 0.159 (11.75) * |
| +Poziotinib 0.3 μM | 0.120 ± 0.013 (1.08) | 0.573 ± 0.164 (5.17) * |
| +Poziotinib 0.6 μM | 0.104 ± 0.009 (0.94) | 0.216 ± 0.082 (1.95) ** |
| +Verapamil 0.6 μM | 0.113 ± 0.011 (1.02) | 0.499 ± 0.173 (4.51) * |
| Doxorubicin | 0.076 ± 0.006 (1.00) | 1.958 ± 0.651 (25.66) |
| +Poziotinib 0.1 μM | 0.075 ± 0.005 (0.98) | 0.750 ± 0.135 (9.83) * |
| +Poziotinib 0.3 μM | 0.076 ± 0.013 (1.00) | 0.361 ± 0.020 (4.74) ** |
| +Poziotinib 0.6 μM | 0.064 ± 0.002 (0.84) | 0.204 ± 0.061 (2.67) *** |
| +Verapamil 0.6 μM | 0.081 ± 0.002 (1.06) | 0.288± 0.093 (3.77) ** |
| Oxaliplatin | 7.004 ± 0.865 (1.00) | 6.981 ± 0.654 (1.00) |
| +Poziotinib 0.1 μM | 7.063 ± 0.984 (1.01) | 7.871 ± 2.158 (1.12) |
| +Poziotinib 0.6 μM | 6.779 ± 0.357 (0.97) | 8.128 ± 0.754 (1.16) |
| +Verapamil 0.6 μM | 5.783 ± 0.374 (0.83) | 7.211 ± 1.664 (1.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, Z.-X.; Yang, Y.; Wang, J.-Q.; Li, J.; Sun, Z.; Teng, Q.-X.; Ashby, C.R., Jr.; Yang, D.-H. Poziotinib Inhibits the Efflux Activity of the ABCB1 and ABCG2 Transporters and the Expression of the ABCG2 Transporter Protein in Multidrug Resistant Colon Cancer Cells. Cancers 2020, 12, 3249. https://doi.org/10.3390/cancers12113249
Zhang Y, Wu Z-X, Yang Y, Wang J-Q, Li J, Sun Z, Teng Q-X, Ashby CR Jr., Yang D-H. Poziotinib Inhibits the Efflux Activity of the ABCB1 and ABCG2 Transporters and the Expression of the ABCG2 Transporter Protein in Multidrug Resistant Colon Cancer Cells. Cancers. 2020; 12(11):3249. https://doi.org/10.3390/cancers12113249
Chicago/Turabian StyleZhang, Yongchao, Zhuo-Xun Wu, Yuqi Yang, Jing-Quan Wang, Jun Li, Zoey Sun, Qiu-Xu Teng, Charles R. Ashby, Jr., and Dong-Hua Yang. 2020. "Poziotinib Inhibits the Efflux Activity of the ABCB1 and ABCG2 Transporters and the Expression of the ABCG2 Transporter Protein in Multidrug Resistant Colon Cancer Cells" Cancers 12, no. 11: 3249. https://doi.org/10.3390/cancers12113249
APA StyleZhang, Y., Wu, Z.-X., Yang, Y., Wang, J.-Q., Li, J., Sun, Z., Teng, Q.-X., Ashby, C. R., Jr., & Yang, D.-H. (2020). Poziotinib Inhibits the Efflux Activity of the ABCB1 and ABCG2 Transporters and the Expression of the ABCG2 Transporter Protein in Multidrug Resistant Colon Cancer Cells. Cancers, 12(11), 3249. https://doi.org/10.3390/cancers12113249





